Effect of Organic Layer Thickness on Black Spruce Aging Mistakes in Canadian Boreal Forests
Abstract
:1. Introduction
2. Methods and Materials
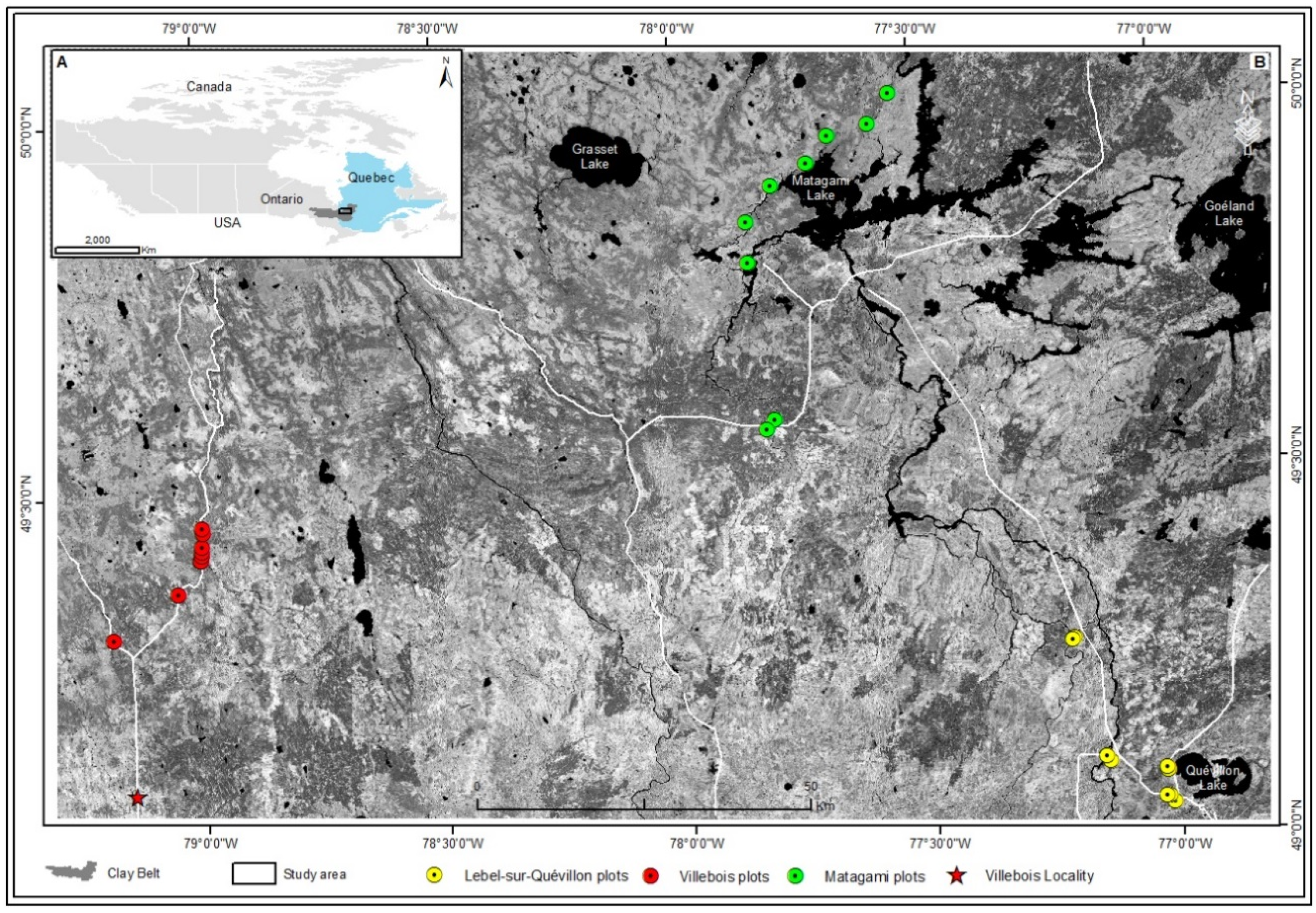
2.1. Study Area
2.2. Site Selection
2.3. Field Measurements and Sample Processing
2.4. Statistical Analysis
3. Results
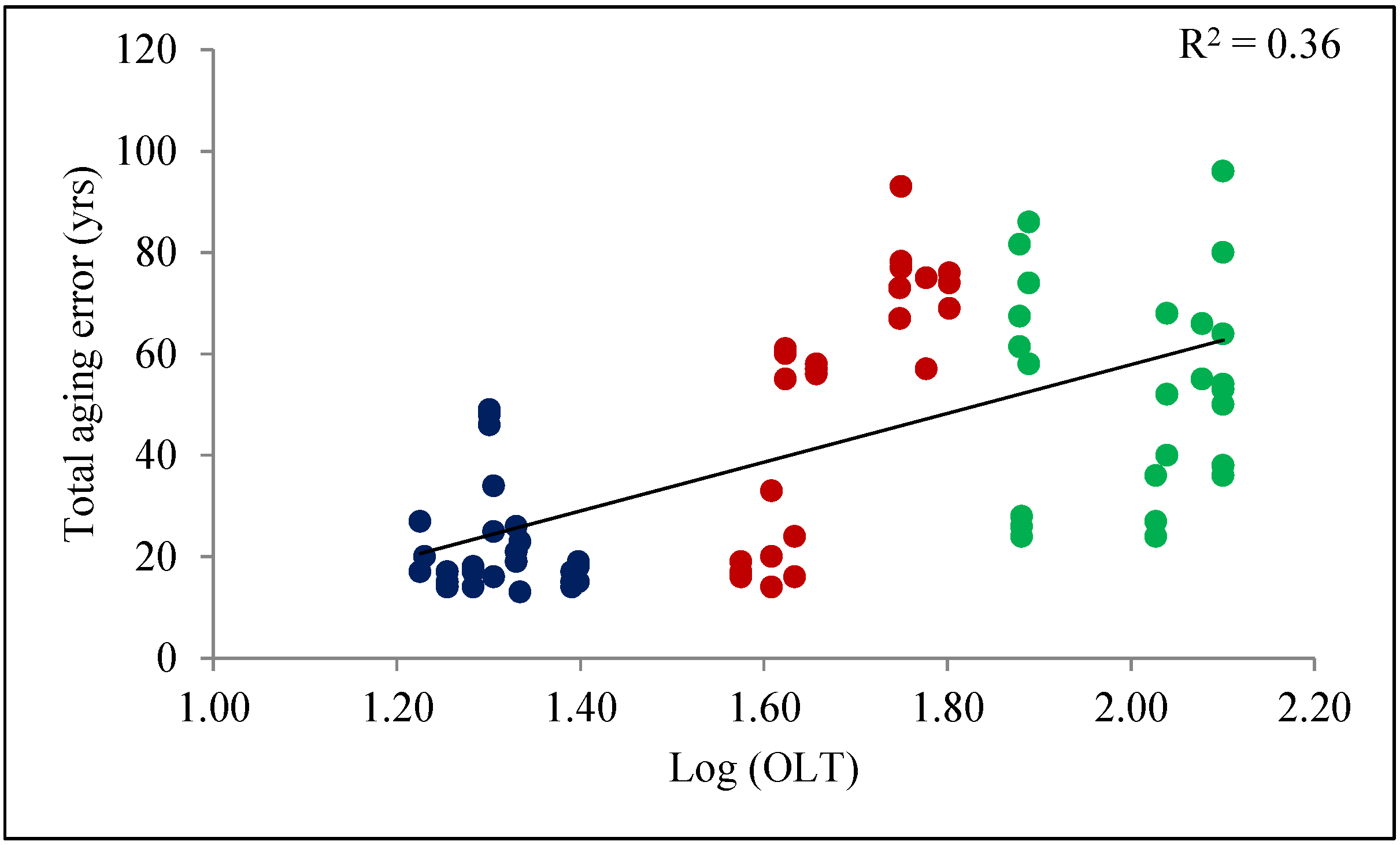
Using Linear Models to Predict Total Aging Error
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koubaa, A.; Isabel, N.; Zhang, S.Y.; Beaulieu, J.; Bousquet, J. Transition from juvenile to mature wood in black spruce (Picea mariana (Mill.) B.S.P.). Wood Fiber Sci. 2005, 37, 445–455. [Google Scholar]
- Crawford, R.M.M.; Jeffree, C.E.; Rees, W.G. Paludification and forest retreat in northern oceanic environments. Ann. Bot. Lond. 2003, 91, 213–226. [Google Scholar] [CrossRef]
- Vygodskaya, N.N.; Groisman, P.Y.; Tchebakova, N.M.; Kurbatova, J.A.; Panfyorov, O.; Parfenova, E.I.; Sogachev, A.F. Ecosystems and climate interactions in the boreal zone of northern Eurasia. Environ. Res. Lett. 2007, 2, 045033. [Google Scholar] [CrossRef]
- Fenton, N.; Lecomte, N.; Légaré, S.; Bergeron, Y. Paludification in black spruce (Picea mariana) forests of eastern Canada: Potential factors and management implications. For. Ecol. Manag. 2005, 213, 151–159. [Google Scholar] [CrossRef]
- Fenton, N.J.; Bergeron, Y. Sphagnum spore availability in boreal forests. Bryologist 2006, 109, 173–181. [Google Scholar] [CrossRef]
- Fenton, N.J.; Bergeron, Y. Sphagnum community change after partial harvest in black spruce boreal forests. For. Ecol. Manag. 2007, 242, 24–33. [Google Scholar] [CrossRef]
