Effect of Timber Harvest Intensities and Fertilizer Application on Stocks of Soil C, N, P, and S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Treatments
- ReM + F—Only stemwood was harvested; all of the forest residues (bark, canopy, and litter layer from the previous rotation) were maintained on the soil after the clear-cutting, and all nutrients were applied as fertilizer and the soil was dressed with limestone;
- ReR + F—All of the forest residues (bark, canopy, and litter layer from the previous rotation) were removed from the plot after the clear-cutting, and all nutrients were applied as fertilizer and the soil was dressed with limestone;
- ACR + F—The canopy (leaves and branches) and bark were removed after clear-cutting, but the litter layer was maintained; all nutrients were applied as fertilizer and the soil was dressed with limestone;
- ACR − N—The canopy (leaves and branches) and bark were removed after the clear-cutting, but the litter layer was maintained; all nutrients except N fertilizer were applied and the soil dressed with limestone. However, a small quantity of N was applied to ensure tree survival;
- ACR − P—The canopy (leaves and branches) and bark were removed after the clear-cutting, but the litter layer was maintained; all nutrients except P fertilizer were applied and the soil was dressed with limestone;
2.3. Field Procedures
2.4. Soil Sampling and Analysis
2.5. Nutrient Accumulation in the Biomass
2.6. Statistical Analysis
3. Results
3.1. Nutrient Balance After Two Crop Rotations
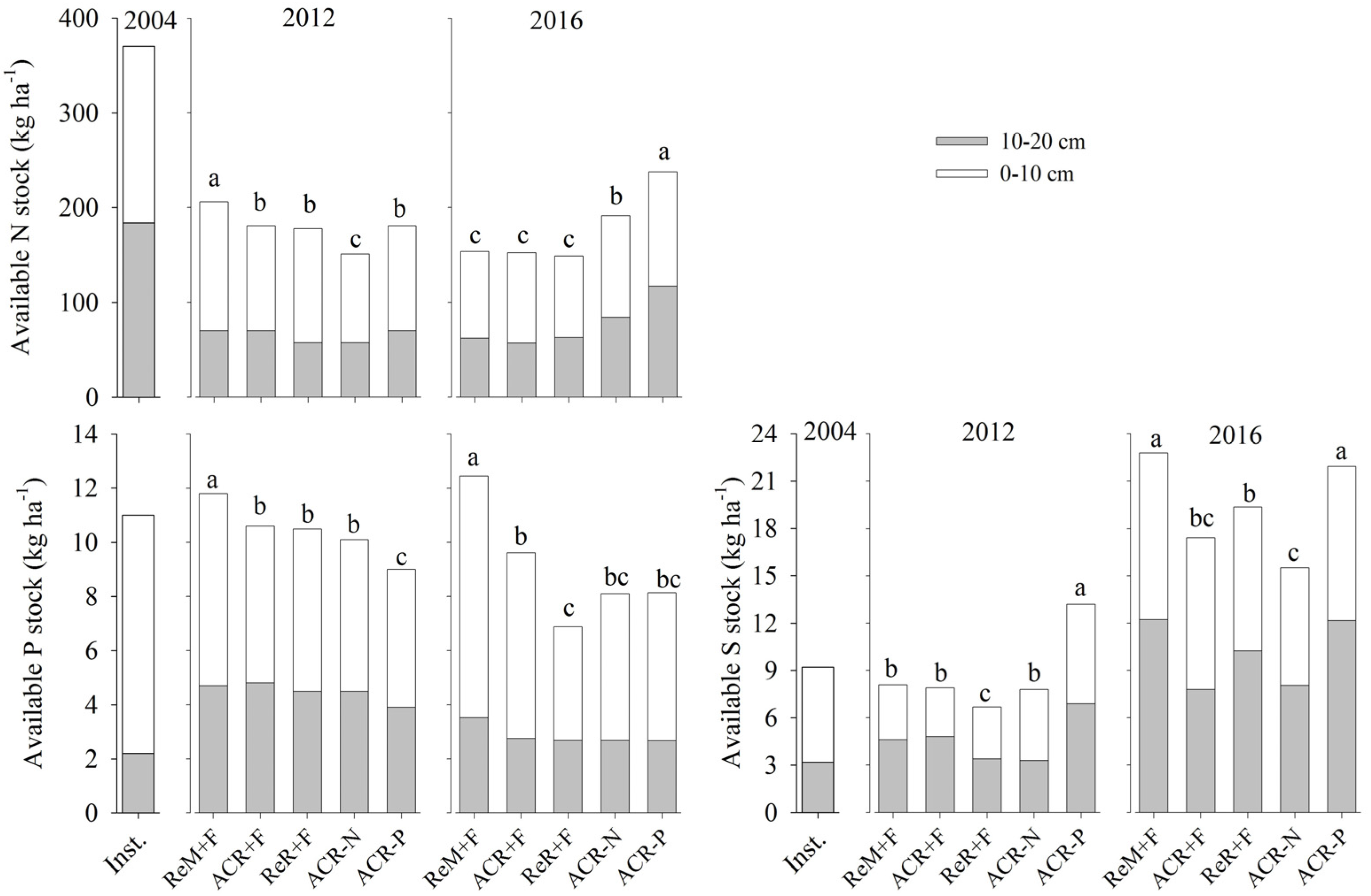
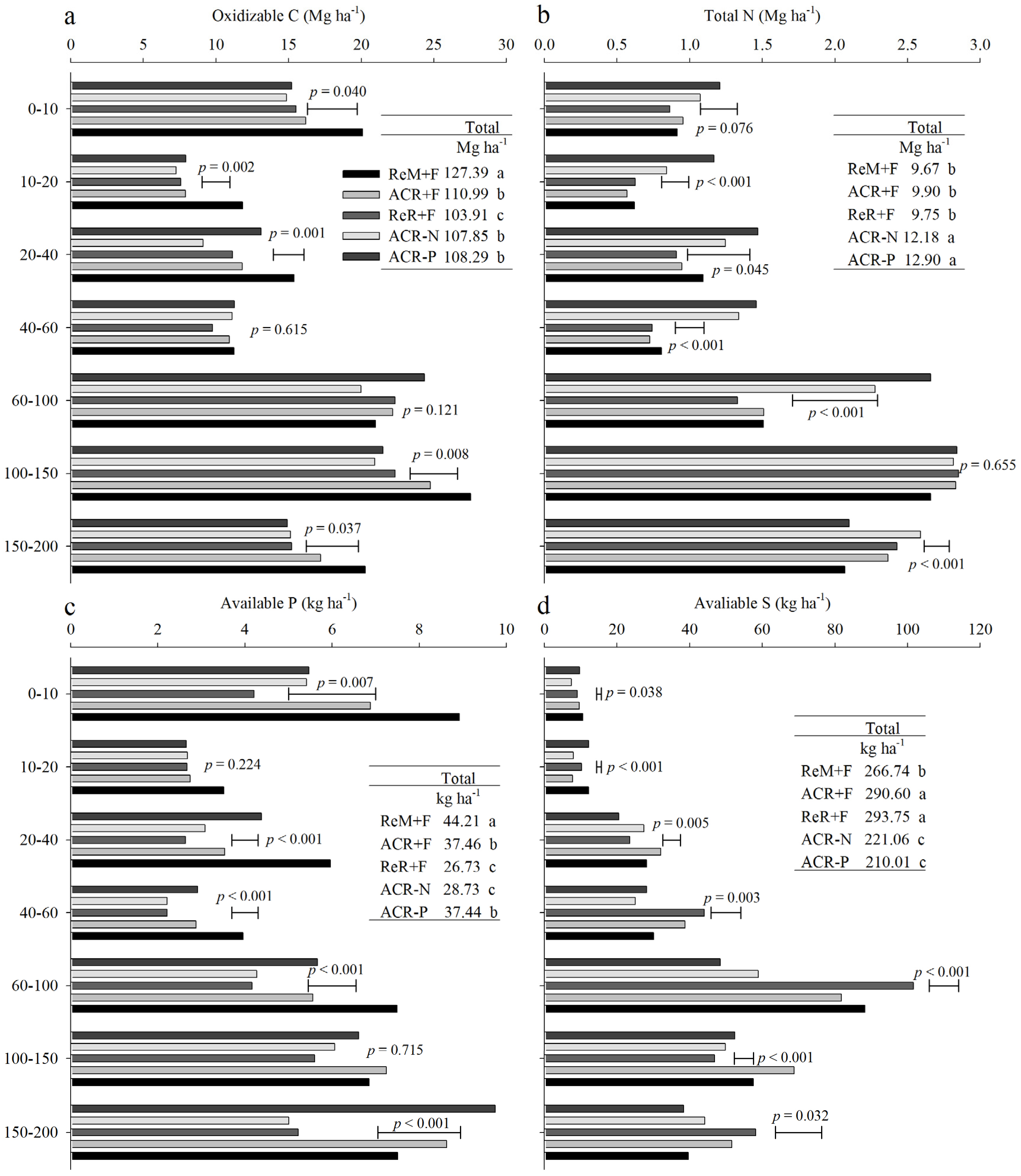
3.2. Soil Nutrient Stocks in 2016
4. Discussion
4.1. Effects of Harvest Residue Strategies on Soil C and Available Nutrient Stocks
4.2. Soil Contribution to Nutrients Absorbed by Trees
4.3. Management Considerations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Elsevier: New York, NY, USA, 2013; pp. 1–664. [Google Scholar]
- Jobbagy, E.; Jackson, R. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, B. Effects of site management on growth, biomass partitioning and light use efficiency in a young stand of Eucalyptus grandis in South Africa. For. Ecol. Manag. 2008, 255, 2324–2336. [Google Scholar] [CrossRef]
