Carbon Storage and Allocation Pattern in Plant Biomass among Different Forest Plantation Stands in Guangdong, China
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Field Inventory
2.2.1. Biomass Inventory of Trees and Shrubs
| Plant | Species | Aboveground biomass | Root biomass | Number of sampled plants | ||
|---|---|---|---|---|---|---|
| Regression equation | R2 | Regression equation | R2 | |||
| Trees (DBH >1 cm or height >1.5 m) | Eucalyptus urophylla | y = 0.1691x0.7472 | 0.9462 | y = 0.1723x0.563 | 0.8942 | 12 |
| Acacia crassicarpa | y = 0.4171x0.6201 | 0.9750 | y = 0.0562x0.6725 | 0.9141 | 12 | |
| Liquidambar formosana | y = 0.2894x0.7405 | 0.8598 | y = 0.136x0.673 | 0.7850 | 9 | |
| Michelia maudiae | y = 0.1419x0.9694 | 0.9932 | y = 0.064x0.6464 | 0.7139 | 5 | |
| Manglietia glauca | y = 0.6355x0.4751 | 0.8123 | y = 0.0635x0.6628 | 0.8994 | 9 | |
| Tsoongiodendron odorum | y = 0.1466x0.8309 | 0.9367 | y = 0.0442x0.7256 | 0.8846 | 7 | |
| Machilus chinensis | y = 1.1447x0.582 | 0.9500 | y = 0.3663x0.5081 | 0.9693 | 7 | |
| Cinnamomum bodinieri | y = 0.376x0.5171 | 0.8521 | y = 0.1017x0.6883 | 0.8714 | 8 | |
| Michelia macclurei | y = 0.201x0.8013 | 0.8022 | y = 0.038x0.7999 | 0.8593 | 9 | |
| Cinnamomum burmannii | y = 0.9665x0.3015 | 0.7535 | y = 0.3376x0.2402 | 0.8446 | 5 | |
| Castanopsis hystrix | y = 0.2926x0.7162 | 0.9555 | y = 0.1017x0.5941 | 0.8238 | 9 | |
| Litsea glutinosa | y = 0.1518x0.7435 | 0.8130 | y = 0.0337x0.7913 | 0.7500 | 8 | |
| Castanopsis fissa | y = 0.3778x0.614 | 0.8782 | y = 0.0718x0.7044 | 0.9548 | 5 | |
| Elaeocarpus japonicus | y = 0.0938x0.8589 | 0.7841 | y = 0.0289x0.8469 | 0.6712 | 5 | |
| Litsea cubeba | y = 0.0497x1.0529 | 0.8348 | y = 0.0019x1.5024 | 0.7549 | 6 | |
| Pinus massoniana | y = 0.0245x1.0209 | 0.9711 | y = 0.0126x0.9024 | 0.9685 | 5 | |
| Trees (DBH <1 cm and height <1.5 m) | Liquidambar formosana | y = 0.0407x0.9147 | 0.9879 | y = 0.0336x0.77 | 0.9997 | 5 |
| Castanopsis hystrix | y = 0.1388x0.7096 | 0.9457 | y = 0.0013x1.2365 | 0.9871 | 5 | |
