Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
| Stand Age (Years) | Stand Density (Trees ha−1) | Mean DBH (cm) | Mean Height (m) | Altitude (m) | Slope Degree (°) | Soil Types |
|---|---|---|---|---|---|---|
| 1 | 1116 | 4.3 | 4.4 | 140–150 | 0–10 | Lateritic red soil |
| 2 | 1348 | 9.6 | 11.8 | 85–170 | 27–34 | Lateritic red soil |
| 3 | 1355 | 10.6 | 12.4 | 37–171 | 12–36 | Lateritic red soil |
| 4–5 | 1197 | 11.8 | 14.2 | 24–161 | 0–36 | Lateritic red soil |
| 6–8 | 1100 | 14.8 | 18.6 | 33–133 | 0–27 | Lateritic red soil |
2.2. Overstory and Understory Biomass
| Component | Allometric Equation | R2 | P | RSS |
|---|---|---|---|---|
| leaf | W = 1.182 | 0.762 | <0.01 | 0.690 |
| branch | W = 0.042D1.835 | 0.894 | <0.01 | 1.530 |
| stem | W = 0.028D2.996 | 0.978 | <0.01 | 0.811 |
| root | W = 0.06D1.771 | 0.851 | <0.01 | 1.831 |
| total | W = 0.138D2.436 | 0.977 | <0.01 | 0.556 |
2.3. Litter Fall Biomass
2.4. Fine-Root Biomass
2.5. Mineral Soil Sampling
2.6. Chemical Analysis
2.7. Statistical Methods
3. Results
3.1. Biomass Carbon Pool
| Components | P1 | pct. | P2 | pct. | P3 | pct. | P4–5 | pct. | P6–8 | pct. |
|---|---|---|---|---|---|---|---|---|---|---|
| Tree Layer | ||||||||||
| leaf | 0.7±0.1 | 22.3 | 1.2 ± 0.2 | 5.0 | 1.2 ± 0.1 | 3.8 | 1.1 ± 0.2 | 2.7 | 1.5 ± 0.2 | 2.1 |
| branch | 0.4 ± 0.0 | 12.0 | 1.9 ± 0.3 | 8.0 | 2.3 ± 0.2 | 7.2 | 2.6 ± 0.3 | 6.2 | 3.5 ± 0.5 | 5.0 |
| stem | 1.6 ± 0.2 | 51.1 | 18.6 ± 2.1 | 77.4 | 25.3 ± 3.8 | 80.1 | 35.6 ± 4.1 | 83.7 | 60.8 ± 8.5 | 86.7 |
| root | 0.5 ± 0.0 | 14.6 | 2.3 ± 0.2 | 9.6 | 2.8 ± 0.2 | 8.9 | 3.2 ± 0.3 | 7.5 | 4.3 ± 0.7 | 6.1 |
| subtotal | 3.1 ± 0.3 | 45.4 | 24.0 ± 2.7 | 77.3 | 31.6 ± 4.2 | 81.3 | 42.6 ± 4.7 | 85.0 | 70.1 ± 9.8 | 92.9 |
| Shrub Layer | ||||||||||
| leaf | 0.1 ± 0.0 | 25.0 | 0.1 ± 0.0 | 28.1 | 0.2 ± 0.0 | 17.5 | 0.2 ± 0.0 | 20.4 | 0.1 ± 0.0 | 19.6 |