- Gonçalves, J.L.N.; Stape, J.L.; Wichert, M.C.P.; Gava, J.L. Conservação e Cultivo de Solos Para Plantações de Florestas (Soil Conservation and Cultivation for Forest Production); Instituto de Pesquisas e Estudos Florestais (IPEF): Piracicaba, (SP), Brazil, 2002; pp. 131–204. [Google Scholar]
- Corbeels, M.; McMurtrie, R.E.; Pepper, D.A.; Mendham, D.S.; Grove, T.S.; O’Connell, A.M. Long-term changes in productivity of Eucalyptus plantations under different harvest residues and nitrogen and management practices: A modeling analysis. For. Ecol. Manag. 2005, 217, 1–18. [Google Scholar] [CrossRef]
- Compton, J.E.; Cole, D.W. Phosphorus cycling and soil P fractions in Douglas-fir and red alder stands. For. Ecol. Manag. 1998, 110, 101–112. [Google Scholar] [CrossRef]
- Hall, S.J.; Matson, P.A. Nutrient status of tropical rainforest influences soil N dynamics after N additions. Ecol. Monogr. 2003, 73, 107–129. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Jones, D.L.; Reynolds, B.; Price, M.H.; Healey, J.R. Whole tree harvesting can reduce second rotation forest productivity. For. Ecol. Manag. 2009, 257, 1104–1111. [Google Scholar] [CrossRef]
- Jones, H.; Beets, P.; Kimberley, M.; Garrett, M. Harvest residue management and fertilization effect on soil carbon and nitrogen in a 15-year old Pinus radiata plantation forest. For. Ecol. Manag. 2011, 262, 339–347. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Marques, E.R.G.; Goncalves, J.L.M.; Hubner, A.; Brandini, C.B.; Ferraz, A.V.; Moreira, R.M. Decomposition rates of forest residues and soil fertility after clear-cutting of Eucalyptus grandis stands in response to site management and fertilizer application. Soil Use Manag. 2016. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Y.; Zhou, J. Carbon quality and the temperature sensitivity of soil organic carbon in a tallgrass prairie. Soil Biol. Biochem. 2012, 50, 142–148. [Google Scholar] [CrossRef]
- Mendham, D.S.; White, D.A.; Battaglia, M.; McGrath, J.F.; Short, T.M.; Ogden, G.N.; Kinal, J. Soil water depletion and replenishment during first- and early second-rotation Eucalyptus globulus plantations with deep soil profiles. Agric. For. Meteorol. 2011, 151, 1568–1579. