Effects of Nitrogen Addition on Leaf Decomposition of Single-Species and Litter Mixture in Pinus tabulaeformis Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
| Forest Type | Nitrogen Treatment | Forest Age (year) | Density (Trees per Hectare) | Mean Diameter at Breast Height (cm) | Mean Tree Height (m) | Slope (Degree) | Elevation (m) |
|---|---|---|---|---|---|---|---|
| Plantation | Control | 60 | 858 | 17.8 | 13.1 | 19 | 1589 |
| Low-N | 60 | 725 | 21.2 | 13.8 | 15 | 1589 | |
| Medium-N | 60 | 692 | 20.2 | 11.6 | 19 | 1589 | |
| High-N | 60 | 658 | 20.5 | 12.4 | 18 | 1589 | |
| Natural forest | Control | 75 | 1267 | 23.9 | 17.7 | 24 | 1680 |
| Low-N | 75 | 1567 | 20.6 | 17.8 | 21 | 1680 | |
| Medium-N | 75 | 1208 | 23.5 | 17.4 | 25 | 1680 | |
| High-N | 75 | 1225 | 23.4 | 19.0 | 23 | 1680 |
| Forest Type | Nitrogen Treatment | Soil Bulk Density (g/cm3) | Soil pH | Total Nitrogen(g/kg) | Soil Organic Carbon (g/kg) | C/N Ratio |
|---|---|---|---|---|---|---|
| Plantation | Control | 1.3 ± 0.1 a | 7.4 ± 0.2 a | 1.0 ± 0.1 a | 22.9 ± 3.7 a | 22.5 ± 0.6 a |
| Low-N | 1.2 ± 0.1 a | 7.5 ± 0.1 a | 1.0 ± 0.1 a | 22.0 ± 3.4 a | 22.5 ± 0.4 a | |
| Medium-N | 1.3 ± 0.1 a | 7.7 ± 0.2 a | 1.0 ± 0.1 a | 22.4 ± 3.2 a | 22.6 ± 0.5 a | |
| High-N | 1.3± 0.1 a | 7.7 ± 0.1 a | 1.0 ± 0.1 a | 23.4 ± 3.8 a | 22.7 ± 0.5 a | |
| Natural forest | Control | 0.9 ± 0.0 b | 7.1 ± 0.3 b | 2.1 ± 0.3 b | 45.3 ±5.9 b | 22.1 ± 1.1 a |
| Low-N | 1.0 ± 0.0 b | 7.1 ± 0.2 b | 2.0 ± 0.3 b | 44.0 ± 6.1 b | 21.8 ± 1.0 a | |
| Medium-N | 1.1 ± 0.1 b | 7.2 ± 0.2 b | 2.0 ± 0.3 b | 44.1 ±5.8 b | 22.0 ± 1.1 a | |
| High-N | 1.1 ± 0.1 b | 7.3 ± 0.2 b | 2.1 ± 0.3 b | 46.1 ± 5.7 b | 22.2 ± 1.1 a |
2.3. Leaf Litter Collection, Decomposition, and Chemical Analysis
2.4. Data and Statistical Analysis
3. Results
3.1. Soil Properties and Initial Litter Chemical Composition
