Effects of Forest Gaps on Litter Lignin and Cellulose Dynamics Vary Seasonally in an Alpine Forest
Abstract
:1. Introduction
2. Materials and Methods
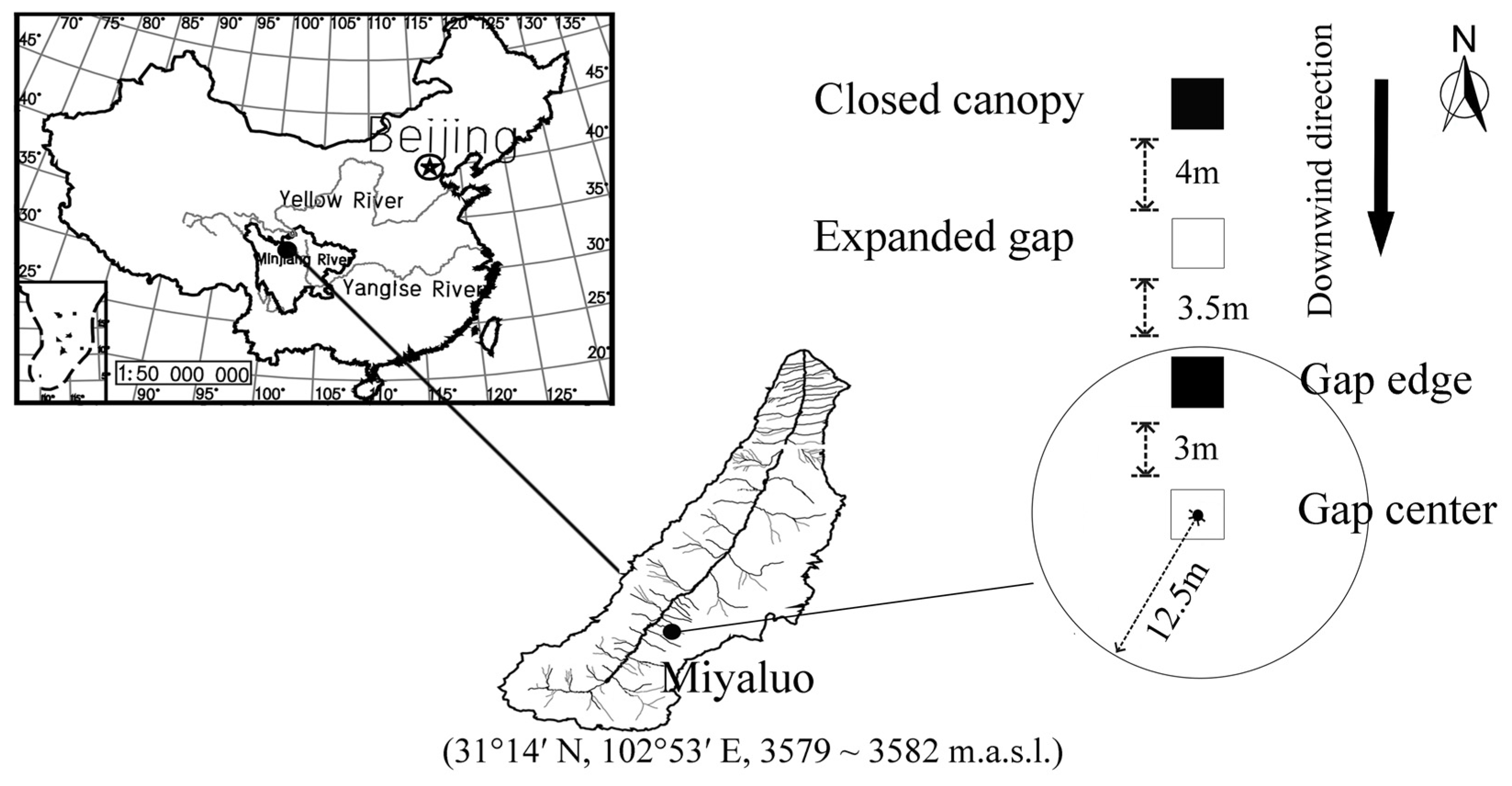
2.1. Site Description
2.2. Experimental Design
| Position | Temperature Characteristics | Snow Formation Period | Snow Cover Period | Snow Melt Period | Winter | Growing Season | 1st Year |
|---|---|---|---|---|---|---|---|
| Air | DMT | −4.11 | −4.46 | 1.43 | −2.38 | 7.46 | 2.54 |
| PAT | 1 | 30 | 82 | 113 | 1417 | 1530 | |
| NAT | −166 | −351 | −15 | −532 | −7 | −539 | |
| FTC | — | — | — | — | — | — | |
| Gap center | DMT | −3.55 | −3.44 | 1.88 | −1.70 | 8.90 | 3.60 |
| PAT | 0 | 14 | 91 | 106 | 1683 | 1789 | |
| NAT | −142 | −262 | −3 | −407 | 0 | −408 | |
| FTC | 0.45 | 0.41 | 0.12 | 0.44 | 0.08 | 0.26 | |
| Gap edge | DMT | −2.88 | −3.14 | 0.93 | −1.70 | 7.66 | 2.98 |
| PAT | 1 | 24 | 53 | 78 | 1451 | 1529 | |
| NAT | −116 | −250 | −9 | −375 | −2 | −378 | |
| FTC | 0.59 | 0.61 | 0.09 | 0.49 | 0.10 | 0.29 | |
| Expanded gap | DMT | −2.91 | −4.20 | 1.94 | −1.73 | 7.87 | 3.03 |
| PAT | 0 | 17 | 93 | 111 | 1493 | 1604 | |
| NAT | −117 | −320 | −2 | −439 | −5 | −444 | |
| FTC | 0.52 | 0.53 | 0.21 | 0.53 | 0.10 | 0.31 | |
| Closed canopy | DMT | −3.87 | −4.05 | 1.51 | −2.14 | 7.70 | 2.78 |
| PAT | 0 | 24 | 79 | 104 | 1458 | 1561 | |
| NAT | −155 | −316 | −8 | −479 | −3 | −482 | |
| FTC | 0.63 | 0.59 | 0.28 | 0.56 | 0.04 | 0.30 |
2.3. Analyses and Calculations
| Species Name | C (%) | N (%) | P (%) | Lignin (%) | Cellulose (%) | C/N | C/P | N/P | Lignin/N | Lignin/Cellulose |
|---|---|---|---|---|---|---|---|---|---|---|
| Cypress | 51.64 ± 1.77 a,b | 0.88 ± 0.01 a | 0.12 ± 0.01 b,c | 14.07 ± 0.74 a | 12.22 ± 0.38 a | 58.86 ± 2.21 b,c | 416.02 ± 14.04 a | 7.08 ± 0.41 a,b | 16.04 ± 1.01 a | 1.15 ± 0.03 a |
| Minjiang Fir | 50.56 ± 2.96 a | 0.88 ± 0.01 a | 0.11 ± 0.01 b | 15.85 ± 0.36 b | 12.19 ± 0.20 a | 57.77 ± 3.53 b | 443.51 ± 36.69 a | 7.68 ± 0.72 b | 18.11 ± 0.42 b | 1.30 ± 0.05 b |
| Larch | 54.35 ± 0.63 b | 0.86 ± 0.04 a | 0.13 ± 0.01 c | 27.21 ± 2.21 c | 16.45 ± 0.44 b | 63.32 ± 3.49 c | 407.08 ± 2.42 a | 6.44 ± 0.38 a | 25.95 ± 1.08 c | 1.36 ± 0.06 b |
