Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study
Abstract
:1. Introduction
- Even single birch trees in spruce stands can modify topsoil properties.
2. Methods
2.1. Study Site
| Species | Age (years) | N ha−1 | dbh (cm) | Basal area (m2 ha−1) | Mixture proportion | H/D ratio | Volume (m³ ha−1) | Crown percent |
|---|---|---|---|---|---|---|---|---|
| Spruce | 60 to 80 | 453 | 29 | 32.0 | 92.6 | 69 | 330 | 60 |
| Birch | 60 | 36 | 30 | 2.6 | 7.4 | - | 25 | 48 |
| Horizon | Depth | Dry bulk density (fine soil) | Stone content | pH-value | Corg/N ratio | CECeff | BS |
|---|---|---|---|---|---|---|---|
| (cm) | (g* cm−3) | (mass %) | H2O | (-) | (µeq g−1) | (%) | |
| L | +3.5 to +4 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Of | +2 to +3.5 | 0.15 | 0 | 5.04 | 20 | 1625.37 | 98.3 |
| Oh | 0 to +2 | 0.28 | 0 to 28 | 5.02 | 16 | 1938.12 | 99.3 |
| Ahe/Ae | 0 to 11 | 0.83 | 36 | 3.94 | 18 | 415.05 | 57.0 |
| Bsh/Bhs | 11 to 17 | 0.51 | 50 | 3.87 | 23 | 68.29 | 9.4 |
| Bv | 17 to 32 | 0.80 | 41 | 4.47 | 19 | 41.68 | 6.0 |
| Bv/Cv | 32+ | n.a. | n.a. | 4.63 | 13 | 135.48 | 17.5 |
2.2. Experimental Design
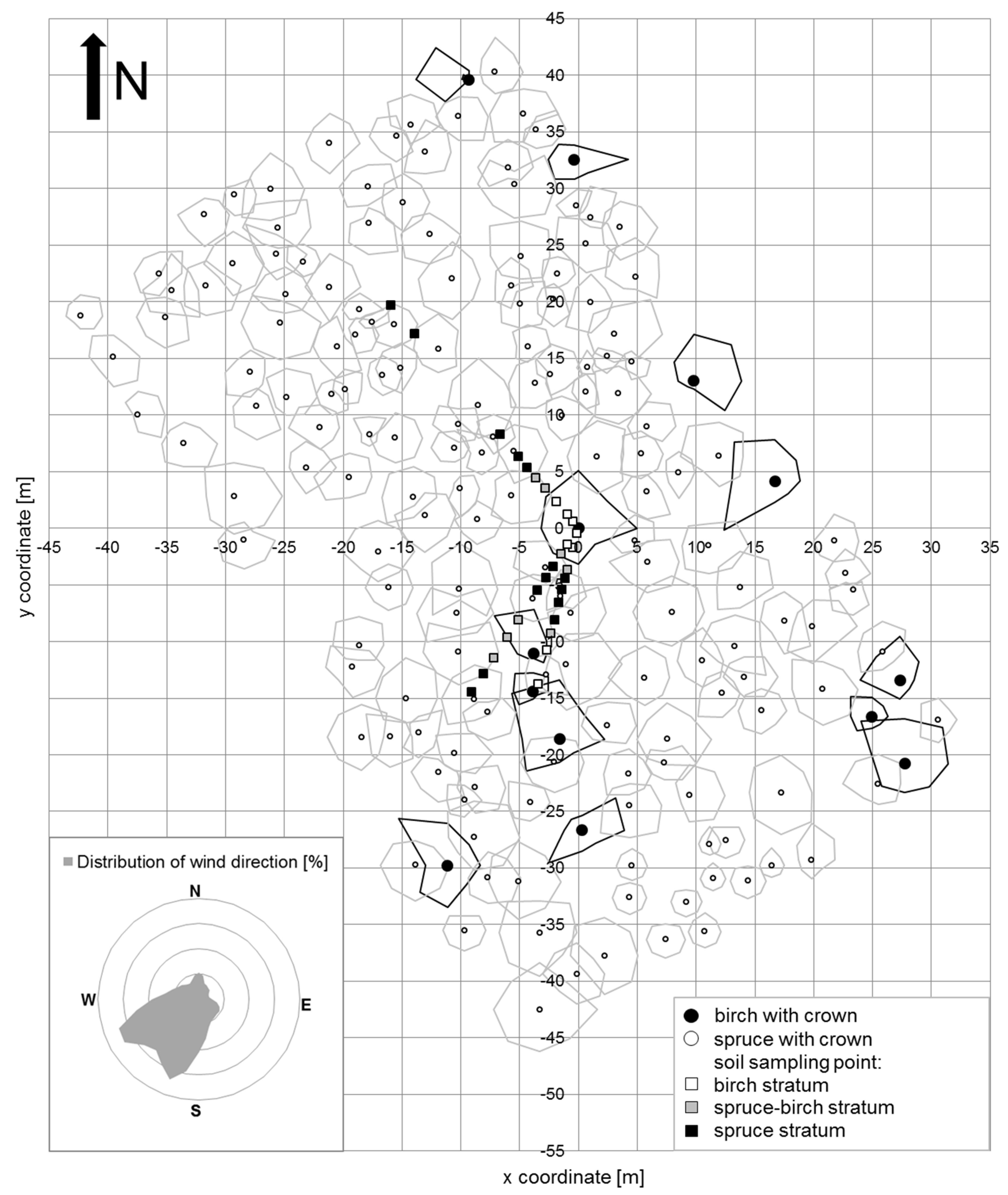
2.3. Soil Sampling and Analysis
2.4. Statistical Analysis
3. Results
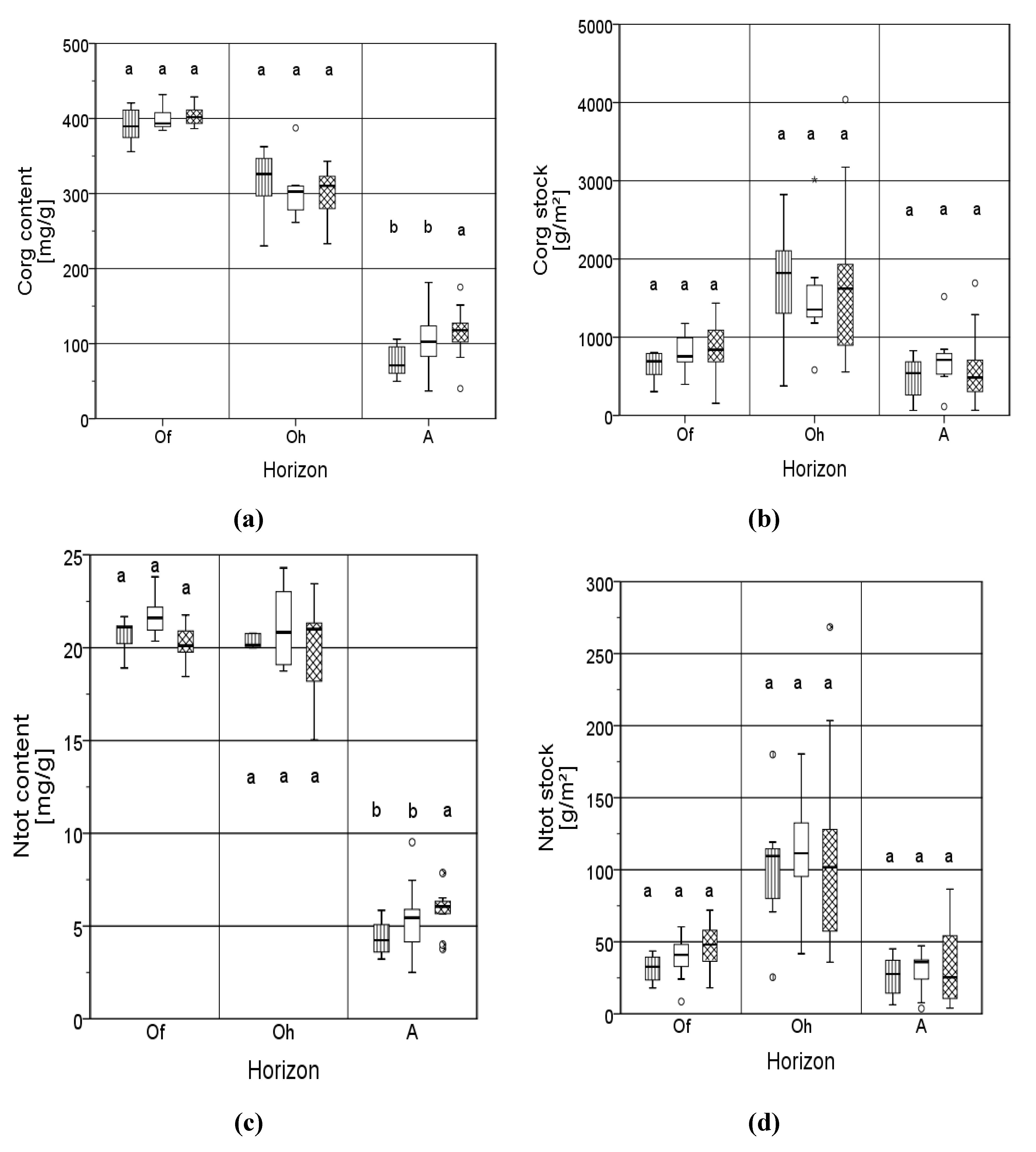
3.1. Carbon
3.2. Nitrogen
3.3. C/N Ratio and Acidity
3.4. Microbial Properties

4. Discussion

5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Jandl, R.M.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Fahlvik, N.; Agestam, E.; Ekö, P.M.; Lindén, M. Development of single-storied mixtures of Norway spruce and birch in Southern Sweden. Scand. J. For. Res. 2011, 26, 36–45. [Google Scholar] [CrossRef]
- Van Breemen, N.; Finzi, A.C.; Canham, C.D. Canopy tree-soil interactions within temperate forests: Effects of soil elemental composition and texture on species distributions. Can. J. For. Res. 1997, 27, 1110–1116. [Google Scholar] [CrossRef]
- Finzi, A.C.; van Breemen, N.; Canham, C.D. Canopy tree-soil interactions within temperate forests: Species effects on soil carbon and nitrogen. Ecol. Appl. 1998, 8, 440–446. [Google Scholar]
- Finzi, A.C.; Canham, C.D.; van Breemen, N. Canopy tree-soil interactions within temperate forests: Species effects on pH and cations. Ecol. Appl. 1998, 8, 447–454. [Google Scholar]
- Samec, P.; Vavřĺček, D.; Šimková, P.; Pňáček, J. Multivariate statistical approach to comparison of the nutrient status of Norway spruce (Picea abies L. Karst.) and top-soil properties in differently managed forest stands. J. For. Sci. 2007, 53, 101–112. [Google Scholar]