| Forest Type | Leaf Litters | Total C (mg·g−1) | Total N (mg·g−1) | Total P (mg·g−1) | Total K (mg·g−1) | Total Ca (mg·g−1) | Total Mg (mg·g−1) | C/N Ratio | N/P Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Plantation | Chinese pine | 425.8 ± 6.5 a | 5.4 ± 0.3 a | 1.3 ± 0.6 a | 2.6 ± 0.1 a | 7.0 ± 0.3 a | 2.0 ± 0.1 a | 79.1 ± 5.5 a | 5.8 ± 1.8 a |
| Natural forest | Mongolian oak | 314.2 ± 4.5 b | 8.1 ± 0.3 b | 0.7 ± 0.1 b | 4.1 ± 0.2 b | 21.8 ± 0.6 b | 3.3 ± 0.1 b | 38.7 ± 1.9 b | 13.2 ± 2.4 a |
| Pine–oak | 367.9 ± 5.0 b | 7.5 ± 0.5 b | 1.0 ± 0.4 a | 3.8 ± 0.3 bc | 14.5 ± 0.7 c | 2.7 ± 0.1 c | 49.34 ± 3.3 b | 10.4 ± 3.5 a | |
| Chinese pine | 413.6 ± 5.2 a | 5.9 ± 0.2 a | 1.1 ± 0.5 a | 3.3 ± 0.1 c | 9.3 ± 0.3 d | 2.2 ± 0.0 a | 70.4 ± 2.1 a | 8.0 ± 3.3 a |
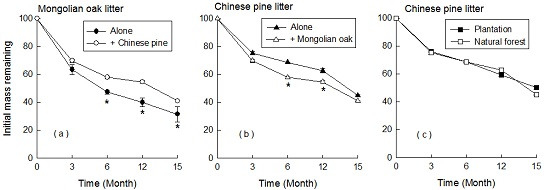
3.2. Patterns of Leaf Litter Decomposition with Ambient N Deposition
| Forest Type | Leaf Litters | Nitrogen Treatment | k-Value | R2 | p |
|---|---|---|---|---|---|
| Plantation | Chinese pine | Control | 0.49 ± 0.04 a | 0.93 | <0.001 |
| Low-N | 0.59 ± 0.04 c | 0.94 | <0.001 | ||
| Medium-N | 0.56 ± 0.05 bc | 0.92 | <0.001 | ||
| High-N | 0.52 ± 0.04 ab | 0.91 | <0.001 | ||
| Natural forest | Mongolian oak | Control | 0.84 ± 0.10 a | 0.86 | <0.001 |
| Low-N | 0.69 ± 0.10 b | 0.79 | <0.001 | ||
| Medium-N | 0.65 ± 0.10 b | 0.77 | <0.001 | ||
| High-N | 0.58 ± 0.11 c | 0.67 | <0.001 | ||
| Pine–oak | Control | 0.59 ± 0.06 a | 0.87 | <0.001 | |
| Low-N | 0.60 ± 0.07 a | 0.86 | <0.001 | ||
| Medium-N | 0.62 ± 0.07 a | 0.86 | <0.001 | ||
| High-N | 0.57 ± 0.08 a | 0.81 | <0.001 | ||
| Chinese pine | Control | 0.52 ± 0.05 a | 0.89 | <0.001 | |
| Low-N | 0.51 ± 0.05 ab | 0.90 | <0.001 | ||
| Medium-N | 0.54 ± 0.05 ab | 0.91 | <0.001 | ||
| High-N | 0.60 ± 0.05 b | 0.92 | <0.001 |
3.3. Response of Leaf Litter Decomposition to N Addition
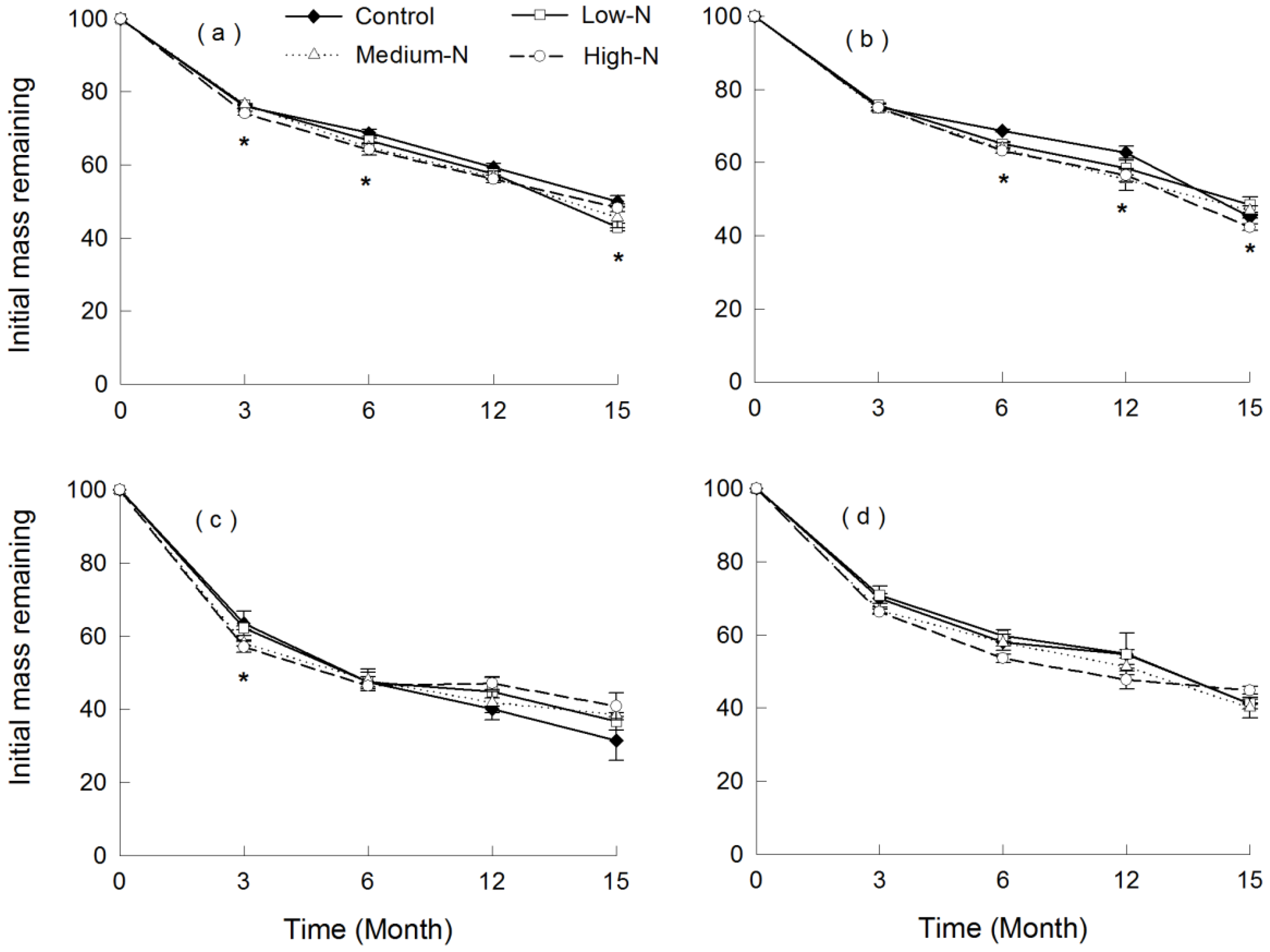
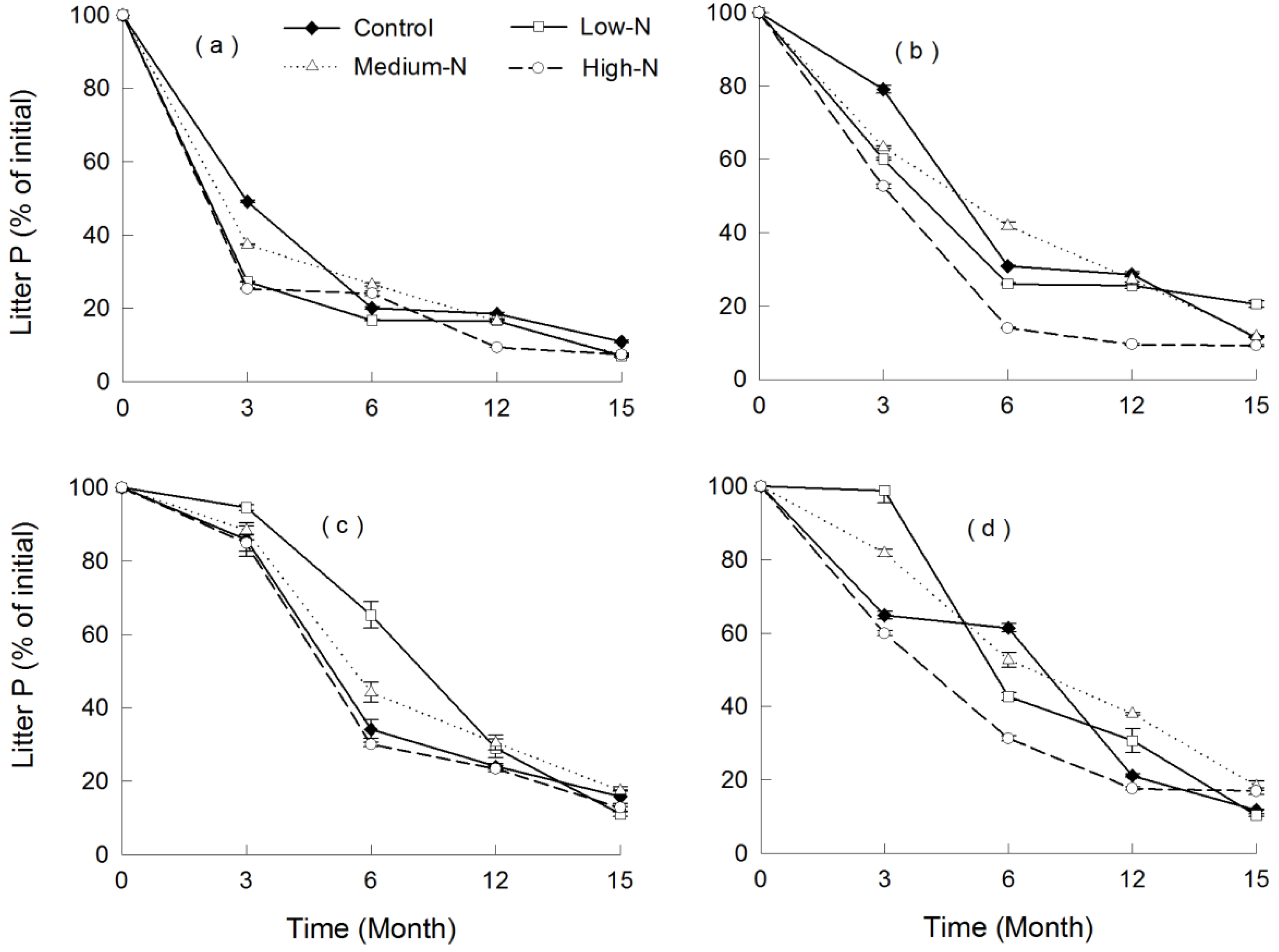
3.4. Nutrient Release


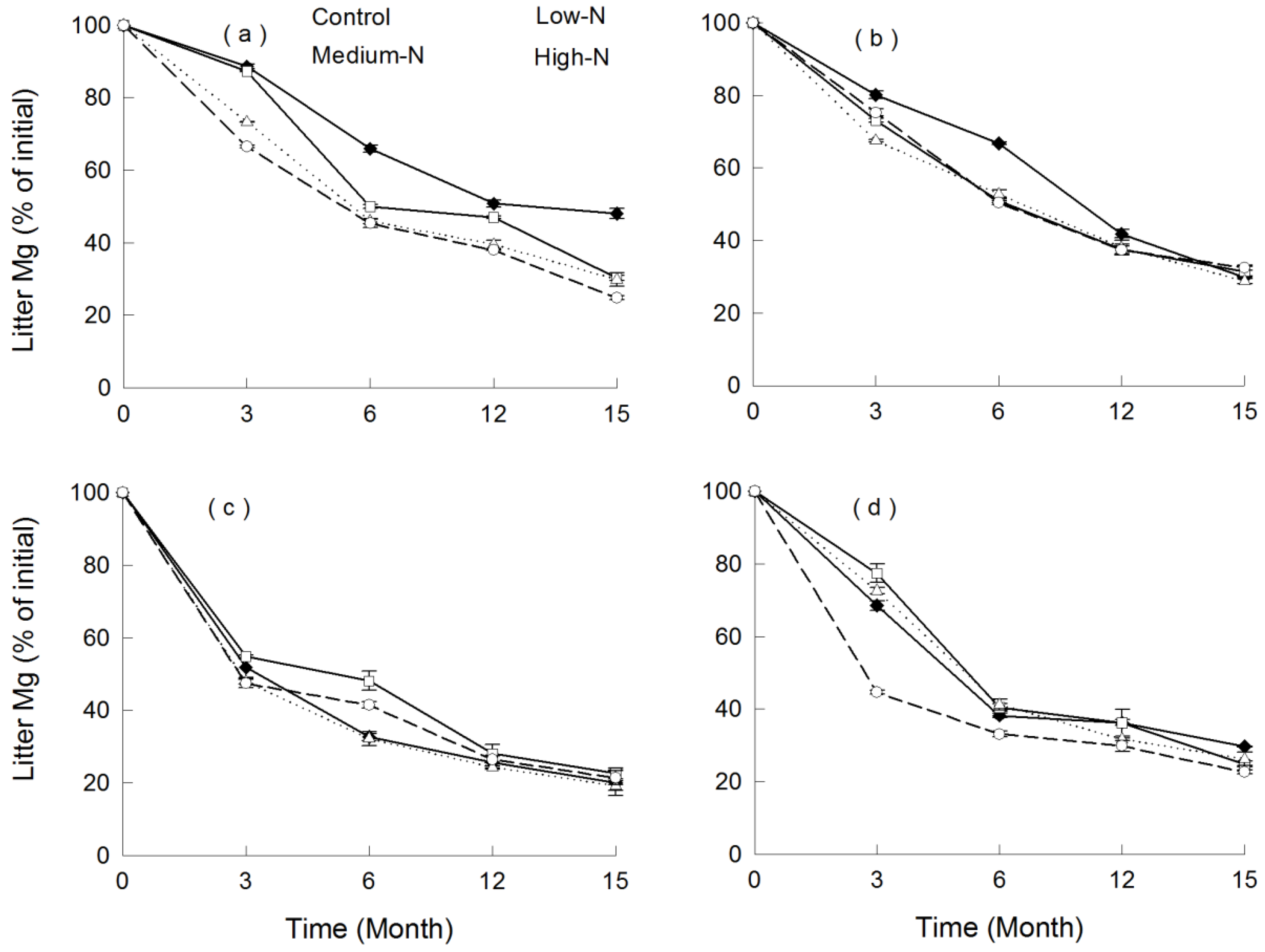