| Red birch | 49.69 ± 1.45 a | 1.33 ± 0.02 b | 0.09 ± 0.01 a | 36.68 ± 0.62 d | 12.47 ± 0.38 a | 37.24 ± 1.35 a | 544.94 ± 31.72 b | 14.63 ± 0.36 c | 25.99 ± 0.37 c | 2.78 ± 0.13 c |
| Source Variance | df | F-Value | p-Value | ||
|---|---|---|---|---|---|
| Cellulose Lignin | Cellulose Lignin | ||||
| Period (P) | 3 | 70.520 | 53.771 | <0.001 ** | <0.001 ** |
| Gap position (G) | 3 | 3.410 | 4.905 | 0.020 * | 0.003 ** |
| Species (S) | 3 | 8.838 | 41.719 | <0.001 ** | <0.001 ** |
| P × G | 9 | 10.741 | 7.727 | <0.001 ** | <0.001 ** |
| P × S | 9 | 9.551 | 18.847 | <0.001 ** | <0.001 ** |
| G × S | 9 | 0.762 | 3.651 | 0.651 | <0.001 ** |
| P × G × S | 27 | 3.918 | 23.413 | <0.001 ** | <0.001 ** |
| Dominant Factors | Snow Formation Period | Snow Cover Period | Snow Melt Period | All of Winter | Growing Season | 1st Year | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Lignin | Cellulose | Lignin | Cellulose | Lignin | Cellulose | Lignin | Cellulose | Lignin | Cellulose | Lignin | |
| 1 | DMT | Initial Lignin/N | Initial Lignin/N | Initial Cellulose | Initial Lignin/Cellulose | Initial Cellulose | Initial Lignin | Initial Lignin/N | Initial Lignin | Initial Cellulose | ||
| (0.139) | (0.265) | (0.270) | (0.289) | (0.227) | (0.773) | (0.562) | (0.083) | (0.605) | (0.551) | |||
| 2 | DMT | NAT | DMT | Initial N | Initial Lignin/N | |||||||
| (0.410) | (0.411) | (0.425) | (0.824) | (0.674) | ||||||||
| 3 | FTC | Initial N | ||||||||||
| (0.544) | (0.487) | |||||||||||
| 4 | Initial P | |||||||||||
2.4. Statistical Analyses
3. Results
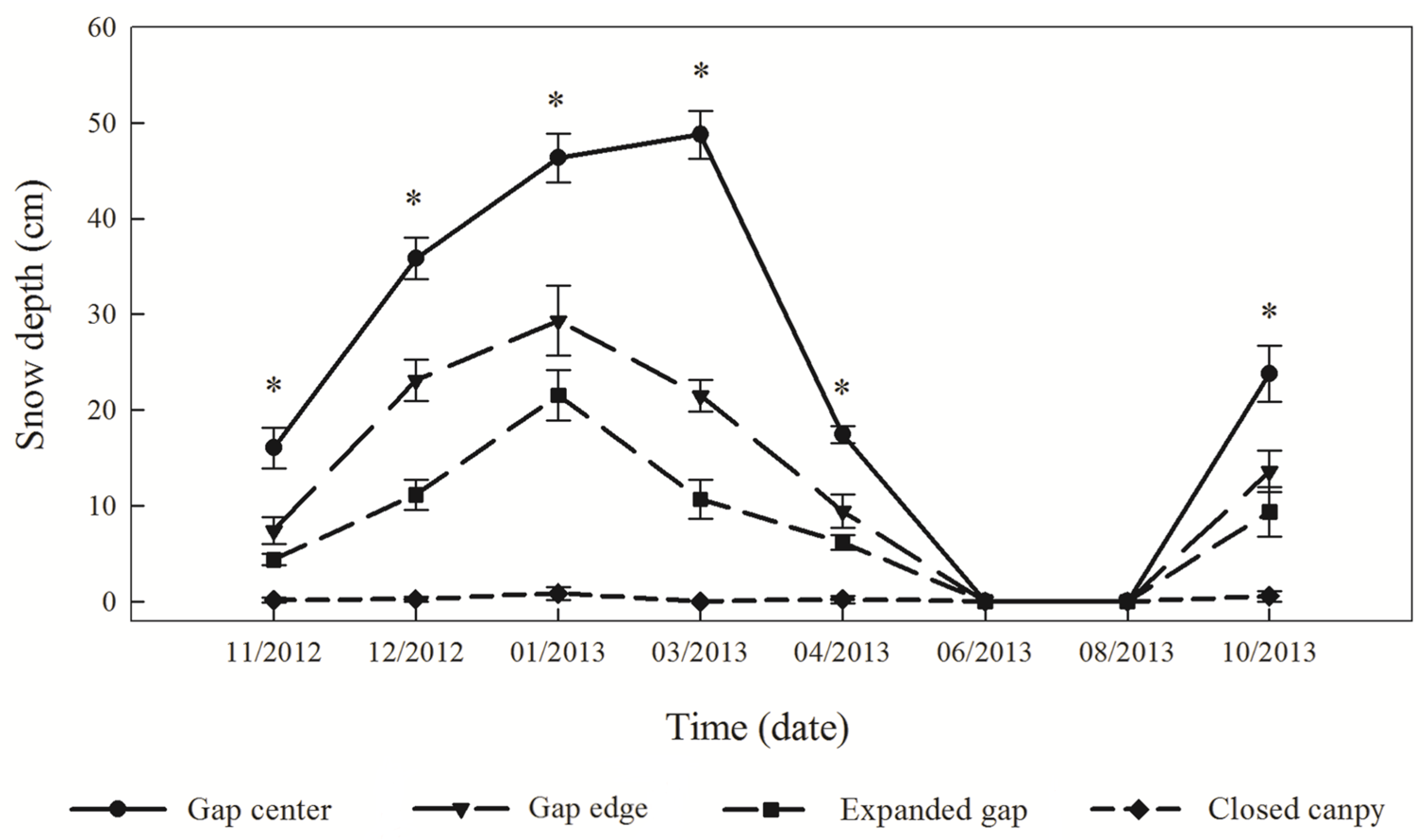
3.1. Snow Depth and Temperature
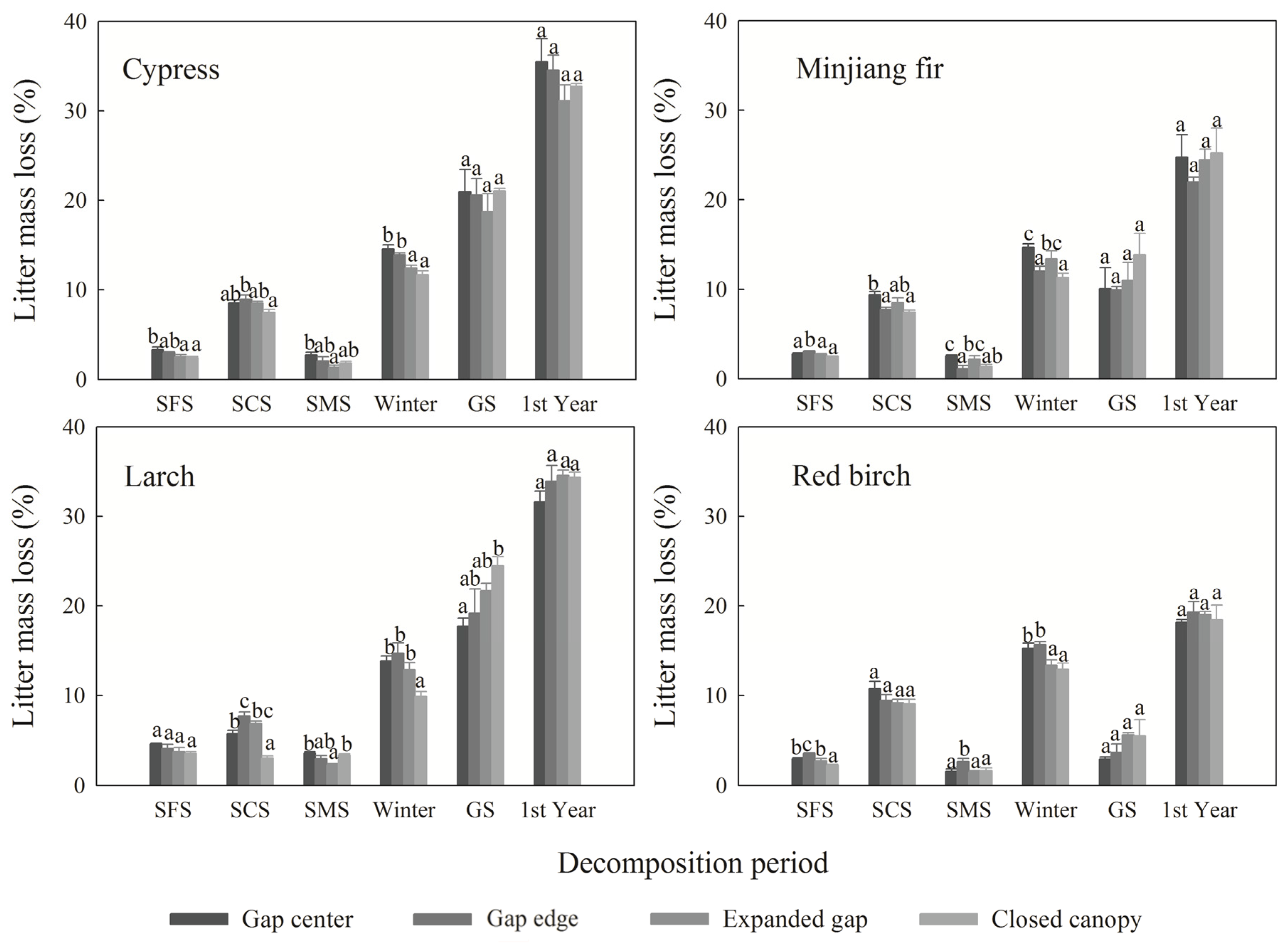
3.2. Cellulose and Lignin Concentrations
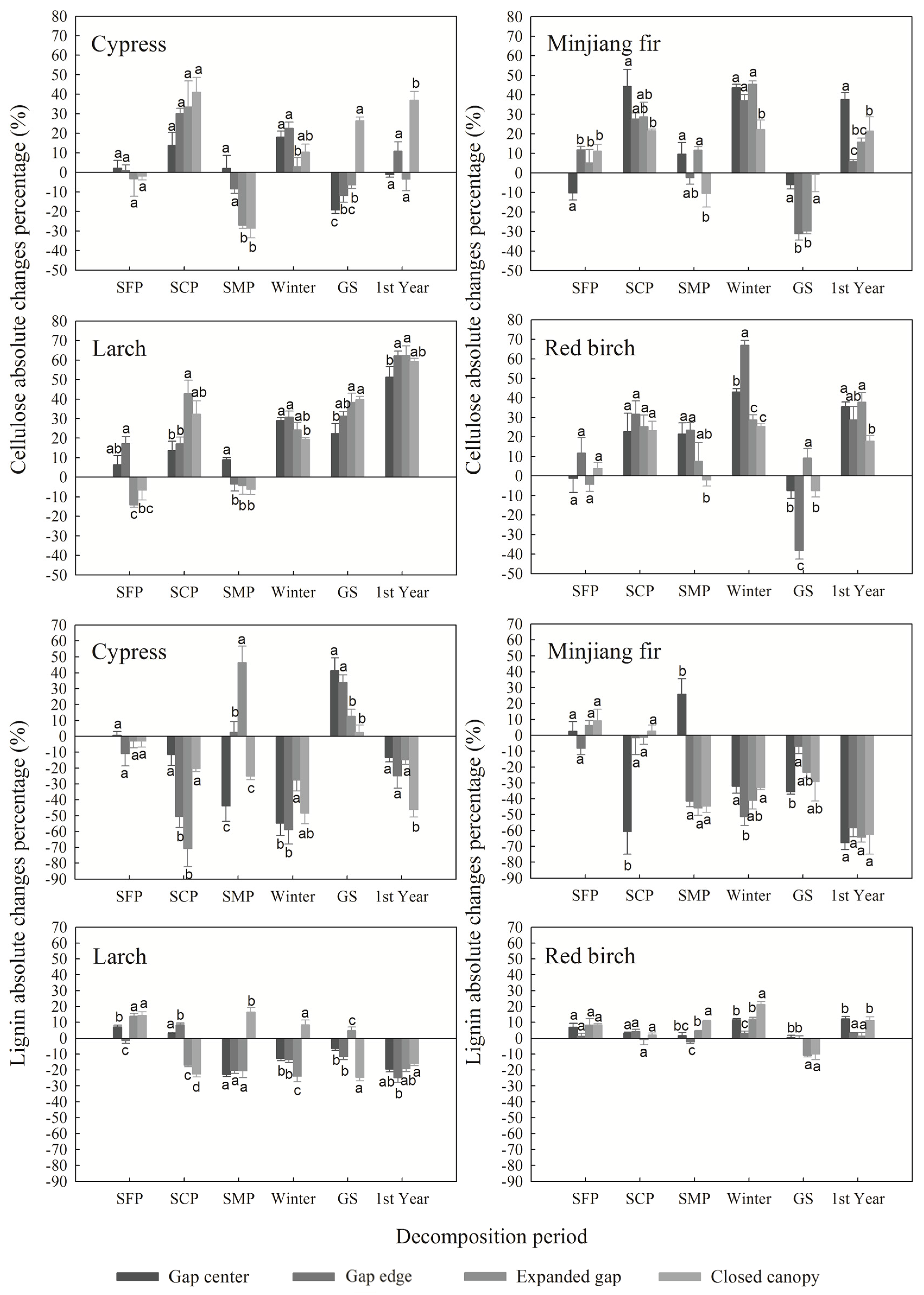
3.3. Cellulose and Lignin Absolute Changes


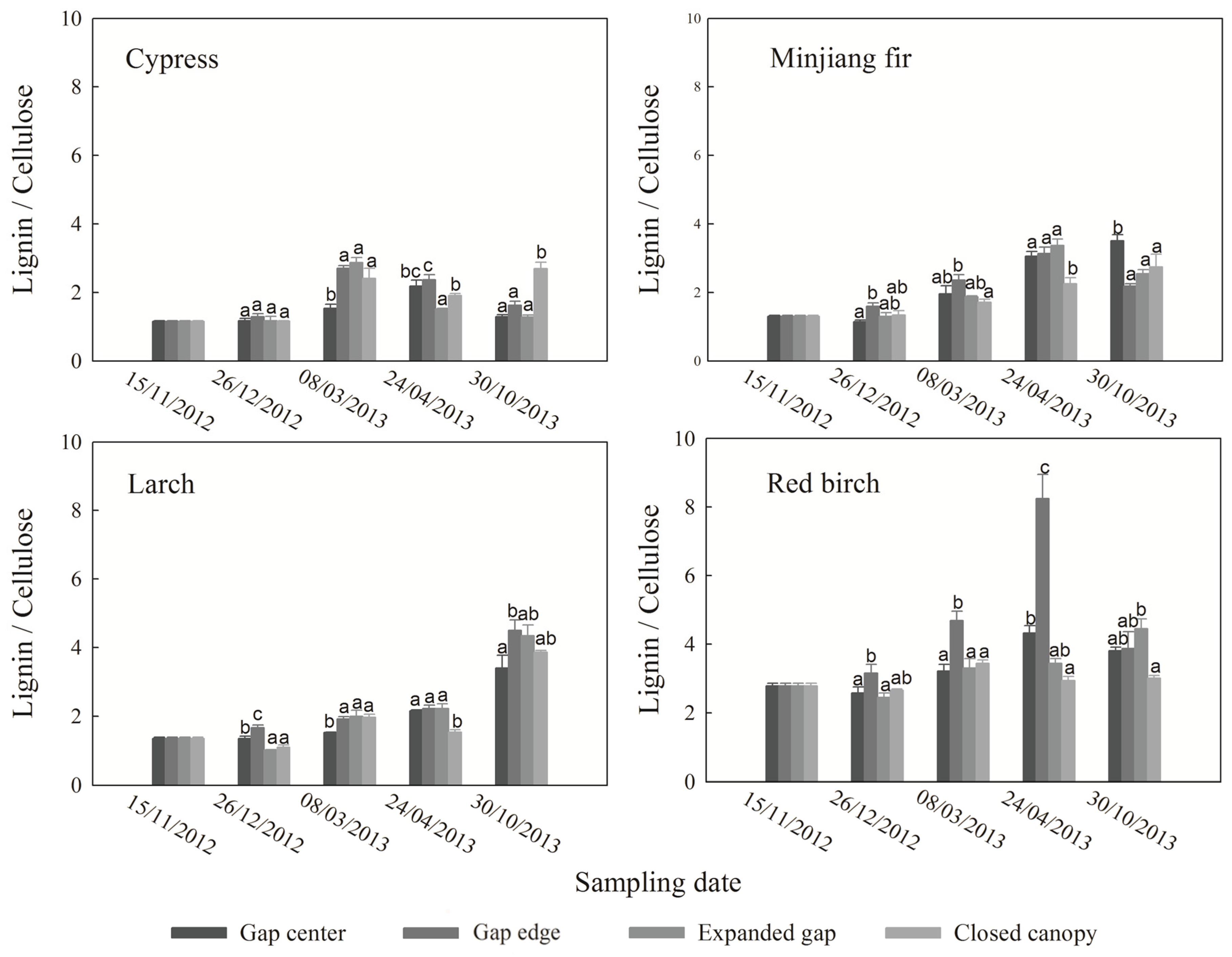
3.4. Ratio between Lignin and Cellulose