- DesRochers, A.; Gagnon, R. Is ring count at ground level a good estimation of black spruce age? Can. J. For. Res. 1997, 27, 263–1267. [Google Scholar]
- Krause, C.; Morin, H. Adventive-root development in mature black spruce and balsam fir in the boreal forests of Quebec, Canada. Can. J. For. Res. 2005, 35, 2642–2654. [Google Scholar] [CrossRef]
- Peters, V.S.; Macdonald, S.E.; Dale, M.R.T. Aging discrepancies of white spruce affect the interpretation of static age structure in boreal mixedwoods. Can. J. For. Res. 2002, 32, 1496–1501. [Google Scholar] [CrossRef]
- Gutsell, S.L.; Johnson, E.A. Accurately ageing trees and examining their height-growth rates: Implications for interpreting forest dynamics. J. Ecol. 2002, 90, 153–166. [Google Scholar] [CrossRef]
- Parent, S.; Morin, H.; Messier, C. Effects of adventitious roots on age determination in Balsam fir (Abies balsamea) regeneration. Can. J. For. Res. 2000, 30, 513–518. [Google Scholar]
- Niklasson, M. A comparison of three age determination methods for suppressed Norway spruce: Implications for age structure analysis. For. Ecol. Manag. 2002, 161, 279–288. [Google Scholar] [CrossRef]
- Groot, A.; Horton, B.J. Age and size structure of natural and second-growth peatland Picea mariana stands. Can. J. For. Res. 1994, 24, 225–233. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Stadt, K.J.; Stan, N. Age structure and growth of understory white spruce under aspen. Can. J. For. Res. 1996, 26, 1002–1007. [Google Scholar] [CrossRef]