4. Discussion
4.1. Decomposition of Single-Species Litter
4.2. Decomposition of Litter Mixture
4.3. Effect of N Addition on Litter Decomposition
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. Ambio J. Hum. Environ. 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Zheng, X.H.; Fu, C.B.; Xu, X.K.; Yan, X.D.; Huang, Y.; Han, S.H.; Hu, F.; Chen, G.X. The Asian nitrogen cycle case study. Ambio A Journal of the Human Environment 2002, 31, 79–87. [Google Scholar] [CrossRef]
- Aber, J.D.; Goodale, C.L.; Ollinger, S.V.; Smith, M.L.; Magill, A.H.; Martin, M.E.; Hallett, R.A.; Stoddard, J.L. Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 2003, 53, 375–389. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A.T. Intrinsic effects of species on leaf litter and root decomposition: A comparison of temperate grasses from North and South America. Oecologia 2006, 150, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W.; et al. Ecological effects of nitrogen deposition in the western United States. Bioscience 2003, 53, 404–420. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Guan, L.L.; Wei, X.H.; Tang, X.L.; Liu, S.G.; Liu, J.X.; Zhang, D.Q.; Yan, J.H. Factors influencing leaf litter decomposition: An intersite decomposition experiment across China. Plant Soil 2008, 311, 61–72. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; Blackwell: Oxford, UK, 1979. [Google Scholar]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar]
- Hobbie, S.E. Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology 2008, 89, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, S.F.; Liu, L.; Ma, C.; Zhang, T.; Zhu, X.M.; Mo, J.M. Effects of experimental nitrogen and phosphorus addition on litter decomposition in an old-growth tropical forest. PLoS ONE 2013, 8, e84101. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Hu, H.L.; Chen, G.; Peng, Y.; Xiao, Y.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS ONE 2014, 9, e88752. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E. Does nitrogen availability control rates of litter decomposition in forests? Plant Soil 1995, 168–169, 83–88. [Google Scholar] [CrossRef]