- Lettl, A.; Hysek, J. Soil microflora in an area where spruce (Picea abies) was killed by SO2 emissions and was succeeded by birch (Betula pendula) and mountain ash (Sorbus aucuparia). Ecol. Eng. 1994, 3, 27–37. [Google Scholar] [CrossRef]
- Saetre, P. Decomposition, microbial community structure, and earthworm effects along a birch-spruce soil gradient. Ecology 1998, 79, 834–846. [Google Scholar]
- Brandtberg, P.O.; Lundkvist, H.; Bengtsson, J. Changes in forest-floor chemistry caused by a birch admixture in Norway spruce stands. For. Ecol. Manag. 2000, 130, 253–264. [Google Scholar] [CrossRef]
- Brandtberg, P.O.; Lundkvist, H. Does an admixture of betula species in Picea abies stands increase organic matter quality and nitrogen release? Scan. J. For. Res. 2004, 19, 127–141. [Google Scholar] [CrossRef]
- Hagen-Thorn, A.; Callesen, I.; Armolaitis, K.; Nihlgård, B. The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land. For. Ecol. Manag. 2004, 195, 373–384. [Google Scholar] [CrossRef]
- Nilsson, M.C.; Wardle, D.A.; Dahlberg, A. Effects of plant litter species composition and diversity on the boreal forest plant-soil system. Oikos 1999, 86, 16–26. [Google Scholar] [CrossRef]
- Brandtberg, P.O.; Simonsson, M. Aluminium and iron chemistry in the O horizon changed by a shift in tree species composition. Biogeochemistry 2003, 63, 207–228. [Google Scholar] [CrossRef]
- Miles, J. Effect of Birch on Moorland. Cambridge: Natural Environment Research Council; Institute of Terrestrial Ecology, Banchory Research Station, Hill of Brathens, Glassel: Banchory, Scotland, 1981; p. 24. [Google Scholar]
- Saetre, P.; Brandtberg, P.O.; Lundkvist, H.; Bengtsson, J. Soil organisms and carbon, nitrogen and phosphorus mineralization in Norway spruce and mixed Norway spruce-birch stands. Biol. Fertil. Soils 1999, 28, 382–388. [Google Scholar] [CrossRef]
- Priha, O.; Grayston, S.J.; Hiukka, R.; Pennanen, T.; Smolander, A. Microbial community structure and characteristics of the organic matter in soils under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Biol. Fertil. Soils 2001, 33, 17–24. [Google Scholar] [CrossRef]
- Saetre, P.; Bååth, E. Spatial variation and patterns of soil microbial community structure in a mixed spruce-birch stand. Soil Biol. Biochem. 2000, 32, 909–917. [Google Scholar] [CrossRef]
- Lavelle, P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. In Transactions of the 15th World Congress on Soil Science; Etchevers, J.D., Aguilar, A., Nunez, R., Alcantar, G., Sanchez, P., Eds.; Society of Soil Science and Mexican Society of Soil Science: Mexico City, Mexico, 1994; pp. 189–220. [Google Scholar]