| Machilus chinensis | y = 0.0587x0.9082 | 0.9991 | y = 0.0319x0.7631 | 0.9930 | 5 | |
| Michelia macclurei | y = 0.0039x1.4435 | 0.9629 | y = 0.002x1.2323 | 0.9192 | 5 | |
| Litsea glutinosa | y = 0.0227x1.0125 | 0.9319 | y = 0.0022x1.2359 | 0.8381 | 6 | |
| Castanopsis fissa | y = 0.0506x0.899 | 0.9724 | y = 0.0082x0.9953 | 0.9844 | 5 | |
| Elaeocarpus japonicus | y = 0.1316x0.6959 | 0.7827 | y = 0.0423x0.6827 | 0.6945 | 5 | |
| Litsea cubeba | y = 0.0043x1.3607 | 0.9080 | y = 0.0004x1.5183 | 0.7597 | 7 | |
| Shrubs | Mallotus apelta | y = 0.1004x0.4018 | 0.7590 | y = 0.0364x0.3676 | 0.6466 | 8 |
| Rhaphiolepis indica | y = 0.0402x0.9471 | 0.6695 | y = 0.0055x1.1122 | 0.8987 | 8 | |
| Gardenia jasminoides | y = 0.0556x0.9636 | 0.8814 | y = 0.0295x0.5991 | 0.6635 | 7 | |
| Cassia tora | y = 0.0267x1.3075 | 0.9649 | y = 0.0117x1.1652 | 0.8955 | 7 | |
| Rhodomyrtus tomentosa | y = 0.4571x0.7346 | 0.6964 | y = 0.2144x0.8813 | 0.6270 | 7 | |
| Melastoma candidum | y = 0.063x0.830 | 0.8260 | y = 0.042x0.358 | 0.6940 | 7 | |
| Ilex asprella | y = 0.1569x0.5899 | 0.8355 | y = 0.0498x0.6248 | 0.8192 | 11 | |
| Clerodendrum fortunatum | y = 0.0351x0.7675 | 0.9201 | y = 0.0092x0.6673 | 0.8393 | 12 | |
2.2.2. Measurements of Herbaceous Biomass and Forest Floor Mass
2.2.3. Measurement of Soil Carbon Content
2.2.4. Calculation of Carbon Stocks
2.3. Statistical Analysis
3. Results
3.1. Carbon Storage of Trees
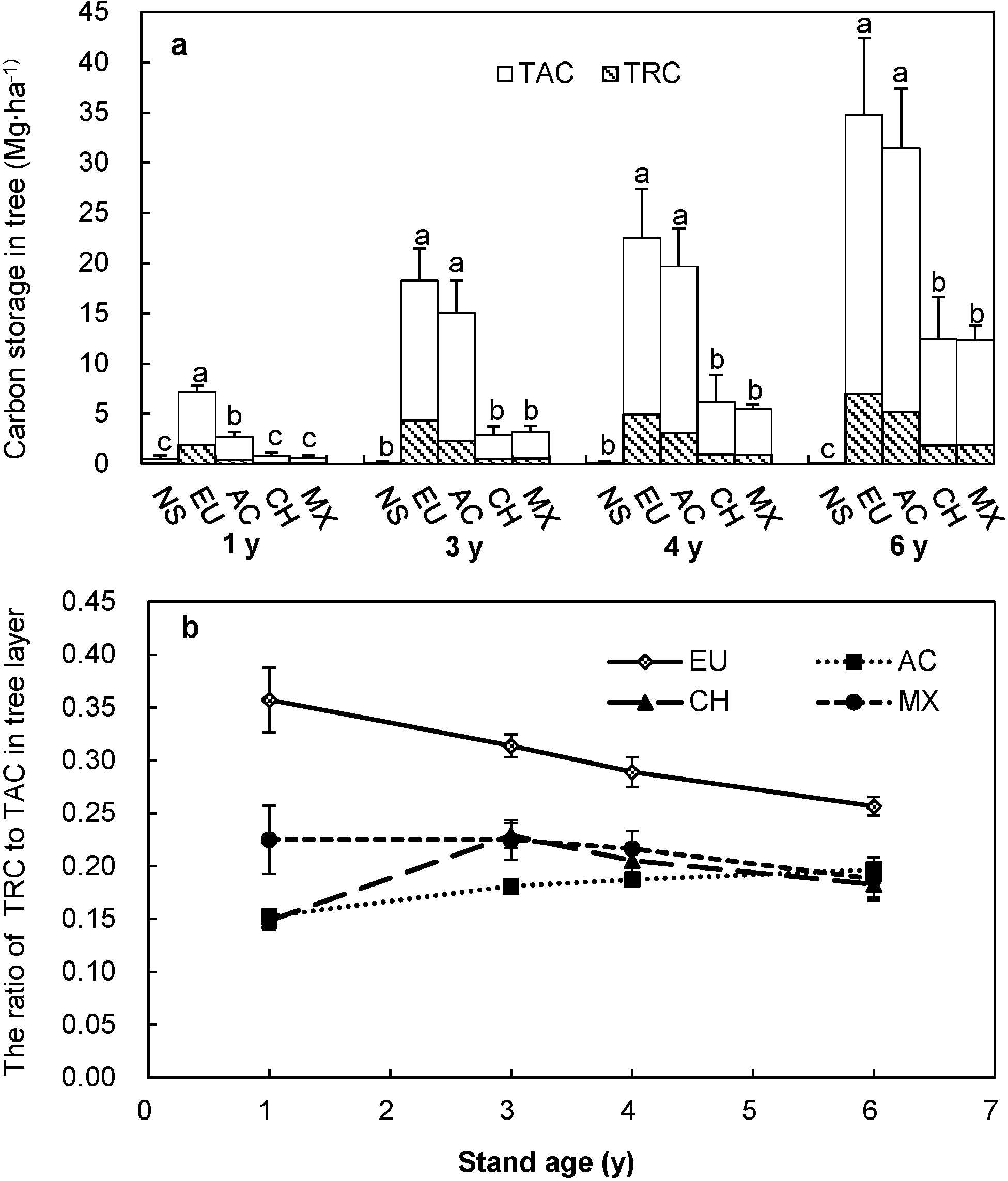
3.2. Carbon Storage of Shrubs
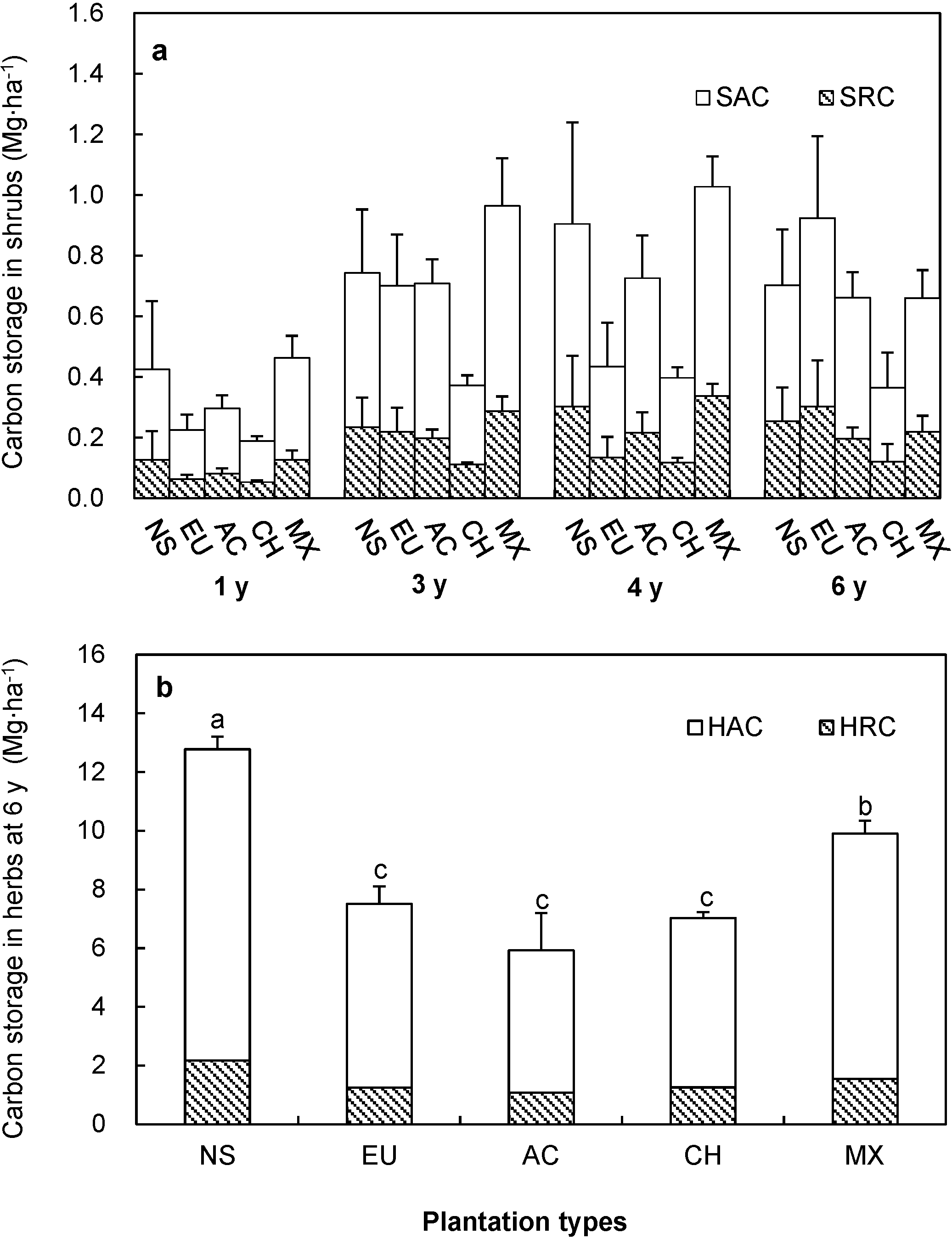
3.3. Carbon Storage of Herbs
3.4. Carbon Storage of Forest Floor Mass
| Plantations | Total Living Plant Carbon (Mg·ha−1) | Forest Floor Carbon (Mg·ha−1) | Total Carbon Storage (Mg·ha−1) | |
|---|---|---|---|---|
| Total Aboveground Carbon | Total Roots Carbon | |||
| NS | 11.05 ± 0.20b | 2.43 ± 0.53b | 1.48 ± 0.36c | 14.96 ± 1.09b |
| EU | 34.64 ± 6.46a | 8.57 ± 1.70a | 3.33 ± 0.95bc | 46.54 ± 8.93a |
| AC | 31.54 ± 4.16a | 6.45 ± 0.64a | 6.41 ± 1.31a | 44.40 ± 5.90a |
| CH | 16.63 ± 3.73b | 3.23 ± 0.51b | 4.80 ± 0.30ab | 24.67 ± 4.04b |