- Hobbie, S.E. Interactions between litter lignin and soil N availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 2000, 3, 484–494. [Google Scholar] [CrossRef]
- Allison, S.D.; LeBauer, D.S.; Ofrecio, M.R.; Reyes, R.; Ta, A.M.; Tran, T. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol. Biochem. 2009, 41, 293–302. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Cao, L.X.; Zhang, R.D.; Yan, L.J.; Mao, Y.; Yang, Y.W. Effects of nitrogen addition and litter properties on litter decomposition and enzyme activities of individual fungi. Appl. Soil Ecol. 2014, 80, 108–115. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bradley, R.L. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Berger, T.W.; Berger, P. Does mixing of beech (Fagus sylvatica) and spruce (Picea abies) litter hasten decomposition? Plant Soil 2014, 377, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Scherer-Lorenzen, M. Functional diversity affects decomposition processes in experimental grasslands. Funct. Ecol. 2008, 22, 547–555. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Quested, H.M.; Cornelissen, J.H.C.; Press, M.C.; Callaghan, T.V.; Aerts, R.; Trosien, F.; Riemann, P.; Gwynn-Jones, D.; Kondratchuk, A.; Jonasson, S.E. Decomposition of sub-arctic plants with nitrogen economics: A functional role for hemi-parasites. Ecology 2003, 84, 3209–3221. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Walker, L.R.; Bonner, K.I. Among- and within-species variation in plant litter decomposition in contrasting long-term chronosequences. Funct. Ecol. 2009, 23, 442–453. [Google Scholar] [CrossRef]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Titus, B.D. Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forest. Plant Soil 2011, 339, 163–175. [Google Scholar] [CrossRef]