| branch | 0.2 ± 0.0 | 40.0 | 0.2 ± 0.0 | 46.9 | 0.5 ± 0.0 | 52.4 | 0.5 ± 0.0 | 52.7 | 0.3 ± 0.0 | 51.0 |
| root | 0.1 ± 0.0 | 35.0 | 0.1 ± 0.0 | 25.0 | 0.3 ± 0.0 | 30.1 | 0.3 ± 0.0 | 26.9 | 0.2 ± 0.0 | 29.4 |
| subtotal | 0.4 ± 0.1 | 5.9 | 0.3 ± 0.0 | 1.0 | 1.0 ± 0.1 | 2.7 | 0.9 ± 0.1 | 1.9 | 0.5 ± 0.1 | 0.7 |
| Herb Layer | ||||||||||
| aboveground | 0.8 ± 0.1 | 57.4 | 0.5 ± 0.0 | 63.9 | 1.1 ± 0.1 | 67.5 | 1.2 ± 0.1 | 59.4 | 1.6 ± 0.2 | 64.6 |
| belowground | 0.6 ± 0.1 | 42.6 | 0.3 ± 0.0 | 36.1 | 0.5 ± 0.0 | 32.5 | 0.8 ± 0.0 | 40.6 | 0.9 ± 0.1 | 35.4 |
| subtotal | 1.4 ± 0.2 | 20.7 | 0.7 ± 0.1 | 2.3 | 1.6 ± 0.1 | 4.0 | 2.1 ± 0.1 | 4.1 | 2.4 ± 0.2 | 3.2 |
| Littefall | 0.9 ± 0.0 | 13.2 | 2.4 ± 0.3 | 7.8 | 2.9 ± 0.3 | 7.5 | 2.01 ± 0.3 | 4.1 | 2.3 ± 0.2 | 3.1 |
| Fine Root | ||||||||||
| 0–20 | 0.8 ± 0.1 | 78.0 | 1.9 ± 0.2 | 52.8 | 1.4 ± 0.1 | 78.5 | 2.1 ± 0.2 | 84.4 | 0.1 ± 0.0 | 54.5 |
| 20–40 | 0.2 ± 0.0 | 22.0 | 1.7 ± 0.2 | 47.2 | 0.34 ± 0.0 | 21.5 | 0.4 ± 0.0 | 15.6 | 0.1 ± 0.0 | 45.5 |
| subtotal | 1.0 ± 0.1 | 14.7 | 3.6 ± 0.3 | 11.5 | 1.7 ± 0.2 | 4.4 | 2.4 ± 0.2 | 4.9 | 0.1 ± 0.0 | 0.1 |
| Total | 6.8 ± 0.6 | 31.0 ± 3.3 | 38.8 ± 4.7 | 50.1 ± 5.4 | 75.5 ± 9.9 |
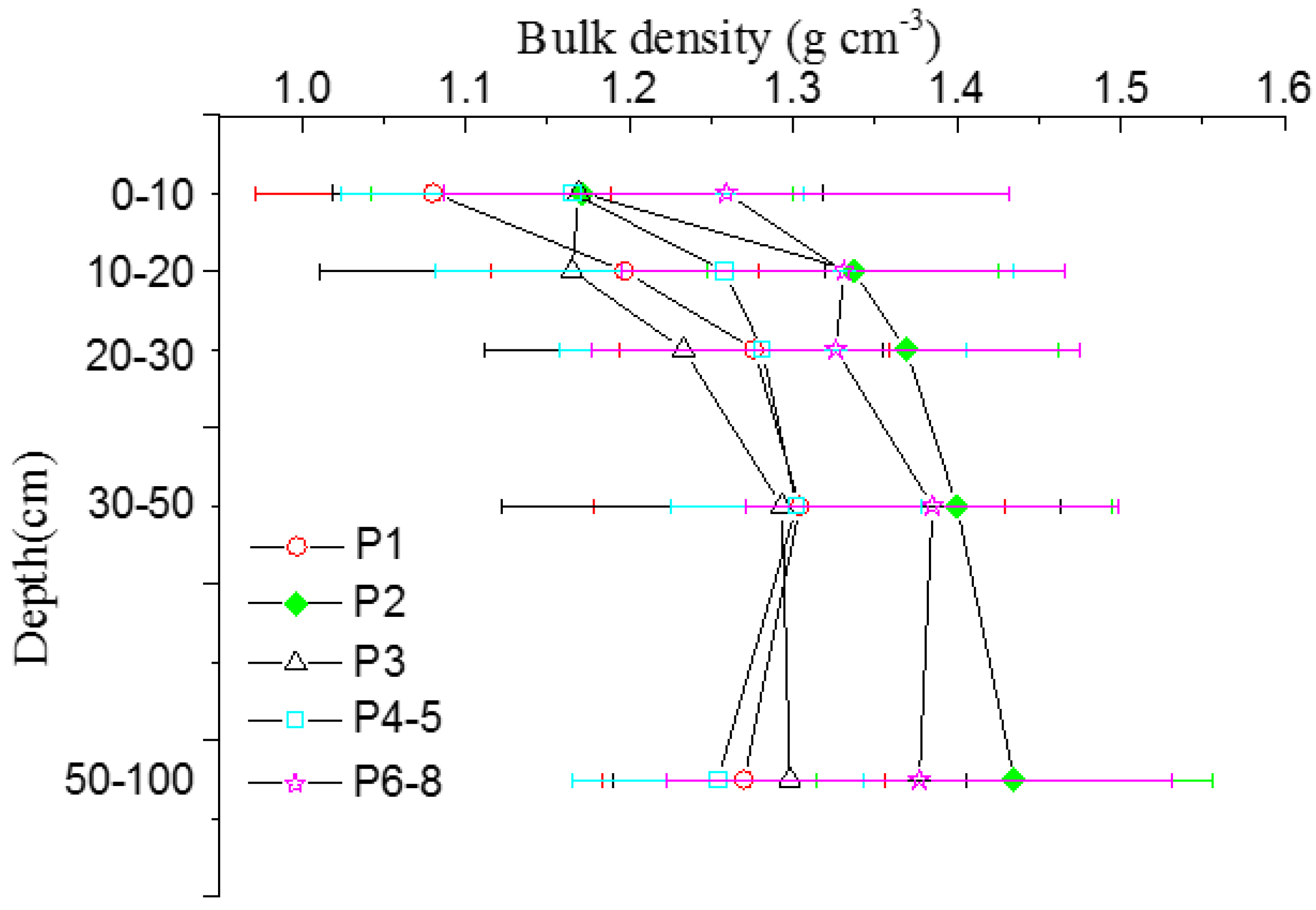
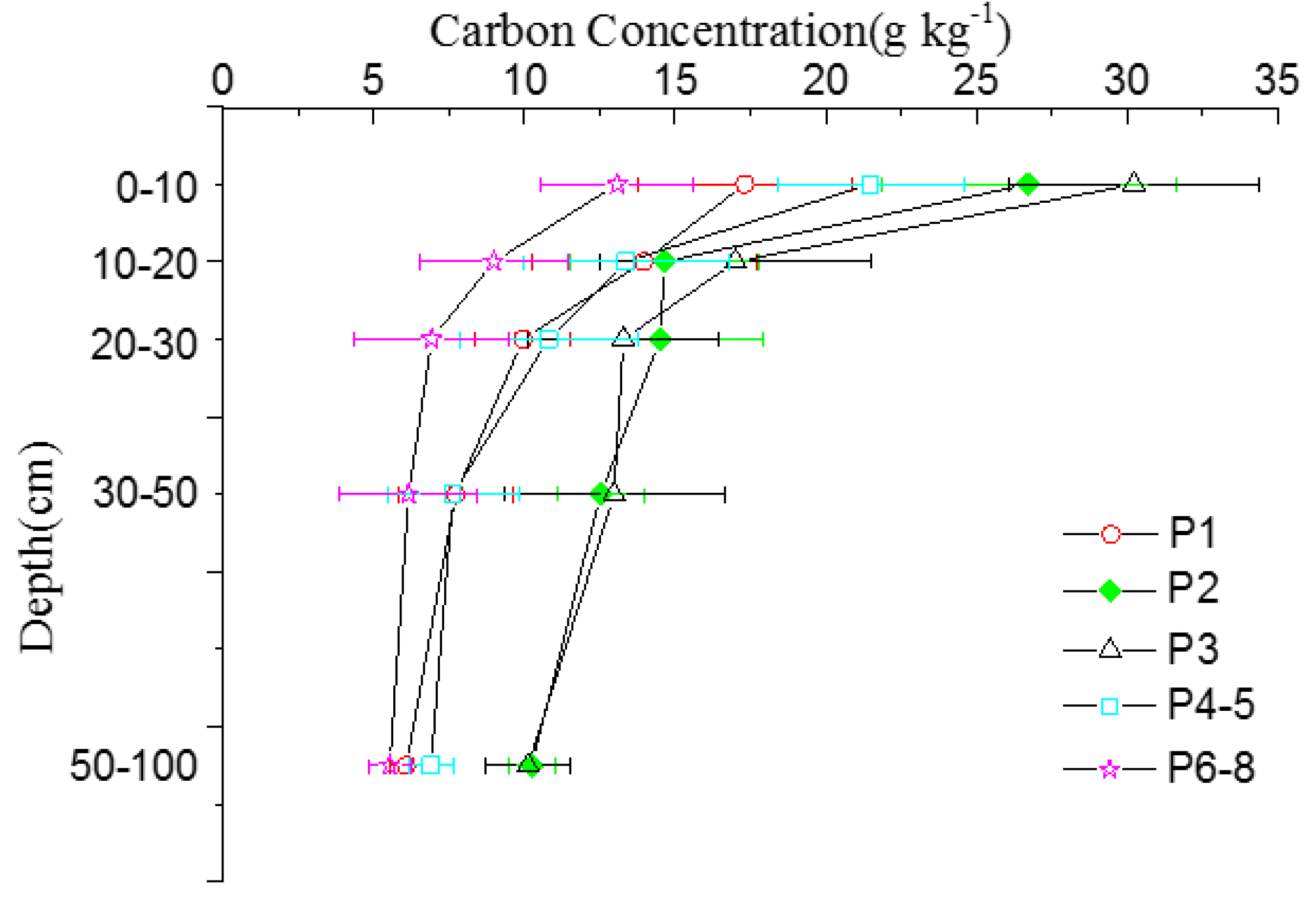
3.2. Mineral Soil Carbon Pool
| Stages | 0–10 | 10–20 | 20–30 | 30–50 | 50–100 | 0–100 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | pct. | C | pct. | C | pct. | C | pct. | C | pct. | C | |
| P1 | 18.3b ± 3.8 | 17.2 | 16.5a ± 3.2 | 15.6 | 12.7ab ± 2.3 | 12.0 | 19.9b ± 3.7 | 18.8 | 38.7b ± 5.6 | 36.5 | 106.1bc ± 10.8 |
| P2 | 29.9a ± 3.6 | 21.1 | 20.8a ± 3.7 | 14.7 | 17.3a ± 2.6 | 12.2 | 27.1ab ± 2.9 | 19.1 | 46.5ab ± 3.6 | 32.8 | 141.6ab ± 13.0 |
| P3 | 32.9a ± 4.5 | 20.0 | 20.0a ± 3.7 | 12.1 | 15.9ab ± 2.6 | 9.6 | 32.9a ± 3.5 | 20.0 | 63.2a ± 4.6 | 38.3 | 164.9a ± 12.4 |
| P4–5 | 24.4ab ± 2.7 | 22.0 | 17.3a ± 2.0 | 15.6 | 13.9ab ± 2.4 | 12.5 | 20.1b ± 2.4 | 18.1 | 35.4b ± 3.6 | 31.9 | 111.1bc ± 10.8 |