| MX | 19.21 ± 1.41b | 3.66 ± 0.12b | 2.92 ± 0.52bc | 25.78 ± 1.14b |
3.5. Carbon Storage and Allocation in Six-Year-Old Plantations
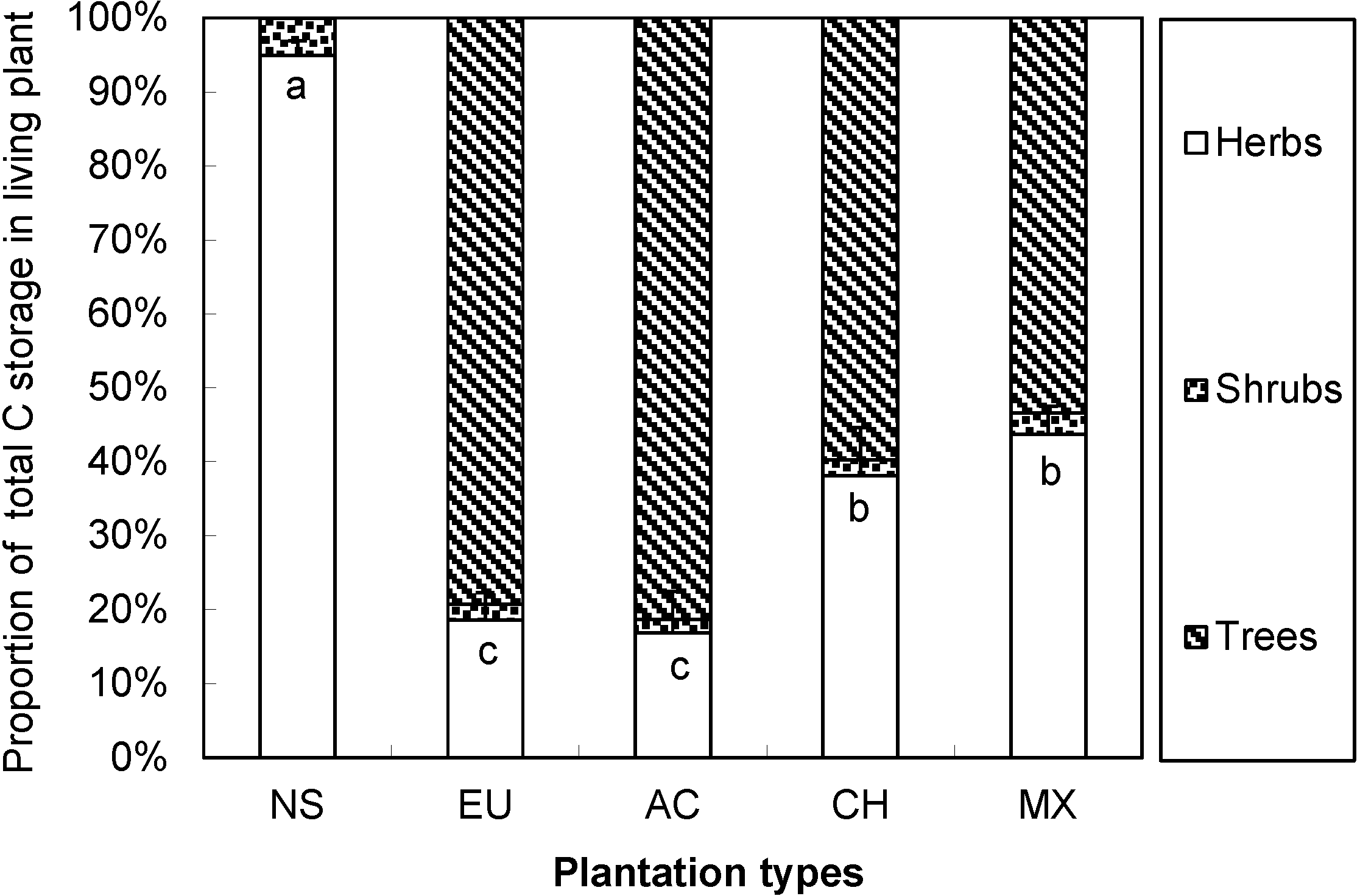
| Plantations | pH | SOC(g/kg) | TN(g/kg) | C:N ratio |
|---|---|---|---|---|
| CK | 4.01 ± 0.04a | 21.70 ± 1.73a | 1.24 ± 0.12a | 18.99 ± 4.44a |
| EU | 3.95 ± 0.02a | 21.16 ± 5.71a | 1.24 ± 0.31a | 17.01 ± 0.28a |
| AC | 3.95 ± 0.10a | 24.20 ± 1.42a | 1.38 ± 0.10a | 16.29 ± 1.75a |
| CH | 3.98 ± 0.03a | 24.86 ± 2.88a | 1.41 ± 0.20a | 17.78 ± 0.75a |
| MX | 3.96 ± 0.04a | 23.00 ± 0.52a | 1.47 ± 0.27a | 16.47 ± 1.93a |
4. Discussion
4.1. Variation of Total Carbon Storage Induced by Tree Traits among Vegetation Types
4.2. Substantial Contribution of Understory Plants to Total Carbon Storage in Plantations
4.3. The Carbon Allocation Pattern Determined by Aboveground Plant Biomass
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. IPCC Four Assessment Report. In Climate Change 2007: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2007; p. 34. [Google Scholar]
- Hunter, I. Above ground biomass and nutrient uptake of three tree species (Eucalyptus camaldulensis, Eucalyptus grandis and Dalbergia sissoo) as affected by irrigation and fertiliser, at 3 years of age, in southern India. For. Ecol. Manag. 2001, 144, 189–199. [Google Scholar] [CrossRef]
- Davis, M.R.; Allen, R.B.; Clinton, P.W. Carbon storage along a stand development sequence in a New Zealand Nothofagus forest. For. Ecol. Manag. 2003, 177, 313–321. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, D.Y. Potential carbon sequestration in China’s forests. Environ. Sci. Policy 2003, 6, 421–432. [Google Scholar] [CrossRef]
- Fang, J.Y.; Guo, Z.D.; Piao, S.L.; Chen, P.A. The estimating of terrestrial vegetation carbon sink in China between 1981 and 2000. Sci. China Earth Sci. 2007, 50, 1341–1350. [Google Scholar] [CrossRef]
- State Forestry Administration. The main results of the 8th national forest resources inventory (from 2009 to 2013). 2014. Available online: http://www.forestry.gov.cn/main/65/content-659670.html (accessed on 3 April 2014). [Google Scholar]