- Garet, J.; Raulier, F.; Pothier, D.; Cumming, S.G. Forest age class structures as indicators of sustainability in boreal forest: Are we measuring them correctly? Ecol. Indic. 2012, 23, 202–210. [Google Scholar] [CrossRef]
- Wilmking, M.; Hallinger, M.; Van Bogaert, R.; Kyncl, T.; Babst, F.; Hahne, W.; Juday, G.P.; De Luis, M.; Novak, K.; Völlm, C. Continuously missing outer rings in woody plants at their distributional margins. Dendrochronologia 2012, 30, 213–222. [Google Scholar] [CrossRef]
- Wong, C.M.; Lertzman, K.P. Errors in estimating tree age: Implications for studies of stand dynamics. Can. J. For. Res. 2001, 31, 1262–1271. [Google Scholar] [CrossRef]
- St-Pierre, H.; Gagnon, R.; Bellefleur, P. Post-fire regeneration of black spruce Picea mariana and jack pine Pinus banksiana in the boreal forest, Quebec. Can. J. For. Res. 1992, 22, 474–481. [Google Scholar] [CrossRef]
- Oliver, C.; Larson, B. Forest Stand Dynamics; Wiley & Sons: New York, NY, USA, 1996; p. 509. [Google Scholar]
- Vasiliauskas, S.; Chen, H.Y.H. How long do trees take to reach breast height after fire in northeastern Ontario? Can. J. For. Res. 2002, 32, 1889–1892. [Google Scholar] [CrossRef]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology, 9th ed.; Wiley & Sons: New York, NY, USA, 1997; pp. 1–537. [Google Scholar]
- Pothier, D.; Savard, F. Actualisation des Tables de Production Pour les Principales Espèces Forestières du Québec; Ministère des Ressources Naturelles du Québec, Direction de la Recherche Forestière: Québec, QC, Canada, 1998; pp. 1–183.