- Zhao, J.L.; Kang, F.F.; Wang, L.X.; Yu, X.W.; Zhao, W.H.; Song, X.S.; Zhang, Y.L.; Chen, F.; Sun, Y.; He, T.F.; et al. Patterns of biomass and carbon distribution across a chronosequence of Chinese pine (Pinus tabulaeformis) forests. PLoS ONE 2014, 9, e94966. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, B.; Ma, X.Q.; Zhao, G.D.; Li, S.N. Evaluation of ecosystem services of Chinese pine forests in China. Sci. China C Life Sci. 2008, 51, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Montané, F.; Romanyà, J.; Rovira, P.; Casals, P. Mixtures with grass litter may hasten shrub litter decomposition after shrub encroachment into mountain grasslands. Plant Soil 2013, 368, 459–469. [Google Scholar] [CrossRef]
- Liu, P.; Sun, O.J.; Huang, J.H.; Li, L.H.; Han, X.G. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol. Fertil. Soils 2007, 44, 211–216. [Google Scholar] [CrossRef]
- Smith, V.C.; Bradford, M.A. Do non-additive effects on decomposition in litter-mix experiments result from differences in resource quality between litter? Oikos 2003, 102, 235–243. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.H.; Sun, O.J.X.; Han, X.G. Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem. Oecologia 2010, 162, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, L.; Austin, A.T. Nitrogen addition stimulates forest litter decomposition and disrupts species interactions in Patagonia, Argentina. Glob. Chang. Biol. 2011, 17, 1963–1974. [Google Scholar] [CrossRef]
- Lin, G.G.; Mao, R.; Zhao, L.; Zeng, D.H. Litter decomposition of a pine plantation is affected by species evenness and soil nitrogen availability. Plant Soil 2013, 373, 649–657. [Google Scholar] [CrossRef]