- Huang, L.; Liu, J.Y.; Shao, Q.Q.; Xu, X.L. Carbon sequestration by forestation across China: Past, present, and future. Renew. Sust. Energ. Rev. 2012, 16, 1291–1299. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Piao, S.L.; Fang, J.Y.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.W.; Li, M.H.; Chen, H.; Lewis, B.J.; Yu, D.B.; Zhou, L.; Zhou, W.M.; Fang, X.M.; Zhao, W.; Dai, L.M. Variation in carbon storage and its distribution by stand age and forest type in boreal and temperate forests in northeastern China. PLoS One 2013, 8, e72201. [Google Scholar] [CrossRef] [PubMed]
- Law, B.E.; Thornton, P.E.; Irvine, J.; Anthoni, P.M.; Tuyl, S.V. Carbon storage and fluxes in ponderosa pine forests at different developmental stages. Glob. Chang. Biol. 2001, 7, 755–777. [Google Scholar] [CrossRef]
- Meilby, H.; Helles, F. A general model for assessment of the carbon sequestration potential of afforestation projects. New For. 2011, 42, 383–396. [Google Scholar] [CrossRef]
- Raich, J.W.; Clark, D.A.; Schwendenmann, L.; Wood, T.E. Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS One 2014, 9, e100275. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.P.; Ryan, M.G. Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosystems 2002, 5, 487–499. [Google Scholar] [CrossRef]
- Van der Werf, A.; Nagel, O.W. Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: Opinion. Plant Soil 1996, 185, 21–32. [Google Scholar] [CrossRef]
- Mäkelä, A. On guiding principles for carbon allocation in eco-physiological growth models. Tree Physiol. 2012, 32, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, D.S.; Song, M.W. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. For. Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Hvistendahl, M. Turing over a new leaf in China’s forests. Science 2012, 337, 26–27. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Li, Z.Y.; Chen, J.; Liu, Y.; Liu, D.P.; Wu, S.F.; Qin, Y.S.; Xu, Z.J. Sustainable management of planted forests in China: comprehensive evaluation, development recommendation and action framework. Chin. For. Sci. Technol. 2008, 7, 1–15. [Google Scholar]