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E. Productivity of acacia and eucalypt plantations in Southeast Asia 1. Biophysical determinants of production: Opportunities and challenges. Intern. For. Rev. 2014, 16, 225–248. [Google Scholar]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Goncalves, J.L.M.; Gava, J.L.; Godinho, T.O.; Melo, E.S.A.C.; Bazani, J.H.; Hubner, A.; Arthur Junior, J.C.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Peng, Y.; Thomas, S.C.; Tian, D. Forest management and soil respiration: Implications for carbon sequestration. Environ. Rev. 2008, 16, 93–111. [Google Scholar] [CrossRef]
- Holub, S.M.; Terry, T.A.; Harrington, C.A.; Harrison, R.B.; Meade, R. Tree growth ten years after residual biomass removal, soil compaction, tillage, and competing vegetation control in a highly productive Douglas-fir plantation. For. Ecol. Manag. 2013, 305, 60–66. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Goncalves, J.L.M.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais (Chemical Analysis for the Evaluation of Fertility in Tropical Soils); Instituto Agronomico de Campinas (IAC): Campinas, (SP), Brazil, 2001; pp. 1–58. [Google Scholar]
- Ribeiro, J.F.; Walter, B.M.T. Fitofisionomias do Bioma Cerrado. Phytophisiognomy of Cerrado biome. In Cerrado: Ambiente e Flora (Cerrado: Environment and Flora); Sano, S.M., Almeida, S.P., Eds.; Embrapa: Brasilia, Brazil, 1998; pp. 1–556. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Sparks, D.L, Ed.; Soil Science Society of America/SSSA and American Society of Agronomy (ASA): Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Schumacher, F.X.; Hall, F.D.S. Logarithmic expression of timber-tree volume. J. Agric. Res. 1933, 47, 719–734. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações (Evaluation of Nutritional Condition of Plants: Principles and Application); Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1989; pp. 1–201. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education Ltd.: DeKalb, IL, USA, 2010. [Google Scholar]
- Hubner, A. Ciclagem de Nutrientes e Produtividade de Madeira em Povoamento de Eucalyptus Grandis Sob Diferentes Manejos de Resíduos Florestais e Fertilização Mineral (Nutrient Cycling and Growth in Eucalyptus Grandis Plantation under Different Forest Residues Management and Fertilization). Ph.D. Thesis, ESALQ-USP, Piracicaba, Brazil, 2015. [Google Scholar]