| P6–8 | 15.0b ± 2.5 | 17.2 | 11.0a ± 1.34 | 12.6 | 8.5b ± 1.4 | 9.7 | 16.0b ± 2.4 | 18.3 | 36.7b ± 3.9 | 42.1 | 87.2c ± 14.2 |
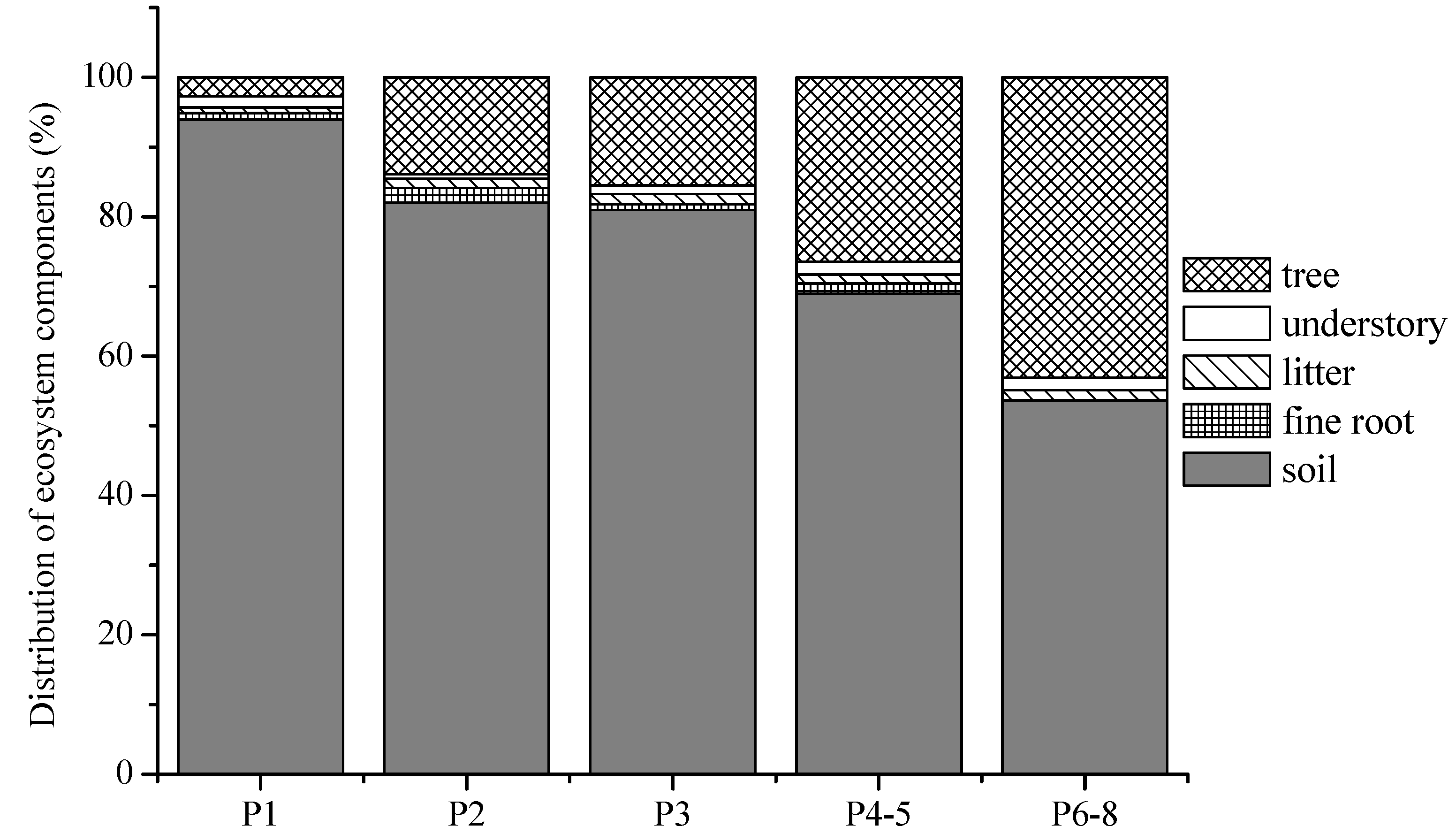
3.3. Carbon Allocation
| Stages | Aboveground | Belowground | Ecosystem | ||
|---|---|---|---|---|---|
| C | pct. | C | pct. | C | |
| P1 | 4.6 | 4.1 | 108.3 | 95.9 | 112.9 |
| P2 | 24.8 | 14.4 | 147.7 | 85.6 | 172.5 |
| P3 | 33.5 | 16.4 | 170.3 | 83.6 | 203.8 |
| P4–5 | 43.3 | 26.9 | 117.8 | 73.1 | 161.1 |
| P6–8 | 70.1 | 43.1 | 92.6 | 56.9 | 162.7 |
4. Discussion
4.1. Biomass Carbon Storage
4.2. Soil C Storage
4.3. Ecosystem Total C Storage
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canadell, J.G.; le Quéré, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, R.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Forest Resource Assessment; Food and Agricultural Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Zhang, J.B.; Shangguan, T.; Meng, Z.Q. Changes in soil carbon flux and carbon stock over a rotation of poplar plantations in northwest China. Ecol. Res. 2011, 26, 153–161. [Google Scholar] [CrossRef]
- Chen, G.S.; Yang, Z.J.; Gao, R.; Xie, J.S.; Guo, J.F.; Huang, Z.Q.; Yang, Y.S. Carbon storage in a chronosequence of Chinese fir plantations in southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Fahey, T.J.; Woodbury, P.B.; Battles, J.J.; Goodale, C.L.; Hamburg, S.P.; Ollinger, S.V.; Woodall, C.W. Forest carbon storage: Ecology, management, and policy. Front. Ecol. Environ. 2010, 8, 245–252. [Google Scholar] [CrossRef]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 2010, 260, 2170–2179. [Google Scholar] [CrossRef]
- Aholoukpè, H.; Dubos, B.; Flori, A.; Deleporte, P.; Amadji, G.; Chotte, J.L.; Blavet, D. Estimating aboveground biomass of oil palm: Allometric equations for estimating frond biomass. For. Ecol. Manag. 2013, 292, 122–129. [Google Scholar] [CrossRef]