- Wang, F.M.; Zhu, W.X.; Xia, H.P.; Fu, S.L.; Li, Z.A. Nitrogen mineralization and leaching in the early stages of a subtropical afforestation in southern China. Restor. Ecol. 2010, 18, 313–322. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, 2nd ed.; World Soil Resources Reports No.103; FAO: Rome, Italy, 2006; p. 67. [Google Scholar]
- Lu, R.K. Method of Analysis in Soil and Agrochemistry; Agricultural Press: Beijing, China, 1999; pp. 31–33. (In Chinese) [Google Scholar]
- Baishya, R.; Barik, S.K.; Upadhaya, K. Distribution pattern of aboveground biomass in natural and plantation forests of humid tropics in northeast India. Trop. Ecol. 2009, 50, 295–304. [Google Scholar]
- Yu, S.Q. Soil respiration of five plantations in south China. Master’s Degree, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 6 July 2014. [Google Scholar]
- Zhang, J.G.; Fu, S.L.; Wen, D.Z.; Zhang, L.L. Relationships of key leaf traits of 16 woody plant species in low subtropical China. J. Trop. Subtrop. Bot. 2009, 17, 395–400. (In Chinese) [Google Scholar]
- Chen, D.M.; Zhang, C.L.; Wu, J.P.; Zhou, L.X.; Lin, Y.B.; Fu, S.L. Subtropical plantations are large carbon sinks, evidence from two monoculture plantations in South China. Agric. For. Meteorol. 2011, 151, 1214–1225. [Google Scholar] [CrossRef]
- Tang, J.X.; Bai, L.H.; Guo, W.F.; Cai, D.X. Preliminary study on growth regularity of Castanopsis hystrix plantation. J. Central South Univ. For. Technol. 2012, 32, 51–60. (In Chinese) [Google Scholar]
- Liu, Z.F.; Wu, J.P.; Zhou, L.X.; Lin, Y.B.; Fu, S.L. Effect of understory fern (Dicranopteris dichotoma) removal on substrate utilization patterns of culturable soil bacterial communities in subtropical Eucalyptus plantations. Pedobiologia 2012, 55, 7–13. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.L.; Shao, Y.H.; Xu, G.L.; Fu, S.L. Effects of vegetation removal on soil properties and decomposer organisms. Soil Biol. Biochem. 2011, 43, 954–960. [Google Scholar] [CrossRef]
