Influence of Tree Spacing on Soil Nitrogen Mineralization and Availability in Hybrid Poplar Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plantation Establishment
| Spacing (m) | Soil Layer (cm) | SOC (g·kg−1) | TN (g·kg−1) | SOC:TN | pH | Bulk Density (g·cm−1) |
|---|---|---|---|---|---|---|
| 6 × 6 | 0–5 | 31.4 | 1.1 | 16.4 | 7.1 | 1.10 |
| 5–10 | 21.2 | 0.6 | 22.3 | 7.2 | 1.19 | |
| 10–20 | 13.9 | 0.6 | 14.2 | 7.4 | 1.28 | |
| 4.5 × 8 | 0–5 | 26.1 | 0.9 | 16.1 | 7.0 | 1.13 |
| 5–10 | 16.4 | 0.5 | 19.5 | 7.0 | 1.21 | |
| 10–20 | 12.0 | 0.4 | 16.2 | 7.3 | 1.35 | |
| 5 × 5 | 0–5 | 23.9 | 0.9 | 15.6 | 7.1 | 1.19 |
| 5–10 | 18.1 | 0.5 | 22.8 | 7.3 | 1.24 | |
| 10–20 | 13.5 | 0.5 | 15.9 | 7.4 | 1.34 | |
| 3 × 8 | 0–5 | 22.9 | 1.1 | 12.7 | 7.0 | 1.22 |
| 5–10 | 14.2 | 0.6 | 13.9 | 7.1 | 1.36 | |
| 10–20 | 11.0 | 0.5 | 13.6 | 7.3 | 1.39 |
2.2. Soil N Mineralization and Sample Collection
2.3. Soil Chemical Analysis and Data Calculation
2.4. Statistical Analysis
3. Results
3.1. Nitrogen Mineralization and Nitrification
| Time Frame | Spacing (m) | N Nitrification Rate (mg·kg−1·day−1) | N Mineralization Rate (mg·kg−1·day−1) | ||||
|---|---|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 10–20 cm | 0–5 cm | 5–10 cm | 10–20 cm | ||
| A | 6 × 6 | 0.40 ± 0.11 a | 0.24 ± 0.05 a | 0.18 ± 0.08 a | 0.40 ± 0.11 a | 0.25 ± 0.14 a | 0.18 ± 0.08 a |
| 4.5 × 8 | 0.31 ± 0.15 a,b | 0.14 ± 0.04 b | 0.08 ± 0.03 b,c | 0.32 ± 0.15 a,b | 0.14 ± 0.05 a | 0.08 ± 0.04 b | |
| 5 × 5 | 0.22 ± 0.07 b | 0.14 ± 0.08 b | 0.11 ± 0.03 b | 0.23 ± 0.07 b | 0.14 ± 0.08 a | 0.11 ± 0.03 a,b | |