- Pulito, A.P.; Goncalves, J.L.M.; Smethurst, P.J.; Arthur Junior, J.C.; Alvares, C.A.; Rocha, J.H.T.; Hubner, A.; Moraes, L.F.; Miranda, A.C.; Kamogawa, M.Y.; et al. Available nitrogen and responses to nitrogen fertilizer in Brazilian Eucalypt plantations on soils of contrasting textures. Forests 2015, 6, 973–991. [Google Scholar] [CrossRef]
- Laclau, J.-P.; Ranger, J.; Goncalves, J.L.M.; Maquere, V.; Krusche, A.V.; M'Bou, A.T.; Nouvellon, Y.; Saint-Andre, L.; Bouillet, J.-P.; Piccolo, M.D.C. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations Main features shown by intensive monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Mendham, D.S.; Ogden, G.N.; Short, T.; O’Connell, T.M.; Grove, T.S.; Rance, S.J. Repeated harvest residue removal reduces E. globulus productivity in the 3rd rotation in south-western Australia. For. Ecol. Manag. 2014, 329, 279–286. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. For. Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Huang, Z.; He, Z.; Wan, X.; Hu, Z.; Fan, S.; Yang, Y. Harvest residue management effects on tree growth and ecosystem carbon in a Chinese fir plantation in subtropical China. Plant Soil 2013, 364, 303–314. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mendham, D.S.; Grove, T.S.; O’Connell, A.M.; Sankaran, K.V.; Rance, S.J. Harvest residue effects on soil organic matter, nutrients and microbial biomass in eucalypt plantations in Kerala, India. For. Ecol. Manag. 2014, 328, 140–149. [Google Scholar] [CrossRef]
- Moreno-Fernandez, D.; Dias-Pines, E.; Barbeito, I.; Sanchez-Gonzalez, M.; Montes, F.; Rubio, A.; Canellas, I. Temporal carbon dynamics over the rotation period of two alternative management systems in Mediterranean mountain Scots pine forests. For. Ecol. Manag. 2015, 348, 186–195. [Google Scholar] [CrossRef]
- Rocha, J.H.T. Reflexos do Manejo de Resíduos Florestais na Produtividade, Nutrição e Fertilidade do Solo em Plantações de Eucalyptus grandis (Implications of Forest Harvest Residues Management on the Productivity, Nutrition and Soil Fertility in Eucalyptus grandis Plantation in Brazil). Master’s Thesis, ESALQ-USP, Piracicaba, Brazil, 2014. [Google Scholar]