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berg, B.; Berg, M.; Bottner, P.; Box, E.; Breymeyer, A.; de Anta, R.C.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W. Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Decomposition rate and chemical changes of scots pine needle litter. II. Influence of chemical composition. Ecol. Bull. 1980, 32, 373–390. [Google Scholar]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Mineral Nutrition; Springer: Berlin, Germany, 2008. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Oakland, CA, USA, 1979; Volume 5. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant litter. In Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Liu, L.; Wu, F.; Yang, W.; Wang, A.; Tan, B.; Yu, S. Soil bacterial diversity in the subalpine/alpine forests of western Sichuan at the early stage of freeze-thaw season. Acta Ecol. Sin. 2010, 30, 5687–5694. [Google Scholar]
- Preston, C.M.; Nault, J.R.; Trofymow, J.; Smyth, C.; Group, C.W. Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 1. Elemental composition, tannins, phenolics, and proximate fractions. Ecosystems 2009, 12, 1053–1077. [Google Scholar] [CrossRef]
- Duguid, M.C.; Frey, B.R.; Ellum, D.S.; Kelty, M.; Ashton, M.S. The influence of ground disturbance and gap position on understory plant diversity in upland forests of southern New England. For. Ecol. Manag. 2013, 303, 148–159. [Google Scholar] [CrossRef]
- Mallik, A.U.; Kreutzweiser, D.P.; Spalvieri, C.M. Forest regeneration in gaps seven years after partial harvesting in riparian buffers of boreal mixedwood streams. For. Ecol. Manag. 2014, 312, 117–128. [Google Scholar] [CrossRef]
- Silver, W.L.; Hall, S.J.; González, G. Differential effects of canopy trimming and litter deposition on litterfall and nutrient dynamics in a wet subtropical forest. For. Ecol. Manag. 2014, 332, 47–55. [Google Scholar] [CrossRef]
- Whitmore, T. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Williams, S.; Gray, T. Decomposition of litter on the soil surface. In Biology of Plant Litter Decomposition; Dickinson, C.H., Pugh, G.J.F., Eds.; Academic Press: New York, USA, 1974. [Google Scholar]