- Brussaard, L. Soil fauna, guilds, functional groups and ecosystem processes. Appl. Soil Ecol. 1998, 9, 123–135. [Google Scholar] [CrossRef]
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forests: A synthesis. Can. J. For. Res. 2001, 31, 1855–1870. [Google Scholar] [CrossRef]
- Kienzler, M.; Alban, D.H.; Perala, D.A. Soil invertebrate and microbial populations under three species on the same soil type. In USDA For. Serv. Res. Note NC-337; U.S. Dept. of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1986; p. 4. [Google Scholar]
- Frischbier, N. Untersuchungen zur einzelbaumverursachten kleinräumigen Variabilität und regenhöhenbasierten Dynamik des Bestandesniederschlages am Beispiel zweier Buchen-Fichten-Mischbestände. Ph.D. Thesis, Technische Universität Dresden, Germany, March 2012; p. 307. [Google Scholar]
- Lindross, A.J.; Derome, J.; Derome, K.; Smolander, A. The effect of Scots pine, Norway spruce and silver birch on the chemical composition of stand throughfall and upper soil percolation water in northern Finland. Boreal Environ. Res. 2011, 16, 240–250. [Google Scholar]
- Kimmins, H.; Blanco, J.A.; Seely, B.; Welham, C.; Scoullar, K. Forecasting Forest Futures. In A Hybrid Modelling Approach to the Assessment of Sustainability of Forest Ecosystems and Their Values; Earthscan: London, UK; Washington, DC, USA, 2010. [Google Scholar]
- Zinke, P.J. The pattern of influence of individual forest trees on soil properties. Ecology 1962, 43, 130–133. [Google Scholar] [CrossRef]
- Crampton, C.B. Podzolization of soils under individual tree canopies in southwestern British Columbia, Canada. Geoderma 1982, 28, 57–61. [Google Scholar] [CrossRef]
- Boettcher, S.E.; Kalisz, P.J. Single-tree influence on soil properties in the mountains of eastern Kentucky. Ecology 1990, 71, 1365–1372. [Google Scholar] [CrossRef]
- Wagner, S. Einzelbaumeffekte—Eine Methode zur ökologischen Forschung. In Beiträge zur Tagung 2004 der Sektion Waldbau DVFFA; Bauhus, J., Csapek, G., Eds.; Waldbau-Institut, Albert-Ludwigs-Unversity Freiburg: Göttingen, Germany, 2005; pp. 127–139. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth and Yield: From Measurement to Model; Springer: Berlin/Heidelberg, Germany, 2009; p. 664. [Google Scholar]
- Schua, K. Standortsökologische Baumarteneffekte in einem Mischbestand aus Gemeiner Fichte (Picea abies [L.] Karst.) und Sand-Birke (Betula pendula Roth) im Erzgebirge. Ph.D.Thesis, Technische Universität Dresden, Germany, January 2012; p. 203. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1995, 28, 25–31. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanzenernähr. Bodenkd. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Jäggi, W. Die Bestimmung der CO2-Bildung als Maß der bodenbiologischen Aktivität. Schweiz. Landwirtsch. Forsch. 1976, 15, 371–380. [Google Scholar]
