Sapflow-Based Stand Transpiration in a Semiarid Natural Oak Forest on China’s Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Meteorological Variables and Soil Water Content
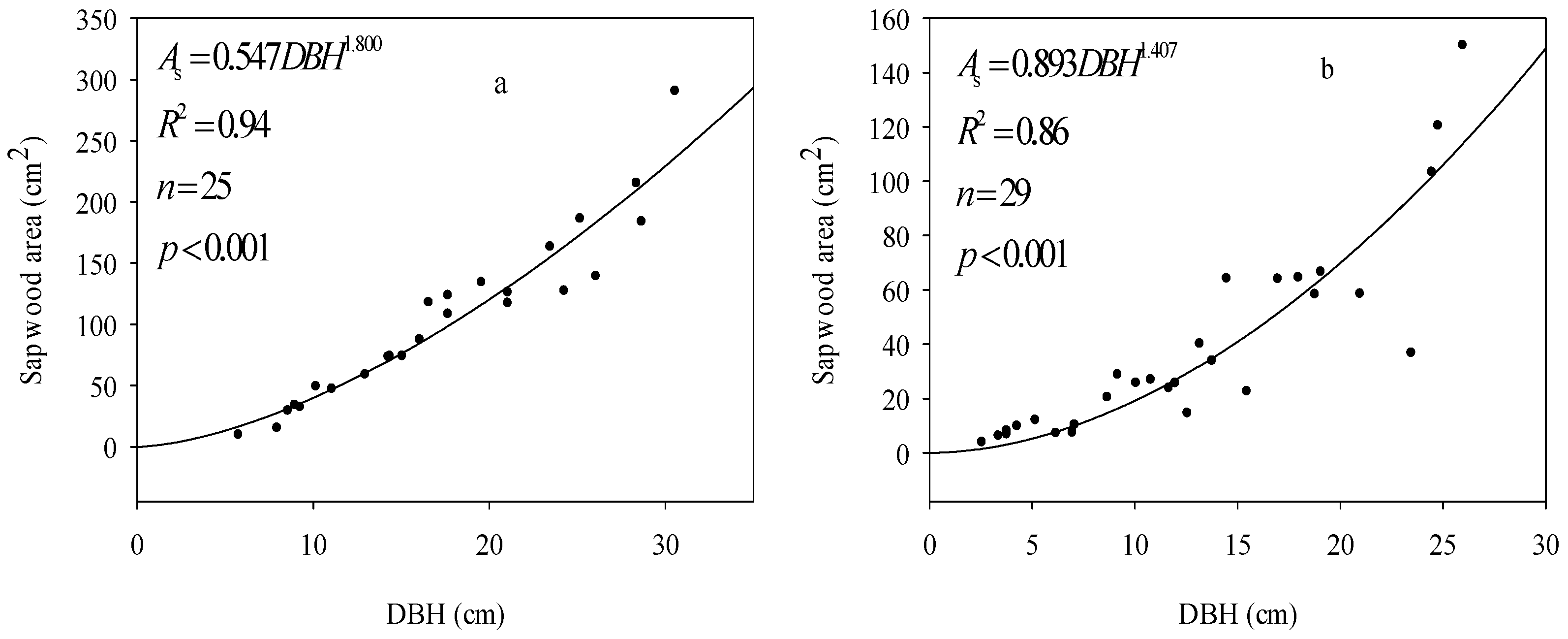
2.3. Sap flow Measurements and Sapwood Area Estimates
2.4. Calculation of Stand Transpiration
3. Results
3.1. Sapwood Area Estimates
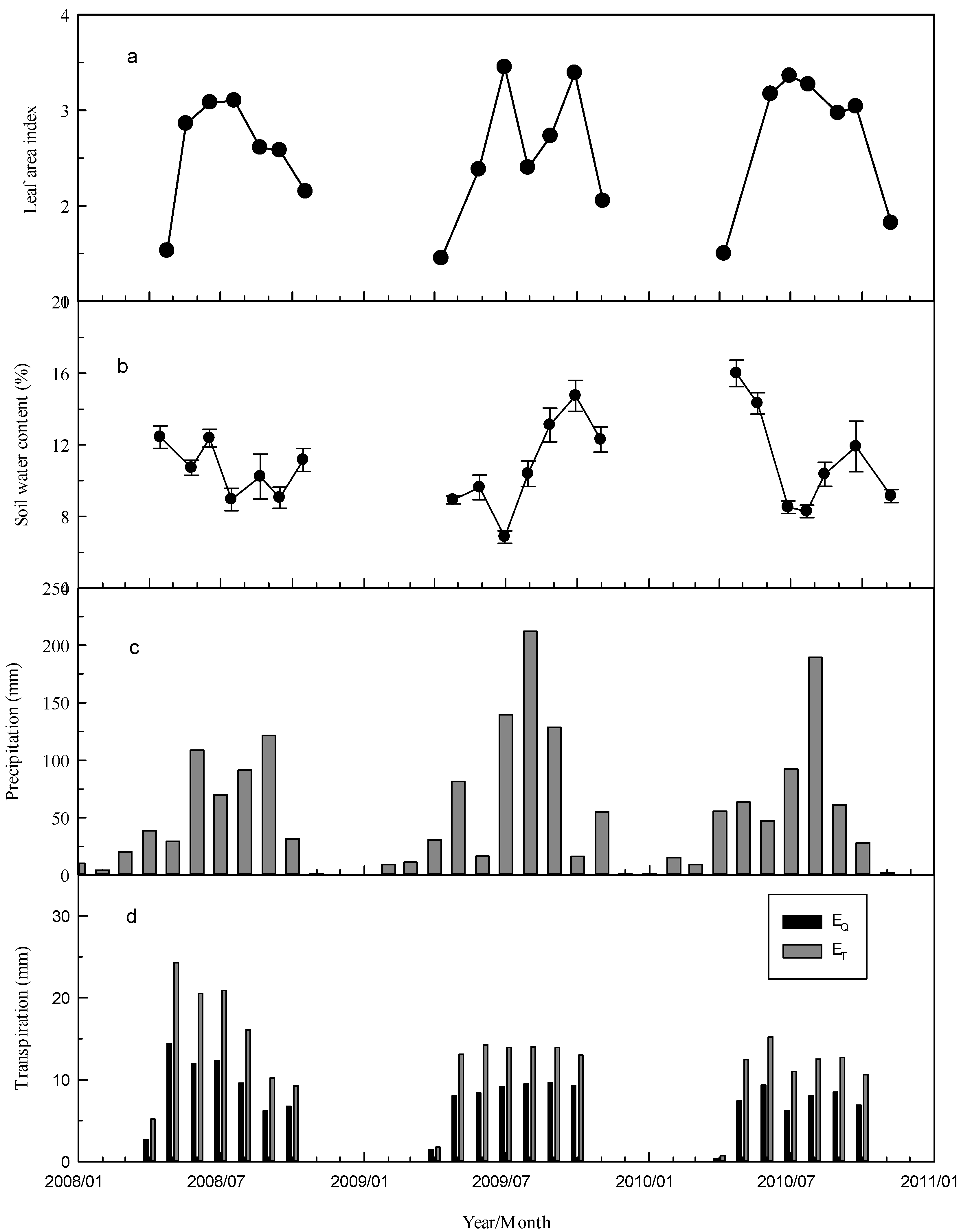
3.2. Meteorological Conditions, LAI, and Soil Water Content
3.3. Transpiration at the Stem-Scale
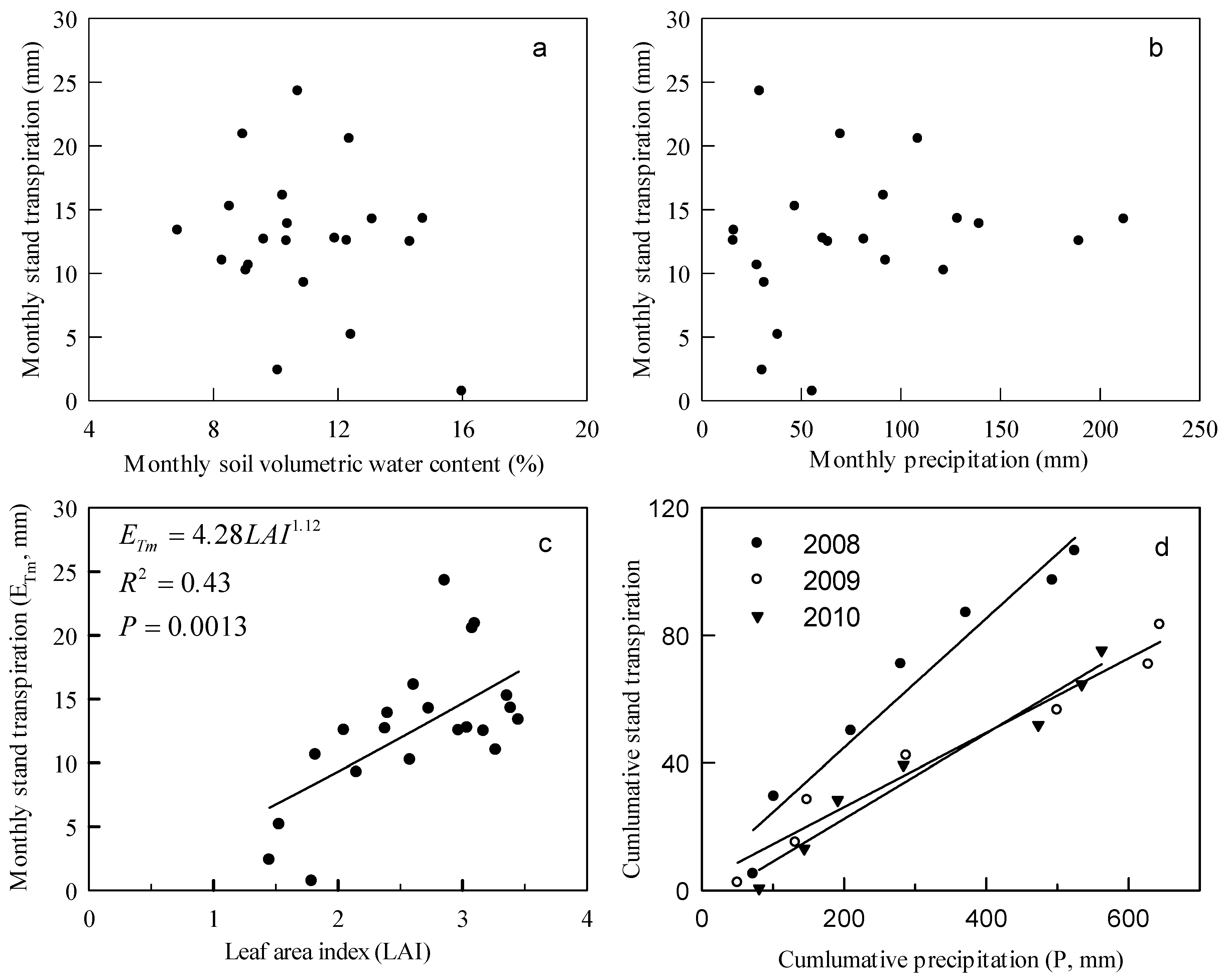
3.4. Stand Transpiration
4. Discussion
4.1. Stem-Scale Transpiration Estimate and Influencing Factors
4.2. Stand Transpiration Characteristics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, J.-Y.; Yoda, K. Climate and vegetation in china iii water balance and distribution of vegetation. Ecol. Res. 1990, 5, 9–23. [Google Scholar] [CrossRef]
- Kira, T. Forest ecosystems of east and southeast Asia in a global perspective. Ecol. Res. 1991, 6, 185–200. [Google Scholar] [CrossRef]
- Aranda, I.; Forner, A.; Cuesta, B.; Valladares, F. Species-specific water use by forest tree species: From the tree to the stand. Agric. Water Manag. 2012, 114, 67–77. [Google Scholar] [CrossRef]
- Kira, T. A climatological interpretation of japanese vegetation zones. In Vegetation Science and Environmental Protection; Miyawaki, A., Tüxen, R., Eds.; Maruzen: Tokyo, Japan, 1977; pp. 21–30. [Google Scholar]
- Wang, K.C.; Dickinson, R.E. A review of global terrestrial evapotranspiration: Observation, modeling, climatology, and climatic variability. Rev. Geophys. 2012, 50, RG2005. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Leuschner, C. Recent drought stress leads to growth reductions in larix sibirica in the western khentey, mongolia. Glob. Chang. Biol. 2010, 16, 3024–3035. [Google Scholar] [CrossRef]
- Yaseef, N.R.; Yakir, D.; Rotenberg, E.; Schiller, G.; Cohen, S. Ecohydrology of a semi-arid forest: Partitioning among water balance components and its implications for predicted precipitation changes. Ecohydrology 2010, 3, 143–154. [Google Scholar] [CrossRef]
- Tian, J.-L.; Liang, Y.-M.; Liu, P.-L. Investigation into the Ecological Agriculture Construction in Hilly-Gully Region of the Loess Plateau; Yellowriver Water Conservancy Press: Zhengzhou, China, 2003; p. 275. (In Chinese) [Google Scholar]