- Canham, C.D.; Marks, P.; Pickett, S.; White, P. The response of woody plants to disturbance patterns of establishment and growth. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P., Eds.; Academic Press: New York, NY, USA, 1985; pp. 197–216. [Google Scholar]
- Perakis, S.S.; Matkins, J.J.; Hibbs, D.E. Interactions of tissue and fertilizer nitrogen on decomposition dynamics of lignin-rich conifer litter. Ecosphere 2012, 3, 1–12. [Google Scholar] [CrossRef]
- Prescott, C.E.; Hope, G.D.; Blevins, L.L. Effect of gap size on litter decomposition and soil nitrate concentrations in a high-elevation spruce fir forest. Can. J. For. Res. 2003, 33, 2210–2220. [Google Scholar] [CrossRef]
- Sariyildiz, T. Effects of gap-size classes on long-term litter decomposition rates of beech, oak and chestnut species at high elevations in Northeast Turkey. Ecosystems 2008, 11, 841–853. [Google Scholar] [CrossRef]
- He, W.; Wu, F.; Zhang, D.; Yang, W.; Tan, B.; Zhao, Y.; Wu, Q. The effects of forest gaps on cellulose degradation in the foliar litter of two shrub species in an alpine fir forest. Plant Soil 2015, 393, 109–122. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, F.; Yang, W.; Zhao, Y.; He, W.; Tan, B. Foliar litter nitrogen dynamics as affected by forest gap in the alpine forest of Eastern Tibet Plateau. PLoS ONE 2014, 9, e97112. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Peng, C.; Zhu, J.; Zhang, J.; Tan, B.; Yang, W. Impact of changes in freezing and thawing on foliar litter carbon release in alpine/subalpine forests along an altitudinal gradient in the Eastern Tibetan Plateau. Biogeosci. Discuss. 2014, 11, 9539–9564. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballaré, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Can. J. Bot. 1991, 69, 1449–1456. [Google Scholar] [CrossRef]
- Couˆteaux, M.M.; Bottner, P.; Berg, B. Litter decomposition, climate and litter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Zhu, J.; He, X.; Wu, F.; Yang, W.; Tan, B. Decomposition of abies faxoniana litter varies with freeze-thaw stages and altitudes in subalpine/alpine forests of southwest China. Scand. J. For. Res. 2012, 27, 586–596. [Google Scholar] [CrossRef]