- Fraver, S.; Bradford, J.B.; Palik, B.J. Improving tree age estimates derived from increment cores: A case study of red pine. For. Sci. 2011, 57, 164–70. [Google Scholar]
- Parisien, M.-A.; Sirois, L.; Parent, S. Landscape-level variability in the age underestimation of understory black spruce in the northern boreal forest of Quebec. Can. J. For. Res. 2005, 35, 633–642. [Google Scholar] [CrossRef]
- Robitaille, A.; Saucier, J.-P. Paysages Régionaux du Quebec Méridional; Les Publications du Québec: Sainte-Foy, QC, Canada, 1998; pp. 1–213. [Google Scholar]
- Veillette, J.J. Evolution and paleohydrology of glacial Lakes Barlow and Ojibway. Quat. Sci. Rev. 1994, 13, 945–971. [Google Scholar] [CrossRef]
- Soil Classification Working Group. The Canadian System of Soil Classification, 3rd ed.; Agriculture and Agri-Food Canad, Publication: Ottawa, ON, Canada, 1998. [Google Scholar]
- Laamrani, A.; Valeria, O.; Cheng, L.Z.; Bergeron, Y.; Camerlynck, C. The use of ground penetrating radar for remote sensing the organic layer—Mineral soil interface in paludified boreal forests. Can. J. Remote Sens. 2013, 39, 74–88. [Google Scholar] [CrossRef]
- Laamrani, A.; Valeria, O.; Fenton, N.; Bergeron, Y. Landscape-scale influence of topography on organic layer accumulation in paludified boreal forests. For. Sci. 2014, 60, 579–590. [Google Scholar]
- Laamrani, A.; Valeria, O.; Fenton, N.; Bergeron, Y.; Cheng, L.Z. The role of mineral soil topography on the spatial distribution of organic layer thickness in a paludified boreal landscape. Geoderma 2014, 221–222, 70–81. [Google Scholar] [CrossRef]
- Bergeron, Y.; Gauthier, S.; Kafka, V.; Lefort, P.; Lesieur, D. Natural fire frequency for the eastern Canadian boreal forest: Consequences for sustainable forestry. Can. J. For. Res. 2001, 31, 384–391. [Google Scholar] [CrossRef]
- Environment Canada. Canadian Climate Normals 1971–2000. Matagami Weather Station. Available online: http://climate.weather.gc.ca/climate_normals/ (accessed on 15 November 2015).
- Simard, M.; Bernier, P.Y.; Bergeron, Y.; Paré, D.; Guérine, L. Paludification dynamics in the boreal forest of the James Bay Lowlands: Effect of time since fire and topography. Can. J. For. Res. 2009, 39, 546–552. [Google Scholar] [CrossRef]
- MRNQ. Norme D’inventaire Écoforestier; Placettes–Échantillons Permanents, Edition 2013; Ministère des Ressources Naturelles du Québec, Direction des Inventaires Forestiers, Secteur des Forêts: Québec, QC, Canada, 2013; p. 213. [Google Scholar]
- Telewski, F.W.; Lynch, A.M. Measuring growth and development of stems. In Techniques and Approaches in Forest Tree Ecophysiology; Lassoie, J.P., Hinckley, T.M., Eds.; CRC Press Inc.: Boston, MA, USA, 1991; pp. 503–555. [Google Scholar]
- Swetnam, T.W.; Thompson, M.A.; Kennedy Sutherland, E. Using Dendrochronology to Measure Radial Growth of Defoliated Trees; Agriculture Handbook; USDA Forest Service: Washington, DC, USA, 1985; pp. 1–39.
- Schweingruber, F.H. Tree Rings: Basics and Applications of Dendrochronology, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 1–276. [Google Scholar]
- Perron, J.-Y. Tarif de Cubage Général-Volume Marchand Brut; Ministère des Ressources Naturelles du Québec: Québec, QC, Canada, 1983; p. 52.