| 3 × 8 | 0.26 ± 0.05 b | 0.16 ± 0.05 b | 0.05 ± 0.02 c | 0.27 ± 0.05 b | 0.16 ± 0.05 a | 0.04 ± 0.02 b | |
| B | 6 × 6 | 0.14 ± 0.03 a | 0.14 ± 0.02 a | 0.07 ± 0.09 a | 0.16 ± 0.03 a | 0.15 ± 0.01 a | 0.08 ± 0.05 a,b |
| 4.5 × 8 | 0.13 ± 0.02 a | 0.11 ± 0.05 a,b | 0.09 ± 0.01 a | 0.15 ± 0.02 a | 0.13 ± 0.07 a,b | 0.09 ± 0.01 a | |
| 5 × 5 | 0.08 ± 0.06 a | 0.07 ± 0.06 b | 0.02 ± 0.01 b | 0.10 ± 0.10 a | 0.06 ± 0.04 b | 0.03 ± 0.03 b | |
| 3 × 8 | 0.09 ± 0.03 a | 0.08 ± 0.04 a,b | 0.03 ± 0.02 b | 0.11 ± 0.03 a | 0.09 ± 0.05 a,b | 0.04 ± 0.03 a,b | |
| C | 6 × 6 | 0.09 ± 0.04 a | 0.06 ± 0.06 a | 0.04 ± 0.02 a | 0.08 ± 0.04 a | 0.05 ± 0.05 a | 0.02 ± 0.01 a |
| 4.5 × 8 | 0.08 ± 0.04 a | 0.04 ± 0.02 a | 0.02 ± 0.01 a | 0.05 ± 0.02 a | 0.04 ± 0.01 a | 0.003 ± 0.00 a | |
| 5 × 5 | 0.05 ± 0.02 a | 0.04 ± 0.03 a | 0.02 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.02 a | 0.001 ± 0.00 a | |
| 3 × 8 | 0.05 ± 0.01 a | 0.03 ± 0.03 a | 0.01 ± 0.01 a | 0.05 ± 0.01 a | 0.04 ± 0.01 a | 0.01 ± 0.01 a | |
| D | 6 × 6 | 0.10 ± 0.06 a | 0.04 ± 0.02 a,b | 0.02 ± 0.02 a | 0.06 ± 0.04 a | 0.03 ± 0.02 a | 0.01 ± 0.01 a |
| 4.5 × 8 | 0.11 ± 0.03 a | 0.07 ± 0.02 a | 0.02 ± 0.00 a | 0.07 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0.00 a | |
| 5 × 5 | 0.06 ± 0.03 a | 0.05 ± 0.01 a,b | 0.02 ± 0.01 a | 0.04 ± 0.02 a | 0.03 ± 0.02 a | 0.02 ± 0.01 a | |
| 3 × 8 | 0.10 ± 0.03 a | 0.03 ± 0.02 b | 0.02 ± 0.01 a | 0.06 ± 0.04 a | 0.02 ± 0.01 a | 0.005 ± 0.00 b | |
| Soil Layer (cm) | Factor | N Nitrification Rate | N Mineralization Rate | ||
|---|---|---|---|---|---|
| F value | p | F value | p | ||
| 0–5 | Spacing | 39.04 | <0.001 | 34.87 | <0.001 |