- Tan, B.; Wu, F.; Yang, W.; Yang, Y.; Wang, A.; Kang, L. Effects of snow pack removal on the dynamics of winter-time soil temperature, carbon, nitrogen, and phosphorus in alpine forests of west sichuan. J. Appl. Ecol. 2011, 22, 2553–2559. [Google Scholar]
- Tan, B.; Wu, F.; Yang, W.; Yu, S.; Liu, L.; Wang, A. The dynamics pattern of soil carbon and nutrients as soil thawing proceeded in the alpine/subalpine forest. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 670–679. [Google Scholar] [CrossRef]
- He, W.; Wu, F.; Yang, W.; Tan, B.; Zhao, Y.; Wu, Q.; He, M. Lignin degradation in foliar litter of two shrub species from the gap center to the closed canopy in an alpine fir forest. Ecosystems 2015, 1–14. [Google Scholar] [CrossRef]
- Aerts, R. The freezer defrosting: Global warming and litter decomposition rates in cold biomes. J. Ecol. 2006, 94, 713–724. [Google Scholar] [CrossRef]
- Song, X.; Jiang, H.; Zhang, H.; Yu, S.; Zhou, G.; Ma, Y.; Chang, S. A review on the effects of global environment change on litter decomposition. Acta Ecol. Sin. 2008, 28, 4414–4423. [Google Scholar]
- Guo, J.; Yang, Y.; Chen, G.; Lin, P.; Xie, J. A review on litter decomposition in forest ecosystem. Sci. Silvae Sin. 2006, 42, 93–100. [Google Scholar]
- Zhu, J.; Yang, W.; He, X. Temporal dynamics of abiotic and biotic factors on leaf litter of three plant species in relation to decomposition rate along a subalpine elevation gradient. PLoS ONE 2013, 8, e62073. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, W.; Zhang, J.; Deng, R. Litter decomposition in two subalpine forests during the freeze-thaw season. Acta Oecol. 2010, 36, 135–140. [Google Scholar] [CrossRef]
- O’Connell, A. Decomposition of slash residues in thinned regrowth eucalpt forest in Western Australia. J. Appl. Ecol. 1997, 111–122. [Google Scholar] [CrossRef]
- Yang, W.; Wang, K.; Kellomäki, S.; Gong, H. Litter dynamics of three subalpine forests in Western Sichuan. Pedosphere 2005, 15, 653–659. [Google Scholar]