- Chambers, J.M. Linear Models. In Statistical Models in S; Chapter 4; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; pp. 95–144. [Google Scholar]
- R Development Core Team-2011. Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 15 November 2015).
- Fayle, D.C.F. Radial Growth in Tree Roots; Technical Report No.9; Faculty of Forestry, University Toronto: Toronto, ON, Canada, 1968; pp. 1–183. [Google Scholar]
- McCarthy, J.W.; Weetman, G. Age and size structure of gap-dynamic, old-growth boreal forest stands in Newfoundland. Silv. Fenn. 2006, 40, 209–230. [Google Scholar] [CrossRef]




| Site/Plot | OLT | DBH | Height | Density | BA | Age (years) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | Class | (cm) | (m) | (Stem/ha) | (m2/ha) | True | 0 m | 1 m | Max | |
| M1 | 17 | A | 13.1 | 14.9 | 2300 | 32.31 | 94 [0] | 85 | 73 | 95 |
| M2 | 25 | A | 13.2 | 14.8 | 2725 | 39.43 | 94 [0] | 88 | 78 | 95 |
| M3 | 19 | A | 14.8 | 17.3 | 2400 | 41.05 | 94 [0] | 87 | 77 | 95 |
| M4 | 42 | B | 10.9 | 9.3 | 1450 | 13.83 | 203 [1] | 176 | 148 | 209 |
| M5 | 63 | B | 11 | 9.8 | 1600 | 15.55 | 220 [1] | 180 | 146 | 228 |
| M6 | 60 | B | 12.2 | 10 | 1350 | 16.28 | 224 [3] | 189 | 143 | 253 |
| M7 | 106 | C | 11.5 | 13.4 | 2275 | 24.19 | 155 [2] | 140 | 126 | 158 |
| M8 | 126 | C | 15 | 13.7 | 1300 | 24.30 | 210 [3] | 189 | 154 | 299 |
| M9 | 120 | C | 11.9 | 11.4 | 950 | 10.84 | 220 [2] | 187 | 151 | 236 |
| Q1 | 20 | A | 13.8 | 14.6 | 1775 | 27.89 | 107 [2] | 91 | 82 | 123 |
| Q2 | 22 | A | 15 | 17.6 | 1925 | 35.78 | 106 [2] | 103 | 92 | 119 |
| Q3 | 18 | A | 13.6 | 15.1 | 2975 | 45.31 | 108 [0] | 102 | 93 | 109 |
| Q4 | 41 | B | 13.6 | 12.5 | 1525 | 23.21 | 103 [0] | 92 | 80 | 103 |
| Q5 | 43 | B | 12.6 | 14.8 | 2325 | 30.38 | 104 [1] | 96 | 85 | 109 |
| Q6 | 38 | B | 10.7 | 12.2 | 1775 | 16.26 | 105 [0] | 98 | 88 | 107 |
| Q7 | 76 | C | 11.5 | 13.1 | 1650 | 17.62 | 100 [0] | 83 | 74 | 102 |
| Q8 | 126 | C | 12 | 12.7 | 2650 | 30.83 | 199 [3] | 173 | 134 | 207 |
| Q9 | 126 | C | 11.7 | 11.5 | 1400 | 15.42 | 140 [0] | 131 | 108 | 171 |
| V1 | 21 | A | 11.5 | 13.2 | 2350 | 25.11 | 88 [0] | 78 | 66 | 88 |
| V2 | 25 | A | 10.8 | 12.3 | 2725 | 25.67 | 89 [0] | 84 | 72 | 90 |
| V3 | 20 | A | 15.1 | 16.2 | 1675 | 31.25 | 156 [2] | 131 | 108 | 168 |
| V4 | 56 | B | 11.3 | 12.4 | 1475 | 15.05 | 295 [0] | 252 | 218 | 296 |
| V5 | 45 | B | 13.2 | 12.4 | 1600 | 23.03 | 149 [2] | 120 | 92 | 173 |
| V6 | 56 | B | 12.7 | 12 | 1375 | 18.41 | 188 [1] | 170 | 136 | 277 |
| V7 | 77 | C | 14.2 | 14.2 | 1000 | 17.09 | 246 [2] | 217 | 166 | 262 |