- Trüby, P; Aldinger, E. Eine Methode zur Bestimmung austauschbarer Kationen in Waldböden. Z. Pflanzenernähr. Bodenkde. 1989, 152, 301–306. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Verlag: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS. In Statistics and Computing; Springer: New York, NY, USA, 2000; p. 528. [Google Scholar]
- Hedderich, J.; Sachs, L. Angewandte Statistik; Springer Verlag: Berlin Heidelberg, Germany, 2012; p. 881. [Google Scholar]
- Jiménez Esquilín, A.E.; Stromberger, M.E.; Shepperd, W.D. Soil scarification and wildfire interactions and effects on microbial communities and carbon. Soil Sci. Soc. Am. J. 2008, 72, 111–118. [Google Scholar] [CrossRef]
- Busse, M.D.; Beattie, S.E.; Powers, R.F.; Sanchez, F.G.; Tiarks, A.E. Microbial community responses in forest mineral soil to compaction, organic matter removal, and vegetation control. Can. J. For. Res. 2006, 36, 577–588. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. The influence of soil fertility on rhizosphere effects in northern hardwood forest soils. Soil Sci. Soc. Am. J. 2008, 72, 453–461. [Google Scholar] [CrossRef]
- Hansson, K.; Olsson, B.A.; Olsson, M.; Johansson, U.; Berggren Kleja, D. Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. For. Ecol. Manag. 2011, 262, 522–530. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, A. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol. Biochem. 2002, 34, 651–660. [Google Scholar] [CrossRef]
- Kanerva, S.; Kitunen, V.; Loponen, J.; Smolander, A. Phenolic compounds and terpenes in soil organic horizon layers under silver birch, Norway spruce and Scots pine. Biol. Fertil. Soils 2008, 44, 547–556. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forest on soil fertility. Rev. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Huhta, V. Effects of liming and deciduous litter on earthworm (Lumbricidae) populations of a spruce forest, with an inoculation experiment on Allobophora caliginosa. Pedobiologia 1979, 19, 340–345. [Google Scholar]
- Bredemeier, M.; Dohrenbusch, A.; Murach, D. Response of soil water chemistry and fine-roots to clean rain in a spruce forest ecosystem at Solling, FRG. Water Air Soil Pollut. 1995, 85, 1605–1611. [Google Scholar] [CrossRef]
- Quian, X.M.; Kottke, I.; Oberwinkler, F.; Kreutzer, K.; Weiss, T. Influence of liming and acidification on the activity of the mycorrhizal communities in a Picea abies (L.) Karst. stand. Plant Soil 1998, 199, 99–109. [Google Scholar] [CrossRef]