- Konestabo, H.S.; Michelsen, A.; Holmstrup, M. Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Appl. Soil Ecol. 2007, 36, 136–146. [Google Scholar] [CrossRef]
- Olsson, P.Q.; Sturm, M.; Racine, C.H.; Romanovsky, V.; Liston, G.E. Five stages of the Alaskan Arctic cold season with ecosystem implications. Arct. Antarct. Alp. Res. 2003, 35, 74–81. [Google Scholar] [CrossRef]
- Ni, X.; Yang, W.; Li, H.; Xu, L.; He, J.; Tan, B.; Wu, F. The responses of early foliar litter humification to reduced snow cover during winter in an alpine forest. Can. J. Soil Sci. 2014, 94, 453–461. [Google Scholar] [CrossRef]
- Ni, X.; Yang, W.; Tan, B.; He, J.; Xu, L.; Li, H.; Wu, F. Accelerated foliar litter humification in forest gaps: Dual feedbacks of carbon sequestration during winter and the growing season in an alpine forest. Geoderma 2015, 241, 136–144. [Google Scholar] [CrossRef]
- Lu, R. Soil and Agro-Chemical Analytical Methods; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 146–195. [Google Scholar]
- Van Soest, P.; Wine, R. Determination of lignin and cellulose in acid-detergent fiber with permanganate. J. Assoc. Off. Anal. Chem. 1968, 51, 780–785. [Google Scholar]
- De Marco, A.; Spaccini, R.; Vittozzi, P.; Esposito, F.; Berg, B.; de Santo, A.V. Decomposition of black locust and black pine leaf litter in two coeval forest stands on Mount Vesuvius and dynamics of organic components assessed through proximate analysis and NMR spectroscopy. Soil Biol. Biochem. 2012, 51, 1–15. [Google Scholar] [CrossRef]
- Preston, C.M.; Nault, J.R.; Trofymow, J. Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of “lignin”. Ecosystems 2009, 12, 1078–1102. [Google Scholar] [CrossRef]
- Baptist, F.; Yoccoz, N.; Choler, P. Direct and indirect control by snow cover over decomposition in alpine tundra along a snowmelt gradient. Plant Soil 2010, 328, 397–410. [Google Scholar] [CrossRef]
- Taylor, B.; Parkinson, D.; Parsons, W. Nitrogen and lignin content as predictors of litter decay rates: A microcosm test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- Deng, R.; Yang, W.; Feng, R.; Hu, J.; Qin, J.; Xiong, X. Mass loss and element release of litter in the subalpine forest over one freeze-thaw season. Acta Ecol. Sin. 2009, 29, 5731–5735. [Google Scholar]