| V8 | 110 | C | 12.1 | 12.2 | 1100 | 13.46 | 153 [3] | 141 | 105 | 169 |
| V9 | 76 | C | 12.3 | 12.7 | 925 | 11.47 | 267 [0] | 229 | 193 | 267 |
| Variables | OLT Class A | OLT Class B | OLT Class C | All data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SE | Min | Max | Mean | SE | Min | Max | Mean | SE | Mean | |
| OLT (cm) | 17 | 25 | 21 | 0.5 | 38 | 63 | 49 | 1.8 | 76 | 126 | 105 | 4.5 | 58 |
| DBH (cm) | 10.2 | 21.9 | 15.5 | 0.7 | 9.1 | 19.1 | 13.6 | 0.5 | 10.6 | 23.6 | 14.6 | 0.8 | 14.6 |
| Height (m) | 10.5 | 18.8 | 15.1 | 0.4 | 7.8 | 16.6 | 11.7 | 0.5 | 9.2 | 18.5 | 12.7 | 0.5 | 13.2 |
| Age [collar] | 87 | 168 | 105 | 4.1 | 100 | 296 | 177 | 12.2 | 99 | 299 | 183 | 9.8 | 155 |
| Age [0m] | 77 | 146 | 94 | 3.1 | 89 | 252 | 150 | 10.0 | 80 | 246 | 160 | 8.6 | 134 |
| Age [1m] | 62 | 120 | 82 | 2.6 | 69 | 218 | 122 | 7.9 | 73 | 203 | 130 | 7.1 | 111 |
| Δ Age [0m] | 6 | 26 | 12 | 0.9 | 6 | 64 | 27 | 3.0 | 7 | 56 | 30 | 2.6 | 23 |
| Δ Age [Total] | 13 | 49 | 22 | 2.0 | 14 | 112 | 50 | 5.6 | 24 | 96 | 54 | 3.9 | 44 |
| Variable | Estimate | SE | t value | Pr (>|t|) | RSE | R2adj | p-value |
|---|---|---|---|---|---|---|---|
| Model (a) | |||||||
| Intercept | −36.765 | 9.490 | −3.874 | 2.2 × 10−4 | 13.97 | 0.71 | <2.2 × 10−16 |
| OLT | 0.441 | 0.165 | 2.670 | 9.3 × 10−4 | |||
| Age0m | 0.601 | 0.079 | 7.591 | 6.2 × 10−11 | |||
| OLT:Age0m | −0.003 | 0.0012 | −2.550 | 1.3 × 10−2 | |||
| Model (b) | |||||||
| Intercept | −35.194 | 13.359 | −2.634 | 0.01 | |||
| OLT | 0.410 | 0.264 | 1.554 | 0.13 | 12.13 | 0.77 | 1.1 × 10−15 |
| Age0m | 0.578 | 0.122 | 4.741 | 2 × 10−5 | |||
| OLT:Age0m | −0.003 | 0.002 | −1.312 | 0.20 |
| Class OLT | Slopes Test Statistic | |||||
|---|---|---|---|---|---|---|
| Std. Error | t-Value | df | α | t-Crit | p-Value | |
| A vs B | 0.115 | 0.202 | 50 | 0.05 | 2.009 | 0.84 |
| A vs C | 0.118 | 0.480 | 50 | 0.05 | 2.011 | 0.63 |
| B vs C | 0.094 | 1.091 | 50 | 0.05 | 2.009 | 0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laamrani, A.; DesRochers, A.; Blackburn, L. Effect of Organic Layer Thickness on Black Spruce Aging Mistakes in Canadian Boreal Forests. Forests 2016, 7, 69. https://doi.org/10.3390/f7030069
Laamrani A, DesRochers A, Blackburn L. Effect of Organic Layer Thickness on Black Spruce Aging Mistakes in Canadian Boreal Forests. Forests. 2016; 7(3):69. https://doi.org/10.3390/f7030069
Chicago/Turabian StyleLaamrani, Ahmed, Annie DesRochers, and Line Blackburn. 2016. "Effect of Organic Layer Thickness on Black Spruce Aging Mistakes in Canadian Boreal Forests" Forests 7, no. 3: 69. https://doi.org/10.3390/f7030069