- Vanguelova, E.; Pitman, R.; Luiro, J.; Helmisaari, H.-S. Long-term effects of whole tree harvesting on soil carbon and nutrient sustainability in the UK. Biogeochemistry 2010, 101, 43–59. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Franci, A.F.; Gonçalves, J.L.M. Effect of forest residue management on fine roots density in an Eucalyptus grandis plantation. “Luiz de Queiroz” College of Agriculture, University of São Paulo: Piracicaba, Brazil, Unpublished data. 2016. [Google Scholar]
- Smolander, A.; Kitunen, V.; Tamminen, P.; Kukkola, M. Removal of logging residue in Norway spruce thinning stands: Long-term changes in organic layer properties. Soil Biol. Biochem. 2010, 42, 1222–1228. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of the Soil, 14th ed.; Bookmam: DeKalb, IL, USA, 2013; pp. 1–716. [Google Scholar]
- Silva, M.D.S.; Paula, T.D.; Moreira, B.C.; Carolino, M.; Cruz, C.; Bazzolli, D.M.S.; Silva, C.C.; Kasuya, M.C.M. Nitrogen-Fixing Bacteria in Eucalyptus globulus Plantations. PLoS ONE 2014, 9, e111313. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.; Gama-Rodrigues, A.C.; Gonçalves, J.L.M.; Gama-Rodrigues, E.F.; Sales, M.V.S.; Aleixo, S. Labile and non-labile fractions of phosphorus and its transformations in soil under Eucalyptus plantations, Brazil. Forests 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Bissani, C.A.; Tedesco, M.J. O enxofre no solo (Sulphur in the soil). In Reunião Brasileira de Fertilidade do Solo, 17nd ed.; Borkert, C.M., Lantmann, A.F., Eds.; EMBRAPA/IAPAR/SBCS: Londrina, Brazil, 1988; pp. 11–29. [Google Scholar]
- Candido, B.M.; Silva, M.L.N.; Curi, N.; Batista, P.V.G. Water erosion post-planting in eucalyptus forests in the Parana river basin, in the eastern Mato Grosso do Sul. Revista Brasileira De Ciencia Do Solo 2014, 38, 1565–1575. [Google Scholar]
| Depth | Sandy | Silt | Clay 1 | pH 2 | CEC7 3 | C 4 | N 5 | P 6 | Exchangeable Cations 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Al | |||||||||
| cm | g·kg−1 | mmolc kg−1 | g·kg−1 | mg·kg−1 | mmolc kg−1 | |||||||
| 0–10 | 802 | 22 | 175 | 3.8 | 63.98 | 9.61 | 1.44 | 4 | 0.25 | 4.28 | 2.81 | 7.50 |
| 10–20 | 811 | 12 | 176 | 3.9 | 51.42 | 10.05 | 1.67 | 3 | 0.27 | 2.80 | 2.17 | 8.43 |