- Don, A.; Kalbitz, K. Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol. Biochem. 2005, 37, 2171–2179. [Google Scholar] [CrossRef]
- Nordén, B.; Berg, B. A non-destructive method (solid state 13 CNMR) for determining organic chemical components of decomposing litter. Soil Biol. Biochem. 1990, 22, 271–275. [Google Scholar] [CrossRef]
- Lemma, B.; Nilsson, I.; Kleja, D.; Olsson, M.; Knicker, H. Decomposition and substrate quality of leaf litters and fine roots from three exotic plantations and a native forest in the southwestern highlands of Ethiopia. Soil Biol. Biochem. 2007, 39, 2317–2328. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, G.; Guo, J.; Lin, P. Decomposition dynamic of fine roots in a mixed forest of Cunninghamia lanceolata and Tsoongiodendron odorum in mid-subtropics. Ann. For. Sci. 2004, 61, 65–72. [Google Scholar] [CrossRef]
- Berg, B.; Hannus, K.; Popoff, T.; Theander, O. Changes in organic chemical components of needle litter during decomposition. Long-term decomposition in a Scots pine forest. I. Can. J. Bot. 1982, 60, 1310–1319. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Fioretto, A.; di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Clein, J.S.; Schimel, J.P. Microbial activity of tundra and taiga soils at sub-zero temperatures. Soil Biol. Biochem. 1995, 27, 1231–1234. [Google Scholar] [CrossRef]
- Aber, J.D.; McClaugherty, C.; Melillo, J. Litter Decomposition in Wisconsin Forests: Mass loss, Organic-Chemical Constituents and Nitrogen; School of Natural Resources, College of Agricultural and Life Sciences, University of Wisconsin: Madison, WI, USA, 1984. [Google Scholar]
- Bokhorst, S.; Metcalfe, D.; Wardle, D. Reduction in snow depth negatively affects decomposers but impact on decomposition rates is substrate dependent. Soil Biol. Biochem. 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Saccone, P.; Morin, S.; Baptist, F.; Bonneville, J.; Colace, M.; Domine, F.; Faure, M.; Geremia, R.; Lochet, J.; Poly, F. The effects of snowpack properties and plant strategies on litter decomposition during winter in subalpine meadows. Plant Soil 2013, 363, 215–229. [Google Scholar] [CrossRef]