- Tsunekawa, A.; Liu, G.; Yamanaka, N.; Du, S. Restoration and Development of the Degraded Loess Plateau, China; Springer: Tokyo, Japan, 2014; p. 288. [Google Scholar]
- Zhang, X.P.; Zhang, L.; Zhao, J.; Rustomji, P.; Hairsine, P. Responses of streamflow to changes in climate and land use/cover in the loess plateau, China. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Wu, Q.-X.; Yang, W.-Z. Forest and Grassland Vegetation Construction and Its Sustainable Development in Loess Plateau; (China’s) Science Press: Beijing, China, 1998; p. 289. (In Chinese) [Google Scholar]
- Cheng, J.M.; Wan, H.E. Vegetation Construction and Soil and Water Conservation in the Loess Plateau of China; China Forestry Publishing House: Beijing, China, 2002; p. 426. (In Chinese) [Google Scholar]
- Kimura, R.; Takayama, N. Climate of the loess plateau. In Restoration and Development of the Degraded Loess Plateau, China; Tsunekawa, A., Liu, G., Yamanaka, N., Du, S., Eds.; Springer: Tokyo, Japan, 2014; pp. 23–33. [Google Scholar]
- Yamanaka, N.; Hou, Q.C.; Du, S. Vegetation of the loess plateau. In Restoration and Development of the Degraded Loess Plateau, China; Tsunekawa, A., Liu, G., Yamanaka, N., Du, S., Eds.; Springer: Tokyo, Japan, 2014; pp. 49–60. [Google Scholar]
- Wang, Y.Q.; Shao, M.A.; Zhu, Y.J.; Liu, Z.P. Impacts of land use and plant characteristics on dried soil layers in different climatic regions on the loess plateau of china. Agric. For. Meteorol. 2011, 151, 437–448. [Google Scholar] [CrossRef]
- Jian, S.Q.; Zhao, C.Y.; Fang, S.M.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese loess plateau. Agric. For. Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, G.B.; Kume, T.; Otsuki, K.; Yamanaka, N.; Du, S. Estimating water use of a black locust plantation by the thermal dissipation probe method in the semiarid region of loess plateau, China. J. For. Res. 2010, 15, 241–251. [Google Scholar] [CrossRef]
- Zhang, J.G.; Guan, J.H.; Shi, W.Y.; Yamanaka, N.; Du, S. Interannual variation in stand transpiration estimated by sap flow measurement in a semiarid black locust plantation, loess plateau, China. Ecohydrology 2015, 8, 137–147. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.D.; Wang, S.Q.; Hou, Q.C. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near yan’an on the loess plateau, China. For. Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Otsuki, K.; Yamanaka, N.; Du, S. Vegetation restoration on loess plateau. In Restoration and Development of the Degraded Loess Plateau, China; Tsunekawa, A., Liu, G., Yamanaka, N., Du, S., Eds.; Springer: Tokyo, Japan, 2014; pp. 233–251. [Google Scholar]
- Cermak, J.; Kucera, J.; Nadezhdina, N. Sap flow measurements with some thermodynamic methods, flow integration within trees and scaling up from sample trees to entire forest stands. Trees-Struct. Funct. 2004, 18, 529–546. [Google Scholar] [CrossRef]
- David, T.S.; Ferreira, M.I.; David, J.S.; Pereira, J.S. Transpiration from a mature eucalyptus globulus plantation in portugal during a spring-summer period of progressively higher water deficit. Oecologia 1997, 110, 153–159. [Google Scholar] [CrossRef]
- Lu, P.; Urban, L.; Zhao, P. Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: Theory and practice. Acta Bot. Sin. 2004, 46, 631–646. [Google Scholar]
- Smith, D.; Allen, S. Measurement of sap flow in plant stems. J. Exp. Bot. 1996, 47, 1833–1844. [Google Scholar] [CrossRef]
- Granier, A.; Biron, P.; Bréda, N.; Pontailler, J.Y.; Saugier, B. Transpiration of trees and forest stands: Short and long-term monitoring using sapflow methods. Glob. Chang. Biol. 1996, 2, 265–274. [Google Scholar] [CrossRef]
- Kume, T.; Otsuki, K.; Du, S.; Yamanaka, N.; Wang, Y.L.; Liu, G.B. Spatial variation in sap flow velocity in semiarid region trees: Its impact on stand-scale transpiration estimates. Hydrol. Process. 2012, 26, 1161–1168. [Google Scholar] [CrossRef]
- Zhang, J.G.; He, Q.Y.; Shi, W.Y.; Otsuki, K.; Yamanaka, N.; Du, S. Radial variations in xylem sap flow and their effect on whole-tree water use estimates. Hydrol. Process. 2015, 29, 4993–5002. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.L.; Kume, T.; Zhang, J.G.; Otsuki, K.; Yamanaka, N.; Liu, G.B. Sapflow characteristics and climatic responses in three forest species in the semiarid loess plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Tateishi, M.; Kumagai, T.; Utsumi, Y.; Umebayasi, T.; Shiiba, Y.; Inoue, K.; Kaji, K.; Cho, K.; Otsuki, K. Spatial variations in xylem sap flux density in evergreen oak trees with radial-porous wood: Comparisons with anatomical observations. Trees-Struct. Funct. 