- Li, H.S.; Wang, J.S.; Liu, X.; Jiang, S.S.; Zhang, C.Y.; Zhao, X.H. Effects and its sustained effect of simulated nitrogen deposition on soil respiration in Pinus tabulaeformis forests in the Taiyue Mountain, China. Acta Sci.Circums. 2014, 34, 238–249. (In Chinese) [Google Scholar]
- Pruden, G.; Powlson, D.S.; Jenkinson, D.S. The measurement of 15N in soil and plant material. Fertil. Res. 1985, 6, 205–218. [Google Scholar] [CrossRef]
- Nanjing Agricultural University. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 1988. (In Chinese) [Google Scholar]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Herrmann, S.; Kahl, T.; Bauhus, J. Decomposition dynamics of coarse woody debris of three important central European tree species. For. Ecosystems 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposition in ecological system. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Hobbie, S.E.; Milchunas, D.G.; Sinsabaugh, R.L. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 2010, 13, 765–781. [Google Scholar] [CrossRef]
- Lv, Y.N.; Wang, C.Y.; Wang, F.Y.; Zhao, G.Y.; Pu, G.Z.; Ma, X.; Tian, X.J. Effects of nitrogen addition on litter decomposition, soil microbial biomass, and enzyme activities between leguminous and non-leguminous forests. Ecol. Res. 2013, 28, 793–800. [Google Scholar] [CrossRef]
- Singh, K.P.; Singh, P.K.; Tripathi, S.K. Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoilat Singrauli, India. Biol. Fertil. Soils 1999, 29, 371–378. [Google Scholar] [CrossRef]
- Song, C.C.; Liu, D.Y.; Yang, G.S.; Song, Y.Y.; Mao, R. Effect of nitrogen addition on decomposition of Calamagrostis angustifolia litters from freshwater marshes of Northeast China. Ecol. Eng. 2011, 37, 1578–1582. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, S.L. Effects of NH4+ and NO3− on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China. Soil Biol. Biochem. 2012, 47, 116–122. [Google Scholar] [CrossRef]
- Li, L.J.; Zeng, D.H.; Yu, Z.Y.; Fan, Z.P.; Yang, D.; Liu, Y.X. Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. J. Arid Environ. 2011, 75, 787–792. [Google Scholar] [CrossRef]