- Tan, B.; Wu, F.; Yang, W.; He, X. Snow removal alters soil microbial biomass and enzyme activity in a Tibetan alpine forest. Appl. Soil Ecol. 2014, 76, 34–41. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Schuur, E.; Vogel, J.G.; Natali, S.M. Moisture drives surface decomposition in thawing tundra. J. Geophys. Res. Biogeosci. 2013, 118, 1133–1143. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Q.; Wu, Y.; He, H. Advances in the studies of forest litter. Chin. J. Ecol. 2003, 23, 60–64. [Google Scholar]
- Song, X.; Jiang, H.; Zhang, H.; Peng, C.; Yu, S. Elevated UV-B radiation did not affect decomposition rates of needles of two coniferous species in subtropical China. Eur. J. Soil Biol. 2011, 47, 343–348. [Google Scholar] [CrossRef]
- Ritter, E. Litter decomposition and nitrogen mineralization in newly formed gaps in a danish beech (Fagus sylvatica) forest. Soil Biol. Biochem. 2005, 37, 1237–1247. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wu, F.; Yang, W.; Xu, L.; Ni, X.; He, J.; Tan, B.; Hu, Y. Effects of Forest Gaps on Litter Lignin and Cellulose Dynamics Vary Seasonally in an Alpine Forest. Forests 2016, 7, 27. https://doi.org/10.3390/f7020027
Li H, Wu F, Yang W, Xu L, Ni X, He J, Tan B, Hu Y. Effects of Forest Gaps on Litter Lignin and Cellulose Dynamics Vary Seasonally in an Alpine Forest. Forests. 2016; 7(2):27. https://doi.org/10.3390/f7020027
Chicago/Turabian StyleLi, Han, Fuzhong Wu, Wanqin Yang, Liya Xu, Xiangyin Ni, Jie He, Bo Tan, and Yi Hu. 2016. "Effects of Forest Gaps on Litter Lignin and Cellulose Dynamics Vary Seasonally in an Alpine Forest" Forests 7, no. 2: 27. https://doi.org/10.3390/f7020027
APA StyleLi, H., Wu, F., Yang, W., Xu, L., Ni, X., He, J., Tan, B., & Hu, Y. (2016). Effects of Forest Gaps on Litter Lignin and Cellulose Dynamics Vary Seasonally in an Alpine Forest. Forests, 7(2), 27. https://doi.org/10.3390/f7020027