- Puhe, J. Die Wurzelentwicklung der Fichte (Picea abies) bei unterschiedlichen chemischen Bodenbedingungen. Ph.D. Thesis, Universität Göttingen, Germany, 1994; p. 128. [Google Scholar]
- Sandnes, A.; Eldhuset, T.D.; Wollebæk, G. Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol. Biochem. 2005, 37, 259–269. [Google Scholar] [CrossRef]
- Ammer, C.; Wagner, S. Problems and options in modelling fine-root biomass of single mature Norway spruce trees at given points from stand data. Can. J. For. Res. 2002, 32, 581–590. [Google Scholar] [CrossRef]
- Ammer, C.; Wagner, S. An approach for modelling the mean fine-root biomass of Norway spruce stands. Trees 2005, 19, 145–153. [Google Scholar] [CrossRef]
- Kalliokoski, T. Root System Traits of Norway Spruce, Scots Pine, and Silver Birch in Mixed Boreal Forests: An Analysis of Root Architecture, Morphology, and Anatomy. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, June 2011; p. 67. [Google Scholar]
- Menyailo, O.V.; Hungate, B.A.; Zech, W. Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 2002, 242, 171–182. [Google Scholar] [CrossRef]
- Merilä, P.; Smolander, A.; Strömmer, R. Soil nitrogen transformations along a primary succession transect on the land uplift coast in western Finland. Soil Biol. Biochem. 2002, 34, 373–385. [Google Scholar] [CrossRef]
- Merilä, P.; Strömmer, R.; Fritze, H. Soil microbial activity and community structure along a primary succession transect on the land-uplift coast in western Finland. Soil Biol. Biochem. 2002, 34, 1647–1654. [Google Scholar] [CrossRef]
- Priha, O.; Smolander, A. Nitrogen transformations in soil under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Soil Biol. Biochem. 1999, 31, 965–977. [Google Scholar] [CrossRef]
- Frankland, J.C. Fungal succession—Unravelling the unpredictable. Mycol. Res. 1998, 102, 1–15. [Google Scholar] [CrossRef]
- Böttcher, J.; Springob, G. A carbon balance model for organic layers of acid forest soils. J. Plant Nutr. Soil Sci. 2001, 164, 399–405. [Google Scholar] [CrossRef]
- Brandtberg, P.O.; Bengtsson, J.; Lundkvist, H. Distributions of the capacity to take up nutrients by Betula spp. and Picea abies in mixed stands. For. Ecol. Manag. 2004, 198, 193–208. [Google Scholar]
- Fischer, H.; Bens, O.; Hüttl, R.F. Veränderung von Humusform, -vorrat und -verteilung im Zuge von Waldumbau-Maßnahmen im Nordostdeutschen Tiefland. Forstwiss. Cent. bl. 2002, 121, 322–334. [Google Scholar] [CrossRef]