| 20–30 | 790 | 34 | 176 | 3.9 | 39.98 | 6.77 | 1.53 | 1 | 0.20 | 1.32 | 1.00 | 6.09 |
| 30–40 | 777 | 23 | 200 | 3.9 | 40.18 | 5.33 | 1.29 | 1 | 0.15 | 0.88 | 0.81 | 7.03 |
| 40–60 | 747 | 14 | 239 | 3.9 | 38.46 | 5.42 | 1.14 | 1 | 0.15 | 0.99 | 0.72 | 7.50 |
| 60–100 | 712 | 12 | 276 | 3.9 | 32.72 | 5.04 | 0.99 | 1 | 0.15 | 0.66 | 0.54 | 6.56 |
| 100–150 | 712 | 11 | 277 | 4.0 | 30.09 | 3.44 | 1.04 | 1 | 0.08 | 0.71 | 0.54 | 2.34 |
| 150–200 | 704 | 20 | 276 | 4.2 | 22.05 | 0.87 | 1.04 | 1 | 0.05 | 0.55 | 0.54 | 2.81 |
| Treatment 1 | Forest Residue 2 | Nutrients 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Canopy | Bark | Litter Layer | N | P | K | Ca | Mg | S | |
| kg·ha −1 | |||||||||
| ReM + F | M | M | M | 130 | 44 | 125 | 480 | 120 | 140 |
| ReR + F | R | R | R | 130 | 44 | 125 | 480 | 120 | 140 |
| ACR + F | R | R | M | 130 | 44 | 125 | 480 | 120 | 140 |
| ACR − N | R | R | M | 10 | 44 | 125 | 480 | 120 | 11 |
| ACR − P | R | R | M | 130 | - | 125 | 480 | 120 | 140 |
| Component | Biomass (t·ha−1) | N (kg·ha−1) | P (kg·ha−1) | S (kg·ha−1) | |
|---|---|---|---|---|---|
| Nutrients Stocks Before 2004 Harvest | |||||
| Litter layer | 24 (1) 1 | 141 (17) | 12 (1) | 12 (1) | |
| Stem wood | 167 (15) | 250 (20) | 52 (3) | 67 (5) | |
| Stem bark | 18 (1) | 62 (3) | 8 (1) | 7 (1) | |
| Canopy | 9 (1) | 100 (9) | 7 (1) | 6 (1) | |
| Root | 33 (5) | 109 (18) | 11 (2) | 14 (2) | |
| Total | 251 (12) | 662 (48) | 90 (10) | 106 (15) | |
| Harvest Outputs in 2004 | |||||
| Treatment ReM + F | 167 (15) | 250 (20) | 52 (3) | 67 (5) | |
| Treatments ACR + F, ACR − N, and ACR − P | 194 (18) | 412 (46) | 67 (4) | 80 (7) | |
| Treatment ReR + F | 218 (18) | 553 (49) | 79 (15) | 92 (8) | |
| Inputs from 2004 to 2012 | |||||
| Atmospheric deposition 2 | 32 | - | - | ||
| Fertilizer application 3: | |||||
| Treatments ReM + F, ACR + F, and ReR + F | 130 | 44 | 143 | ||
| Treatment ACR − N | 10 | 44 | 11 | ||
| Treatment ACR − P | 130 | 0 | 143 | ||
| Harvest Outputs in 2012 | |||||
| Treatment ReM + F | 191 (10) | 313 (17) | 60 (3) | 71 (4) | |
| Treatment ACR + F | 196 (20) | 412 (37) | 67 (7) | 71 (7) | |
| Treatment ReR + F | 220 (16) | 542 (39) | 79 (6) | 80 (6) | |
| Treatment ACR − N | 200 (32) | 426 (60) | 69 (11) | 70 (11) | |