2008, 22, 23–30. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, P.T.; Xiong, W.; Shen, Z.X.; Guo, M.C.; Shi, Z.J.; Du, A.; Wang, L.M. Water-yield reduction after afforestation and related processes in the semiarid Liupan mountains, northwest China. J. Am. Water Resour. Assoc. 2008, 44, 1086–1097. [Google Scholar] [CrossRef]
- Shi, W.Y.; Yan, M.J.; Zhang, J.G.; Guan, J.H.; Du, S. Soil CO2 emissions from five different types of land use on the semiarid loess plateau of China, with emphasis on the contribution of winter soil respiration. Atmos. Environ. 2014, 88, 74–82. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An introduction to Environmental Biophysics; Springer: New York, NY, USA, 1998. [Google Scholar]
- Granier, A. Evaluation of transpiration in a douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Clearwater, M.J.; Meinzer, F.C.; Goldstein, G. Heat dissipation sensors of variable length for the measurement of sap flow in trees with deep sapwood. Tree Physiol. 2002, 22, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Yan, M.J.; Shi, W.Y.; Du, S. Seasonal dynamics and azimuthal variations of sap flow in quercus liaotungensis and their effects on transpiration estimates. J. Soil Water Conserv. 2011, 25, 193–197. [Google Scholar]
- Vertessy, R.; Benyon, R.; O’sullivan, S.; Gribben, P. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol. 1995, 15, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.B.; Hanson, P.J.; Mulholland, P.J.; Baldocchi, D.D.; Wullschleger, S.D. A comparison of methods for determining forest evapotranspiration and its components: Sap-flow, soil water budget, eddy covariance and catchment water balance. Agric. For. Meteorol. 2001, 106, 153–168. [Google Scholar] [CrossRef]
- Pataki, D.; Oren, R. Species differences in stomatal control of water loss at the canopy scale in a mature bottomland deciduous forest. Adv. Water Resour. 2003, 26, 1267–1278. [Google Scholar] [CrossRef]
- Kumagai, T.; Aoki, S.; Shimizu, T.; Otsuki, K. Sap flow estimates of stand transpiration at two slope positions in a Japanese cedar forest watershed. Tree Physiol. 2007, 27, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ewers, B.E.; Mackay, D.S.; Gower, S.T.; Ahl, D.E.; Burrows, S.N.; Samanta, S.S. Tree species effects on stand transpiration in northern Wisconsin. Water Resour. Res. 2002, 38, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.G.; Kume, T.; Otsuki, K.; Yamanaka, N.; Du, S. Sap flow dynamics of dominant trees in quercus liaotungensis forest in the semiarid loess plateau region. Sci. Silvae Sin. 2011, 47, 63–69. [Google Scholar]