- Schua, K.; Fischer, H.; Lehmann, B.; Wagner, S. Wirkungen einzelbaumweise eingemischter Trauben-Eichen (Quercus petraea (Matt.) Liebl.) auf den Oberbodenzustand in Kiefernbeständen (Pinus sylvestris L.). Allg. Forst- und Jagd.-Ztg. 2007, 178, 172–179. [Google Scholar]
- Smolander, A.; Loponen, J.; Suominen, K.; Kitunen, V. Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol. Biochem. 2005, 37, 1309–1318. [Google Scholar] [CrossRef]
- Saetre, P. Spatial patterns of ground vegetation, soil microbial biomass and activity in a mixed spruce-birch stand. Ecography 1999, 22, 183–192. [Google Scholar] [CrossRef]
- Priha, O.; Smolander, A. Microbial biomass and activity in soil and litter under Pinus sylvestris, Picea abies and Betula pendula at originally similar field afforestation sites. Biol. Fertil. Soils 1997, 24, 45–51. [Google Scholar] [CrossRef]
- Kiikkilä, O.; Kitunen, V.; Smolander, A. Dissolved soil organic matter from surface organic horizons under birch and conifers: Degradation in relation to chemical characteristics. Soil Biol. Biochem. 2006, 38, 737–746. [Google Scholar] [CrossRef]
- Johansson, M.B. The chemical composition of needle and leaf litter from Scots pine, Norway spruce and white birch in Scandinavian forests. Forestry 1995, 68, 49–62. [Google Scholar] [CrossRef]
- Chauvat, M.; Titsch, D.; Zaytsev, A.S.; Wolters, V. Changes in soil faunal assemblages during conservation from pure to mixed forest stands. For. Ecol. Manag. 2011, 262, 317–324. [Google Scholar] [CrossRef]
- Frischbier, N; Wagner, S. Detection, quantification and modelling of small-scale lateral translocation of throughfall in tree crowns of European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). J. Hydrol. 2015, 522, 228–238. [Google Scholar]
- Müller, K.H.; Wagner, S. Fine root dynamics in gaps of Norway spruce stands in the German Ore Mountains. Forestry 2003, 76, 149–158. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schua, K.; Wende, S.; Wagner, S.; Feger, K.-H. Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study. Forests 2015, 6, 1949-1965. https://doi.org/10.3390/f6061949
Schua K, Wende S, Wagner S, Feger K-H. Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study. Forests. 2015; 6(6):1949-1965. https://doi.org/10.3390/f6061949
Chicago/Turabian StyleSchua, Karoline, Stefan Wende, Sven Wagner, and Karl-Heinz Feger. 2015. "Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study" Forests 6, no. 6: 1949-1965. https://doi.org/10.3390/f6061949
APA StyleSchua, K., Wende, S., Wagner, S., & Feger, K.-H. (2015). Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study. Forests, 6(6), 1949-1965. https://doi.org/10.3390/f6061949