- Wang, F.M.; Xu, X.; Zou, B.; Guo, Z.H.; Li, Z.A.; Zhu, W.X. Biomass accumulation and carbon sequestration in four different aged Casuarina equisetifolia coastal shelterbelt plantations in south China. PLoS ONE 2013, 8, e77449. [Google Scholar] [CrossRef]
- Cao, J.X.; Wang, X.P.; Tian, Y.; Wen, Z.Y.; Zha, T.S. Pattern of carbon allocation across three different stages of stand development of a Chinese pine (Pinus tabulaeformis) forest. Ecol. Res. 2012, 27, 883–892. [Google Scholar] [CrossRef]
- Kaul, M.; Mohren, G.M.J.; Dadhwal, V.K. Carbon storage and sequestration potential of selected tree species in India. Mitig. Adapt. Strateg. Glob. Change 2010, 15, 489–510. [Google Scholar] [CrossRef]
- Gonzalez, M.; Augusto, L.; Gallet-Budynek, A.; Xue, J.; Yauschew-Raguenes, N.; Guyon, D.; Trichet, P.; Delerue, F.; Niollet, S.; Andreasson, F.; et al. Contribution of understory species to total ecosystem aboveground and belowground biomass in temperate Pinus pinaster Ait. forests. For. Ecol. Manag. 2013, 289, 38–47. [Google Scholar] [CrossRef]
- Powers, R.F.; Busse, M.D.; McFarlane, K.J.; Zhang, J.W.; Young, D.H. Long-term effects of silviculture on soil carbon storage: Does vegetation control make a difference? Forestry 2013, 86, 47–58. [Google Scholar] [CrossRef]
- Kumada, S.; Kawanishi, T.; Hayashi, Y.; Ogomori, K.; Kobayashi, Y.; Takahashi, N.; Saito, M.; Hamano, H.; Kojima, T.; Yamada, K. Litter carbon dynamics analysis in forests in an arid ecosystem with a model incorporating the physical removal of litter. Ecol. Model. 2008, 215, 190–199. [Google Scholar] [CrossRef]
- Kumada, S.; Kawanishi, T.; Hayashi, Y.; Hamano, H.; Kawarasaki, S.; Aikawa, S.-i.; Takahashi, N.; Egashira, Y.; Tanouchi, H.; Kojima, T.; et al. Effects of different mobilities of leaf and woody litters on litter carbon dynamics in arid ecosystems in Western Australia. Ecol. Model. 2009, 220, 2792–2801. [Google Scholar] [CrossRef]