- Chastain, R.A., Jr.; Currie, W.S.; Townsend, P.A. Carbon sequestration and nutrient cycling implications of the evergreen understory layer in Appalachian forests. For. Ecol. Manag. 2006, 231, 63–77. [Google Scholar] [CrossRef]
- Wan, S.Z. The Effects of Forest Management on Carbon Allocation in Eucalyptus Plantation. Ph.D. Degree, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China, 6 July 2014. [Google Scholar]
- Yang, K.; Guan, D.S. Biomass distribution and its functioning of forest understory vegetation. Chin. J. Ecol. 2006, 25, 1252–1256. (In Chinese) [Google Scholar]
- Fang, Y.T.; Mo, J.M.; Huang, Z.L.; Ouyang, X.J. Carbon accumulation and distribution in Pinus massoniana and Schima superba mixed forest ecosystem in Dinghushan Biosphere Reserve. J. Trop. Subtrop. Bot. 2003, 11, 47–52. (In Chinese) [Google Scholar]
- Zhou, C.Y.; Wei, X.H.; Zhou, G.Y.; Yan, J.H.; Wang, X.; Wang, C.L.; Liu, H.G.; Tang, X.L.; Zhang, D.Q. Impacts of a large-scale reforestation program on carbon storage dynamics in Guangdong, China. For. Ecol. Manag. 2008, 255, 847–854. [Google Scholar] [CrossRef]
- Wan, S.Z.; Zhang, C.L.; Chen, Y.Q.; Zhao, J.; Wang, X.L.; Wu, J.P.; Zhou, L.X.; Lin, Y.B.; Liu, Z.F.; Fu, S.L. The understory fern Dicranopteris Dichotoma facilitates the overstory Eucalyptus trees in subtropical plantations. Ecosphere 2014, 5, 1–12. [Google Scholar]
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Shibistova, O.; Yohannes, Y.; Boy, J.; Richter, A.; Wild, B.; Watzka, M.; Guggenberger, G. Rate of belowground carbon allocation differs with successional habit of two Afromontane trees. PLoS One 2012, 7, e45540. [Google Scholar] [CrossRef] [PubMed]
- Walcroft, A.S.; Whitehead, D.; Kelliher, F.M.; Arneth, A.; Silvester, W.B. The effects of long-term, partial shading on growth and photosynthesis in Pinus radiate D. Don trees. For. Ecol. Manag. 2002, 163, 51–163. [Google Scholar] [CrossRef]