- Jung, E.; Otieno, D.; Lee, B.; Lim, J.; Kang, S.; Schmidt, M.; Tenhunen, J. Up-scaling to stand transpiration of an asian temperate mixed-deciduous forest from single tree sapflow measurements. Plant Ecol. 2011, 212, 383–395. [Google Scholar] [CrossRef]
- Köstner, B.; Schulze, E.-D.; Kelliher, F.; Hollinger, D.; Byers, J.; Hunt, J.; McSeveny, T.; Meserth, R.; Weir, P. Transpiration and canopy conductance in a pristine broad-leaved forest of nothofagus: An analysis of xylem sap flow and eddy correlation measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef]
- Martin, T.A.; Brown, K.J.; Cermak, J.; Ceulemans, R.; Kucera, J.; Meinzer, F.C.; Rombold, J.S.; Sprugel, D.G.; Hinckley, T.M. Crown conductance and tree and stand transpiration in a second-growth abies amabilis forest. Can. J. For. Res. 1997, 27, 797–808. [Google Scholar] [CrossRef]
- Granier, A.; Biron, P.; Lemoine, D. Water balance, transpiration and canopy conductance in two beech stands. Agric. For. Meteorol. 2000, 100, 291–308. [Google Scholar] [CrossRef]
- Horna, V.; Schuldt, B.; Brix, S.; Leuschner, C. Environment and tree size controlling stem sap flux in a perhumid tropical forest of central sulawesi, Indonesia. Ann. For. Sci. 2011, 68, 1027–1038. [Google Scholar] [CrossRef]
- Ford, C.R.; McGuire, M.A.; Mitchell, R.J.; Teskey, R.O. Assessing variation in the radial profile of sap flux density in pinus species and its effect on daily water use. Tree Physiol. 2004, 24, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, R.; Cermak, J.; Llorens, P. Variation in the radial patterns of sap flux density in pubescent oak (quercus pubescens) and its implications for tree and stand transpiration measurements. Tree Physiol. 2007, 27, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Nadezhdina, N.; Cermak, J.; Ceulemans, R. Radial patterns of sap flow in woody stems of dominant and understory species: Scaling errors associated with positioning of sensors. Tree Physiol. 