- Montané, F.; Romanyà, J.; Rovira, P.; Casals, P. Aboveground litter quality changes may drive soil organic carbon increase after shrub encroachment into mountain grasslands. Plant Soil 2010, 337, 151–165. [Google Scholar] [CrossRef]
- Rieger, I.; Lang, F.; Kleinschmit, B.; Kowarik, I.; Cierjacks, A. Fine root and aboveground carbon stocks in riparian forests: The roles of diking and environmental gradients. Plant Soil 2013, 370, 497–509. [Google Scholar] [CrossRef]
- Varik, M.; Aosaar, J.; Ostonen, I.; Lõhmus, K.; Uri, V. Carbon and nitrogen accumulation in belowground tree biomass in a chronosequence of silver birch stands. For. Ecol. Manag. 2013, 302, 62–70. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Claus, A.; George, E. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can. J. For. Res.-Rev. Can. Rech. For. 2005, 35, 1617–1625. [Google Scholar] [CrossRef]
- Borja, I.; de Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Zhong, M.C. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Polglase, P.; Nyakuengama, J.; Khanna, P. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Deluca, T.H.; Boisvenue, C. Boreal forest soil carbon: Distribution, function and modelling. Forestry 2012, 85, 161–184. [Google Scholar] [CrossRef]
- Booth, T.H. Eucalypt plantations and climate change. For. Ecol. Manag. 2013, 301, 28–34. [Google Scholar] [CrossRef]
- State Forestry Administration. Report of Forest Resources in China: The Seventh Check; Chinese Forestry Publishing House: Beijing, China, 2009. (In Chinese)