| Time frame | 3.87 | 0.018 | 3.11 | 0.040 | |
| Spacing × Time frame | 0.95 | 0.497 | 0.73 | 0.681 | |
| 5–10 | Spacing | 43.70 | <0.001 | 38.18 | <0.001 |
| Time frame | 4.62 | 0.009 | 5.85 | 0.003 | |
| Spacing × Time frame | 2.67 | 0.020 | 2.41 | 0.032 | |
| 10–20 | Spacing | 58.32 | <0.001 | 41.60 | <0.001 |
| Time frame | 13.27 | <0.001 | 10.31 | <0.001 | |
| Spacing × Time frame | 6.17 | <0.001 | 5.65 | <0.001 | |
3.2. Soil Inorganic N Concentration
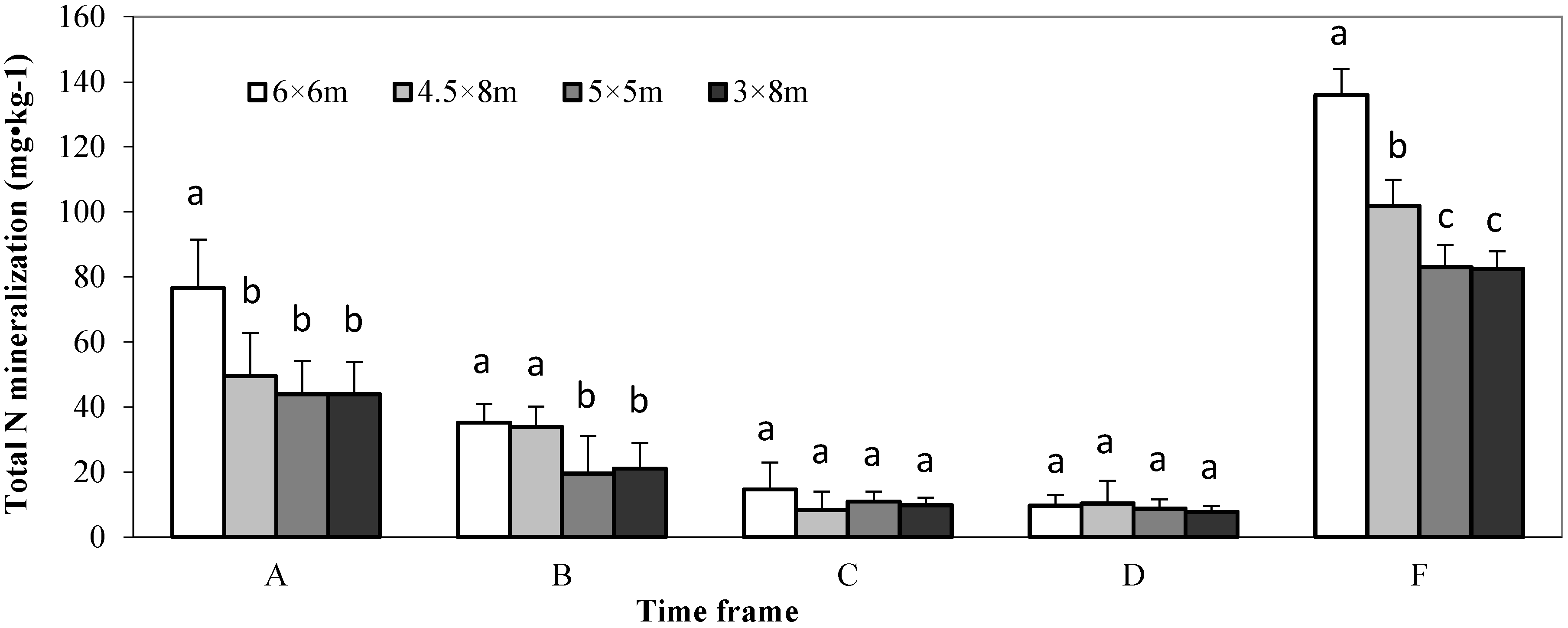
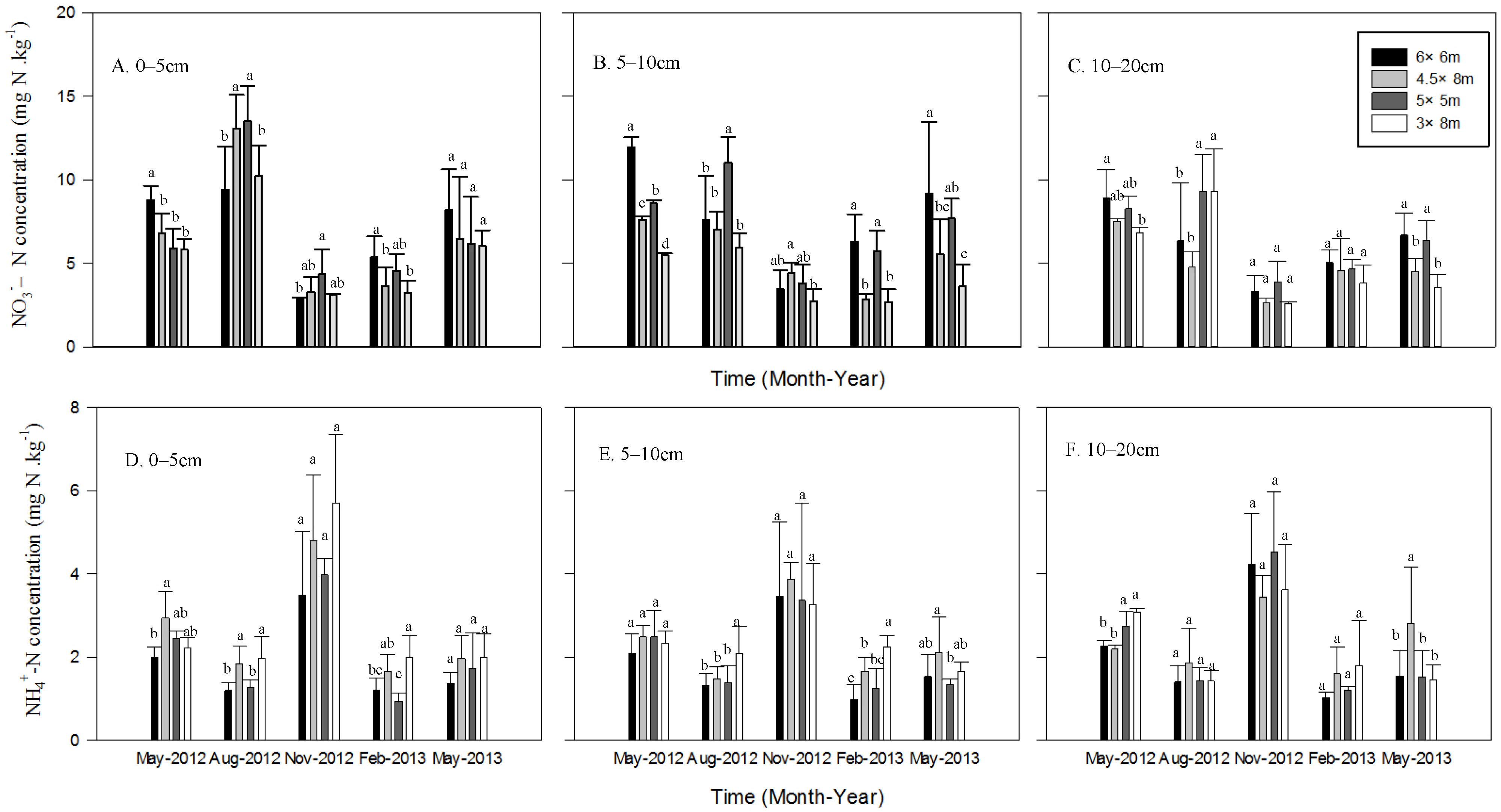
3.3. Soil Inorganic N Content
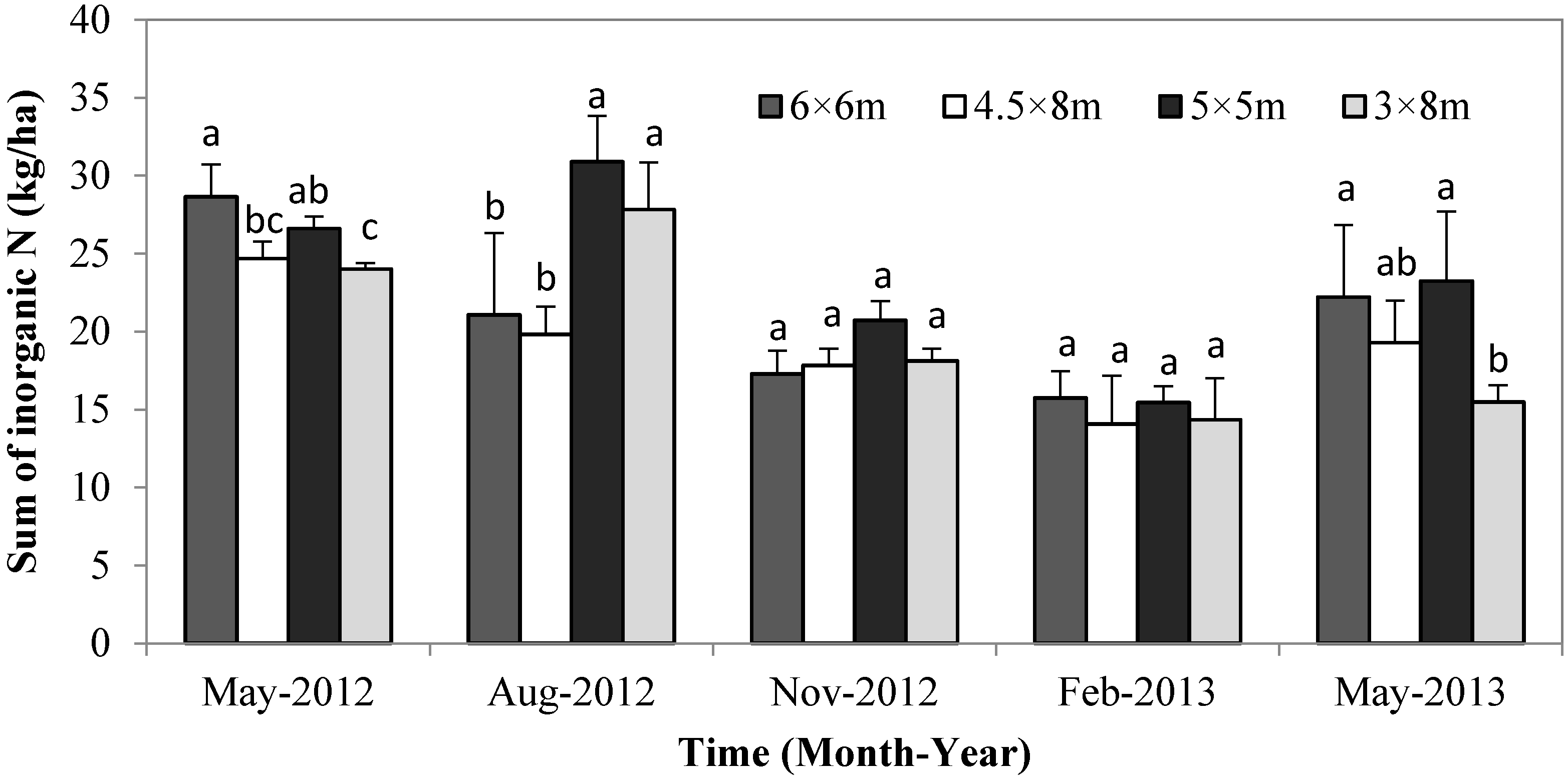
4. Discussion
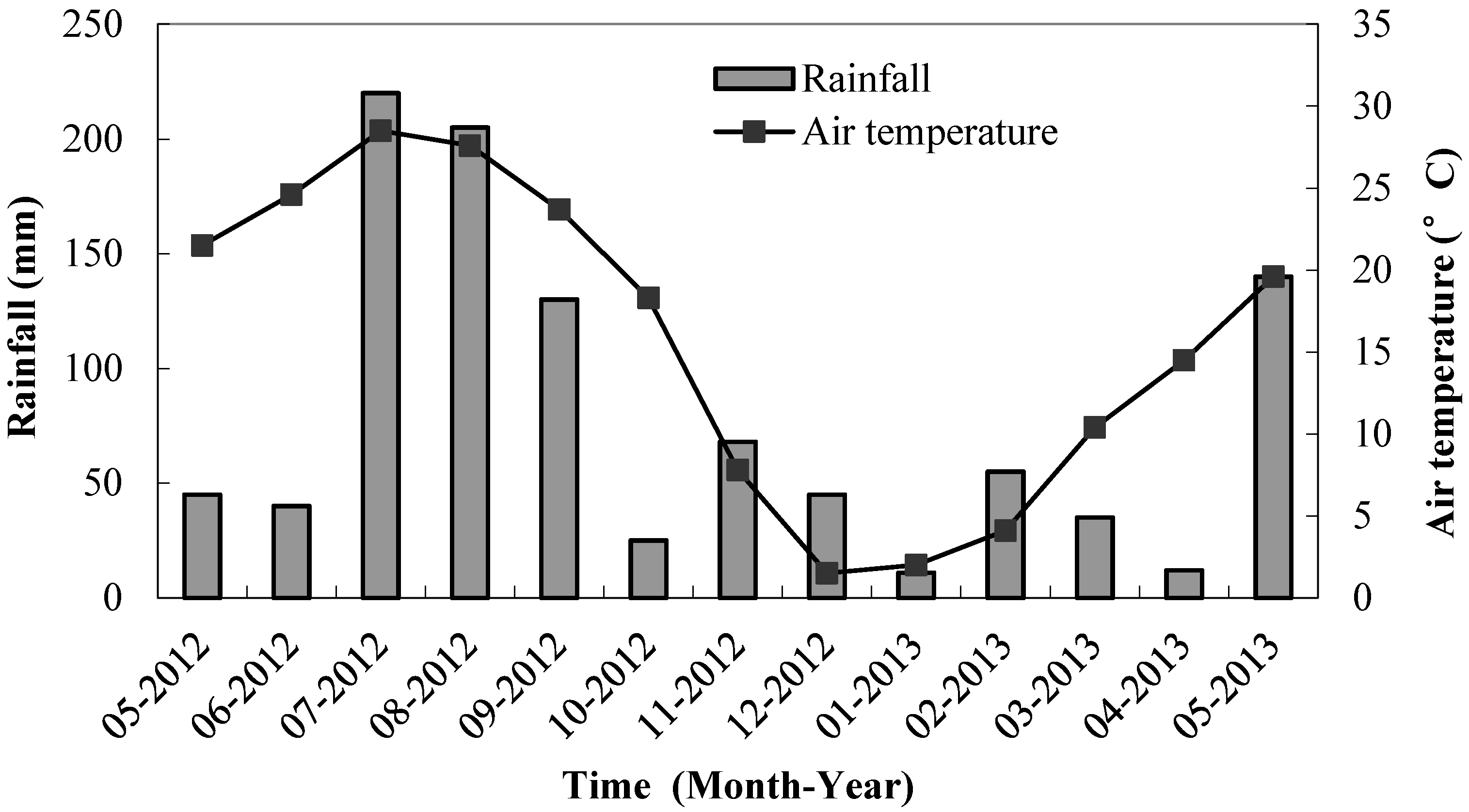
4.1. Dynamics of Inorganic N and N Mineralization
4.2. Effects of Tree Spacing on Soil N Availability
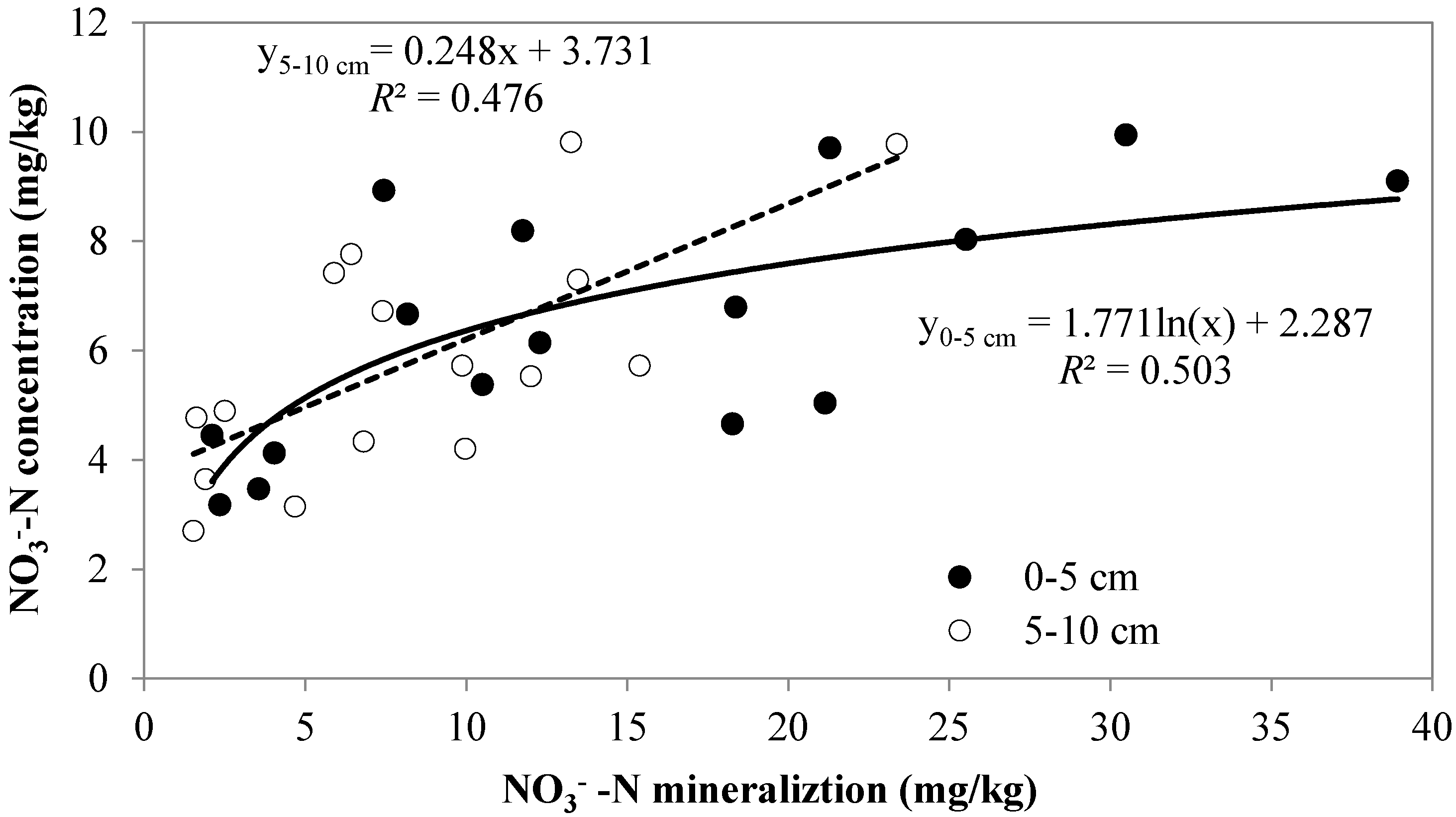
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, G.; Brennan, P. First thinning in sub-tropical eucalypt plantations grown for high-value solidwood products: A review. Aust. For. 2006, 69, 305–312. [Google Scholar] [CrossRef]
- Armstrong, A.; Johns, C.; Tubby, I. Effects of spacing and cutting cycle on the yield of poplar grown as an energy crop. Biomass Bioenergy 1999, 17, 305–314. [Google Scholar] [CrossRef]
- Benomar, L.; DesRochers, A.; Larocque, G.R. Comparing growth and fine root distribution in monocultures and mixed plantations of hybrid poplar and spruce. J. For. Res. 2013, 24, 247–254. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xu, X.Z.; Lu, S.X.; Tang, L.Z. Growth dynamics and biomass production in short-rotation poplar plantations: 6-Year results for three clones at four spacings. Biomass Bioenergy 1999, 17, 415–425. [Google Scholar] [CrossRef]