2002, 22, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Pataki, D.E. Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia 2001, 127, 549–559. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hicks, B.B.; Camara, P. A canopy stomatal resistance model for gaseous deposition to vegetated surfaces. Atmos. Environ. (1967) 1987, 21, 91–101. [Google Scholar] [CrossRef]
- Brun, L.J.; Kanemasu, E.T.; Powers, W.L. Evapotranspiration from soybean and sorghum fileds. Agron. J. 1972, 64, 145–148. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Zeppel, M.; Liu, C.; Guo, J.; Zhu, J.; Zhang, X.; Zhang, J.; Zha, T. Response of transpiration to rain pulses for two tree species in a semiarid plantation. Int. J. Biometeorol. 2014, 58, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, A.P.; Worledge, D.; Battaglia, M. Constraints on transpiration of eucalyptus globulus in southern tasmania, Australia. Agric. For. Meteorol. 2008, 148, 453–465. [Google Scholar] [CrossRef]
- Tognetti, R.; Giovannelli, A.; Lavini, A.; Morelli, G.; Fragnito, F.; d’Andria, R. Assessing environmental controls over conductances through the soil-plant-atmosphere continuum in an experimental olive tree plantation of southern Italy. Agric. For. Meteorol. 2009, 149, 1229–1243. [Google Scholar] [CrossRef]
- Breda, N.; Granier, A. Intra- and interannual variations of transpiration, leaf area index and radial growth of a sessile oak stand (quercus petraea). Ann. For. Sci. 1996, 53, 521–536. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Hanson, P.; Todd, D. Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For. Ecol. Manag. 2001, 143, 205–213. [Google Scholar] [CrossRef]
- Schiller, G.; Cohen, S.; Ungar, E.D.; Moshe, Y.; Herr, N. Estimating water use of sclerophyllous species under east-mediterranean climate: III. Tabor oak forest sap flow distribution and transpiration. For. Ecol. Manag. 2007, 238, 147–155. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; Xu, D. Regulations of water use for transpiration of larix principi-rupprechtii plantation and its response on environmental factors in southern Ningxia hilly area. Sci. Silvae Sin. 2003, 39, 1–7. [Google Scholar]

| Variable | Year | ||
|---|---|---|---|
| 2008 | 2009 | 2010 | |
| Diameter at breast height (DBH, cm) of all trees | 11.80 ± 5.81 | 11.93 ± 5.23 | 12.20 ± 5.51 |