- Xue, P. Growth and biomass of six-year-old Eucalyptus urophylla plantation in Leizhou forestry bureau. Eucalypt Sci. Technol. 2009, 26, 18–21. (In Chinese) [Google Scholar]
- Genet, H.; Bréda, N.; Dufréne, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 2010, 30, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.N. Investigation on the growth law and biomass of Castanopsis hystrix natural forest. J. Fujian For. Sci. Technol. 1998, 25, 20–23. (In Chinese) [Google Scholar]
- Yan, E.R.; Wang, X.H.; Huang, J.J. Shifts in plant nutrient use strategies under secondary forest succession. Plant Soil. 2006, 289, 187–197. [Google Scholar] [CrossRef]
- Epron, D.; Laclau, J.P.; Almeida, J.C.; Goncalves, J.L.M.; Ponton, S.; Sette, C.R., Jr.; Delgado-Rojas, J.S.; Bouillet, J.P.; Nouvellon, Y. Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations? Tree Physiol. 2011, 32, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Schere-lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [PubMed]
- Peichl, M.; Arain, M.A. Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. For. Ecol. Manag. 2007, 253, 68–80. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Z.; Rao, X.; Wang, X.; Liang, C.; Lin, Y.; Zhou, L.; Cai, X.-a.; Fu, S. Carbon Storage and Allocation Pattern in Plant Biomass among Different Forest Plantation Stands in Guangdong, China. Forests 2015, 6, 794-808. https://doi.org/10.3390/f6030794
Chen Y, Liu Z, Rao X, Wang X, Liang C, Lin Y, Zhou L, Cai X-a, Fu S. Carbon Storage and Allocation Pattern in Plant Biomass among Different Forest Plantation Stands in Guangdong, China. Forests. 2015; 6(3):794-808. https://doi.org/10.3390/f6030794
Chicago/Turabian StyleChen, Yuanqi, Zhanfeng Liu, Xingquan Rao, Xiaoling Wang, Chenfei Liang, Yongbiao Lin, Lixia Zhou, Xi-an Cai, and Shenglei Fu. 2015. "Carbon Storage and Allocation Pattern in Plant Biomass among Different Forest Plantation Stands in Guangdong, China" Forests 6, no. 3: 794-808. https://doi.org/10.3390/f6030794