- Perez Harguindeguy, N.; Blundo, C.M.; Gurvich, D.E.; Díaz, S.; Cuevas, E. More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through leaf chemistry. Plant Soil 2008, 303, 151–159. [Google Scholar] [CrossRef]
- Blair, J.M.; Parmalee, W.; Baere, M.H. Decay rates, nitrogen fluxes, and decomposer communities of single- and mixed- species foliar litter. Ecology 1990, 71, 1976–1985. [Google Scholar] [CrossRef]
- Fog, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Mo, J.M.; Brown, S.; Xue, J.H.; Fang, Y.T.; Li, Z.A. Response of litter decomposition to simulated nitrogen deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 285, 135–151. [Google Scholar] [CrossRef]
- Adersson, M.; Kjoller, A.; Struwe, S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.S.; Zhao, X.H. Effects of simulated nitrogen deposition on the soil enzyme activities in a Pinus tabulaeformis forest at the Taiyue Mountain. Acta Ecol. Sin. 2015, 35, 4613–4624. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and condi–tions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Bu, W.; Zhao, B.; Zhao, X.; Zhang, C.; Fan, J.; Gadow, K.V. Effects of Nitrogen Addition on Leaf Decomposition of Single-Species and Litter Mixture in Pinus tabulaeformis Forests. Forests 2015, 6, 4462-4476. https://doi.org/10.3390/f6124381
Wang J, Bu W, Zhao B, Zhao X, Zhang C, Fan J, Gadow KV. Effects of Nitrogen Addition on Leaf Decomposition of Single-Species and Litter Mixture in Pinus tabulaeformis Forests. Forests. 2015; 6(12):4462-4476. https://doi.org/10.3390/f6124381
Chicago/Turabian StyleWang, Jinsong, Wensheng Bu, Bo Zhao, Xiuhai Zhao, Chunyu Zhang, Juan Fan, and Klaus V. Gadow. 2015. "Effects of Nitrogen Addition on Leaf Decomposition of Single-Species and Litter Mixture in Pinus tabulaeformis Forests" Forests 6, no. 12: 4462-4476. https://doi.org/10.3390/f6124381