- Cassidy, M.; Palmer, G.; Smith, R.G.B. The effect of wide initial spacing on wood properties in plantation grown Eucalyptus pilularis. New For. 2013, 44, 919–936. [Google Scholar] [CrossRef]
- Benomar, L.; DesRochers, A.; Larocque, G.R. The effects of spacing on growth, morphology and biomass production and allocation in two hybrid poplar clones growing in the boreal region of Canada. Trees 2012, 26, 939–949. [Google Scholar] [CrossRef]
- Debell, D.S.; Harrington, C.A.; Clendenen, G.W.; Zasada, J.C. Tree growth and stand development of four Populus clones in large monoclonal plots. New For. 1997, 14, 1–18. [Google Scholar] [CrossRef]
- Kang, K.Y.; Zhang, S.Y.; Mansfield, S.D. The effects of initial spacing on wood density, fibre and pulp properties in jack pine (Pinus banksiana Lamb.). Holzforschung 2004, 58, 455–463. [Google Scholar] [CrossRef]
- Yan, Y.F.; Fang, S.Z.; Tian, Y.; Song, H.; Dun, X.J. The response of understory plant diversity and nutrient accumulation to stand structure of poplar plantation. Chin. J. Ecol. 2014, 33, 1170–1177. [Google Scholar]
- Oh, T.K.; Cho, M.G.; Chung, J.M.; Jung, H.; Jeon, K.S.; Moon, H.S.; Lee, S.J.; Shinogi, Y. Effect of thinning intensity on soil nitrogen dynamics in Pinus densiflora stand. J. Fac. Agric. Kyushu. Univ. 2012, 57, 473–479. [Google Scholar]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xie, B.D.; Liu, D.; Liu, J.J. Effects of mulching materials on nitrogen mineralization, nitrogen availability and poplar growth on degraded agricultural soil. New For. 2011, 41, 147–162. [Google Scholar] [CrossRef]