| DBH of oak trees | 12.76 ± 1.09 | 12.91 ± 1.10 | 13.32 ± 1.13 |
| Stand basal area (BA, m2∙ha−1) | 19.04 | 19.45 | 20.02 |
| BA of oak trees (m2∙ha−1) | 11.09 | 11.33 | 12.04 |
| Stand leaf area index (LAI) | 1.53–3.1 | 1.45–3.45 | 1.5–3.36 |
| Soil moisture (θ, %) | 9.0–12.4 | 6.9–14.7 | 8.3–16.0 |
| Mean daily solar radiation (Rs, MJ∙m−2) | 17.0 ± 0.51 | 17.2 ± 0.55 | 17.1 ± 0.53 |
| Mean daily air temperature (T, °C) | 16.2 ± 0.33 | 16.9 ± 0.33 | 16.0 ± 0.43 |
| Mean daily daytime vapor pressure deficit (VPDm, kPa) | 1.22 ± 0.05 | 1.20 ± 0.06 | 0.99 ± 0.05 |
| Total gross precipitation (P, mm) | 526 | 700 | 564 |
| Month | ETd vs. VPDm | R2 | ETd vs. Rs | R2 |
|---|---|---|---|---|
| May | ETd = 0.726(1 − Exp(−1.634VPDm)) | 0.89 | ETd = 0.063 + 0.023Rs | 0.88 |
| Jun | ETd = 0.612(1 − Exp(−1.209VPDm)) | 0.63 | ETd = 0.245 + 0.013Rs | 0.51 |
| Jul | ETd = 0.633(1 − Exp(−2.424VPDm)) | 0.59 | ETd = 0.190 + 0.018Rs | 0.61 |
| Aug | ETd = 0. 639(1 − Exp(−2.744VPDm)) | 0.88 | ETd = 0.141 + 0.023Rs | 0.74 |
| Sep | ETd = 0.714(1 − Exp(−2.425VPDm)) | 0.93 | ETd = 0.068 + 0.034Rs | 0.89 |
| Oct | ETd = 0.553(1 − Exp(−1.335VPDm)) | 0.44 | ETd = −0.56 + 0.031Rs | 0.61 |
| Species | E (mm) | Species Contribution (%) | ||||
|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2008 | 2009 | 2010 | |
| Oak | 63.93 | 55.52 | 46.81 | 60.05 | 66.65 | 62.19 |
| Apricot | 22.24 | 18.40 | 18.57 | 18.61 | 22.73 | 23.53 |
| Others | 20.30 | 9.38 | 9.89 | 16.99 | 11.60 | 12.54 |
| Total | 106.47 | 83.3 | 75.27 | 100 | 100 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.-J.; Zhang, J.-G.; He, Q.-Y.; Shi, W.-Y.; Otsuki, K.; Yamanaka, N.; Du, S. Sapflow-Based Stand Transpiration in a Semiarid Natural Oak Forest on China’s Loess Plateau. Forests 2016, 7, 227. https://doi.org/10.3390/f7100227
Yan M-J, Zhang J-G, He Q-Y, Shi W-Y, Otsuki K, Yamanaka N, Du S. Sapflow-Based Stand Transpiration in a Semiarid Natural Oak Forest on China’s Loess Plateau. Forests. 2016; 7(10):227. https://doi.org/10.3390/f7100227
Chicago/Turabian StyleYan, Mei-Jie, Jian-Guo Zhang, Qiu-Yue He, Wei-Yu Shi, Kyoichi Otsuki, Norikazu Yamanaka, and Sheng Du. 2016. "Sapflow-Based Stand Transpiration in a Semiarid Natural Oak Forest on China’s Loess Plateau" Forests 7, no. 10: 227. https://doi.org/10.3390/f7100227