- Nouvellon, Y.; Laclau, J.P.; Epron, D.; le Maire, G.; Bonnefond, J.M.; Goncalves, J.L.M.; Bouillet, J.P. Production and carbon allocation in monocultures and mixed-species plantations of Eucalyptus grandis and Acacia mangium in Brazil. Tree Physiol. 2012, 32, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, D.; Song, M. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. For. Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Drake, P.L.; Mendham, D.S.; Ogden, G.N. Plant carbon pools and fluxes in coppice regrowth of Eucalyptus globulus. For. Ecol. Manag. 2013, 305, 161–170. [Google Scholar] [CrossRef]
- Kuyah, S.; Dietz, J.; Muthuri, C.; van Noordwijk, M.; Neufeldt, H. Allometry and partitioning of above-and below-ground biomass in farmed eucalyptus species dominant in Western Kenyan agricultural landscapes. Biomass Bioenergy 2013, 55, 276–284. [Google Scholar] [CrossRef]
- Volkova, L.; Weston, C. Redistribution and emission of forest carbon by planned burning in Eucalyptus obliqua (L. Hérit.) forest of south-eastern Australia. For. Ecol. Manag. 2013, 304, 383–390. [Google Scholar] [CrossRef]
- Wink, C.; Reinert, D.J.; Muller, I.; Reichert, J.M.; Jacomet, L. The Eucalyptus sp age plantations influencing the carbon stocks. Cienc Florest 2013, 23, 333–343. [Google Scholar]
- State Soil Survey Service of China. China Soil; China Agricultural Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Du, H.; Zeng, F.P.; Wang, K.L.; Song, T.Q.; Wen, Y.G.; Li, C.G.; Peng, W.X.; Liang, H.W.; Zhu, H.G.; Zeng, Z.X. Dynamics of biomass and productivity of three major plantation types in southern China. Acta Ecol. Sin. 2014, 34, 2712–2724. (In Chinese) [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3-Chemical Methods; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Li, X.; Yi, M.J.; Son, Y.; Park, P.S.; Lee, K.H.; Son, Y.M.; Kim, R.H.; Jeong, M.J. Biomass and Carbon Storage in an Age-Sequence of Korean Pine (Pinus koraiensis) Plantation Forests in Central Korea. J. Plant Biol. 2011, 54, 33–42. [Google Scholar] [CrossRef]