- Gelfand, I.; Yakir, D. Influence of nitrite accumulation in association with seasonal patterns and mineralization of soil nitrogen in a semi-arid pine forest. Soil Biol. Biochem. 2008, 40, 415–424. [Google Scholar] [CrossRef]
- Pajuste, K.; Frey, J. Nitrogen mineralization in podzol soils under boreal Scots pine and Norway spruce stands. Plant Soil 2003, 257, 237–247. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Nortcliff, S. Seasonal fluctuations of native available N and soil management implications. Fertil. Res. 1995, 42, 13–26. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Hättenschwiler, S.; Olander, L.; Allison, S. Nitrogen and nature. Ambio 2002, 31, 97–101. [Google Scholar] [PubMed]
- Fang, S.Z.; Zhai, X.C.; Wan, J.; Tang, L.Z. Clonal variation in growth, chemistry and calorific value of new poplar hybrids at nursery stage. Biomass Bioenergy 2013, 54, 303–311. [Google Scholar] [CrossRef]
- Thomas, D.; Henson, M.; Joe, B.; Boyton, S.; Dickson, R. Review of growth and wood quality of plantation grown Eucalyptus dunnii Maiden. Aust. For. 2009, 72, 3–11. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J.M.; Kucuk, M. Effects of tree species and topography on soil chemistry, leaf litter quality, and decomposition in Northeast Turkey. Soil Biol. Biochem. 2005, 37, 1695–1706. [Google Scholar] [CrossRef]
- Binkley, D.; Aber, J.; Pastor, J.; Nadelhoffer, K. Nitrogen availability in some Wisconsin forests: Comparisons of resin bags and on-site incubations. Biol. Fertil. Soils 1986, 2, 77–82. [Google Scholar] [CrossRef]