| Treatment ACR − P | 180 (23) | 386 (48) | 61 (8) | 65 (8) | |
| Inputs from 2012 to 2016 | |||||
| Atmospheric deposition | 16 | - | - | ||
| Fertilizer application: | |||||
| Treatments ReM + F, ACR+F, and ReR + F | 130 | 44 | 143 | ||
| Treatment ACR − N | 10 | 44 | 11 | ||
| Treatment ACR − P | 130 | 0 | 143 | ||
| Accumulated in the Biomass in 2016 | |||||
| Treatment ReM + F | 100 (3) | 255 (20) | 25 (4) | 40 (2) | |
| Treatment ACR + F | 86 (3) | 207 (26) | 21 (1) | 37 (2) | |
| Treatment ReR + F | 85 (7) | 202 (17) | 18 (2) | 37 (4) | |
| Treatment ACR − N | 84 (5) | 195 (12) | 18 (1) | 36 (1) | |
| Treatment ACR − P | 75 (2) | 172 (3) | 15 (2) | 32 (1) | |
| Treatment | N (kg·ha−1) | P (kg·ha−1) | S (kg·ha−1) |
|---|---|---|---|
| Net Nutrient Balance 1 | |||
| ReM + F | −255 | −24 | 148 |
| ACR + F | −516 | −48 | 135 |
| ReR + F | −787 | −70 | 111 |
| ACR − N | −770 | −48 | −128 |
| ACR − P | −490 | −128 | 141 |
| Difference in Soil Nutrients Stocks from 2004 to 2012 (0–20 cm) | |||
| ReM + F | −164 | 0.8 | −1.1 |
| ACR + F | −189 | −0.4 | −1.3 |
| ReR + F | −192 | −0.5 | −2.5 |
| ACR − N | −219 | −0.9 | −1.4 |
| ACR − P | −189 | −2.0 | 4.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegale, M.L.C.; Rocha, J.H.T.; Harrison, R.; Goncalves, J.L.d.M.; Almeida, R.F.; Piccolo, M.D.C.; Hubner, A.; Arthur Junior, J.C.; De Vicente Ferraz, A.; James, J.N.; et al. Effect of Timber Harvest Intensities and Fertilizer Application on Stocks of Soil C, N, P, and S. Forests 2016, 7, 319. https://doi.org/10.3390/f7120319
Menegale MLC, Rocha JHT, Harrison R, Goncalves JLdM, Almeida RF, Piccolo MDC, Hubner A, Arthur Junior JC, De Vicente Ferraz A, James JN, et al. Effect of Timber Harvest Intensities and Fertilizer Application on Stocks of Soil C, N, P, and S. Forests. 2016; 7(12):319. https://doi.org/10.3390/f7120319
Chicago/Turabian StyleMenegale, Marcella L.C., Jose Henrique T. Rocha, Robert Harrison, Jose Leonardo de M. Goncalves, Rodrigo F. Almeida, Marisa De C. Piccolo, Ayeska Hubner, Jose Carlos Arthur Junior, Alexandre De Vicente Ferraz, Jason N. James, and et al. 2016. "Effect of Timber Harvest Intensities and Fertilizer Application on Stocks of Soil C, N, P, and S" Forests 7, no. 12: 319. https://doi.org/10.3390/f7120319