- Harper, R.J.; Okom, A.E.A.; Stilwell, A.T.; Tibbett, M.; Dean, C.; George, S.J.; Sochacki, S.J.; Mitchell, C.D.; Mann, S.S.; Dods, K. Reforesting degraded agricultural landscapes with Eucalypts: Effects on carbon storage and soil fertility after 26 years. Agric. Ecosyst. Environ. 2012, 163, 3–13. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Palmiotto, P.A.; Boon, P.; Ohara, J.; Asbjornsen, H. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 1996, 187, 159–219. [Google Scholar] [CrossRef]
- Jagodzinski, A.M.; Kalucka, I. Fine roots biomass and morphology in a chronosequence of young Pinus sylvestris stands growing on a reclaimed lignite mine spoil heap. Dendrobiology 2010, 64, 19–30. [Google Scholar]
- Ostonen, I.; Lohmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comparison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Sainju, U.; Good, R. Vertical root distribution in relation to soil properties in New Jersey Pinelands forests. Plant Soil 1993, 150, 87–97. [Google Scholar] [CrossRef]
- Schmid, I.; Kazda, M. Root distribution of Norway spruce in monospecific and mixed stands on different soils. For. Ecol. Manag. 2002, 159, 37–47. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Wang, S.L.; Zhang, C.M.; Gong, C.; Hu, Q. Carbon storage in evergreen broad-leaf forests in mid-subtropical region of China at four succession stages. J. For. Res. 2013, 24, 677–682. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Yu, Z.L.; Zhao, S.D. Carbon storage and budget of major Chinese forest types. Acta Phytoecol. Sin. 2000, 24, 518–522. (In Chinese) [Google Scholar]
- Seely, B.; Welham, C.; Blanco, J.A. Towards the application of soil organic matter as an indicator of forest ecosystem productivity: Deriving thresholds, developing monitoring systems, and evaluating practices. Ecol. Indic. 2010, 10, 999–1008. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G.M. Soil organic carbon and water retention following conversion of grasslands to pine plantations in the Ecuadoran Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above-and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Hooker, T.D.; Compton, J.E. Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonment. Ecol. Appl. 2003, 13, 299–313. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Change Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Lemma, B.; Kleja, D.B.; Nilsson, I.; Olsson, M. Soil carbon sequestration under different exotic tree species in the southwestern highlands of Ethiopia. Geoderma 2006, 136, 886–898. [Google Scholar] [CrossRef]
- Grunzweig, J.M.; Gelfand, I.; Fried, Y.; Yakir, D. Biogeochemical factors contributing to enhanced carbon storage following afforestation of a semi-arid shrubland. Biogeosciences 2007, 4, 891–904. [Google Scholar] [CrossRef]
- Noh, N.J.; Son, Y.; Lee, S.K.; Seo, K.W.; Heo, S.J.; Yi, M.J.; Park, P.S.; Kim, R.H.; Son, Y.M.; Lee, K.H. Carbon and nitrogen storage in an age-sequence of Pinus densiflora stands in Korea. Sci. China Life Sci. 2010, 53, 822–830. [Google Scholar] [PubMed]
- Chen, D.; Zhang, Y.; Lin, Y.; Zhu, W.; Fu, S. Changes in belowground carbon in Acacia crassicarpa and Eucalyptus urophylla plantations after tree girdling. Plant Soil 2010, 326, 123–135. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Zeng, F.; Peng, W.; Wang, K.; Zhang, H.; Liu, L.; Song, T. Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China. Forests 2015, 6, 1763-1778. https://doi.org/10.3390/f6061763
Du H, Zeng F, Peng W, Wang K, Zhang H, Liu L, Song T. Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China. Forests. 2015; 6(6):1763-1778. https://doi.org/10.3390/f6061763
Chicago/Turabian StyleDu, Hu, Fuping Zeng, Wanxia Peng, Kelin Wang, Hao Zhang, Lu Liu, and Tongqing Song. 2015. "Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China" Forests 6, no. 6: 1763-1778. https://doi.org/10.3390/f6061763