- Shibata, H.; Urakawa, R.; Toda, H.; Inagaki, Y.; Tateno, R.; Koba, K.; Nakanishi, A.; Fukuzawa, K.; Yamasaki, A. Changes in nitrogen transformation in forest soil representing the climate gradient of the Japanese archipelago. J. For. Res. 2011, 16, 374–385. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis. Part 1—Physical Mineralogical Methods; INC; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Trindade, H.; Coutinho, J.; Jarvis, S.; Moreira, N. Nitrogen mineralization in sandy loam soils under an intensive doublecropping forage system with dairy-cattle slurry applications. Eur. J. Agron. 2001, 15, 281–293. [Google Scholar] [CrossRef]
- Uri, V.; Lohmus, K.; Kund, M.; Tullus, H. The effect of land use type on net nitrogen mineralization on abandoned agricultural land: Silver birch stand versus grassland. For. Ecol. Manag. 2008, 255, 226–233. [Google Scholar] [CrossRef]
- Pandey, C.B.; Rai, R.B.; Singh, L. Seasonal dynamics of mineral N pools and N mineralization in soils under homegarden trees in South Andaman, India. Agrofor. Syst. 2007, 71, 57–66. [Google Scholar] [CrossRef]
- Wei, X.R.; Shao, M.A.; Fu, X.L.; Göran, I.; Yin, X.Q. The effects of land use on soil N mineralization during the growing season on the northern Loess Plateau of China. Geoedroma 2011, 160, 590–598. [Google Scholar] [CrossRef]
- Yan, E.R.; Wan, X.H.; Guo, M.; Zhong, Q.; Zhou, W.; Li, Y.F. Temporal patterns of net soil N mineralization and nitrification through secondary succession in the subtropical forests of eastern China. Plant Soil 2009, 320, 181–194. [Google Scholar] [CrossRef]
- Zhou, L.S.; Huang, J.H.; Lü, F.M.; Han, X. Effects of prescribed burning and seasonal and interannual climate variation on nitrogen mineralization in a typical steppe in Inner Mongolia. Soil Biol. Biochem. 2009, 41, 796–803. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Han, X.G.; Parsons, W.F.J.; Zhu, W.X. On the nature of environmental gradients: Temporal and spatial variability of soils and vegetation in the New Jersey pinelands. J. Ecol. 1997, 85, 785–798. [Google Scholar] [CrossRef]
- Zhu, W.X.; Carreiro, M.M. Temporal and spatial variations in nitrogen transformations in deciduous forest ecosystems along an urban-rural gradient. Soil Biol. Biochem. 2004, 36, 267–278. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, Q.B.; Li, L.H.; Han, X.G. Seasonal variations in nitrogen mineralization under three land use types in a grassland landscape. Acta Oecol. 2008, 3, 322–330. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Comeau, P.G.; Wang, Y.H. Thinning effects on microbial biomass, N mineralization, and tree growth in a mid-rotation fire-origin lodgepole pine stand in the lower foothills of Alberta, Canada. For. Sci. 2008, 54, 465–474. [Google Scholar]
- Merila, P.; Smolander, A.; Strommer, R. Soil nitrogen transformations along a primary succession transect on the land-uplift coast in Western Finland. Soil Biol. Biochem. 2002, 34, 373–385. [Google Scholar] [CrossRef]
- Binkley, D.; Hart, S. The components of nitrogen availability assessments in forest soils. Adv. Soil Sci. 1989, 10, 57–116. [Google Scholar]
- Nadelhoffer, K.J.; Giblin, A.E.; Shaver, G.R.; Laundre, J.A. Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology 1991, 72, 242–253. [Google Scholar] [CrossRef]
- Schmidt, I.K.; Jonasson, S.; Michelsen, A. Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Appl. Soil Ecol. 1999, 11, 147–160. [Google Scholar] [CrossRef]
- McKinley, D.C.; Rice, C.W.; Blair, J.M. Conversion of grassland to coniferous woodland has limited effects on soil nitrogen cycle processes. Soil Biol. Biochem. 2008, 40, 2627–2633. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xie, B.D.; Liu, J.J. Soil nutrient availability, poplar growth and biomass production on degraded agricultural soil under fresh grass mulch. For. Ecol. Manag. 2008, 255, 1802–1809. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Fang, S.; Tian, Y.; Deng, S.; Tang, L.; Chuong, D.N. Influence of Tree Spacing on Soil Nitrogen Mineralization and Availability in Hybrid Poplar Plantations. Forests 2015, 6, 636-649. https://doi.org/10.3390/f6030636
Yan Y, Fang S, Tian Y, Deng S, Tang L, Chuong DN. Influence of Tree Spacing on Soil Nitrogen Mineralization and Availability in Hybrid Poplar Plantations. Forests. 2015; 6(3):636-649. https://doi.org/10.3390/f6030636
Chicago/Turabian StyleYan, Yafei, Shengzuo Fang, Ye Tian, Shiping Deng, Luozhong Tang, and Dao Ngoc Chuong. 2015. "Influence of Tree Spacing on Soil Nitrogen Mineralization and Availability in Hybrid Poplar Plantations" Forests 6, no. 3: 636-649. https://doi.org/10.3390/f6030636
APA StyleYan, Y., Fang, S., Tian, Y., Deng, S., Tang, L., & Chuong, D. N. (2015). Influence of Tree Spacing on Soil Nitrogen Mineralization and Availability in Hybrid Poplar Plantations. Forests, 6(3), 636-649. https://doi.org/10.3390/f6030636








