1. Introduction
Clonal forestry comprises the process of clonal timber production from candidate plus-tree selection, germplasm capture and clonal field testing through to clonal tree propagation, plantation establishment and site management [
1]. Clonal tree propagation, as part of a clonal forestry program, aims to maximize productivity, quality and uniformity in plantations by capturing the total genetic variance [
2,
3]. However, maturation of clonal stock plants can limit the adventitious rooting of cuttings and reduce the subsequent growth of plantation trees [
4,
5,
6]. Maturation-related loss of rooting competence is a major limiting factor in woody plant propagation [
7,
8] and, consequently, in the establishment of clonal forestry plantations.
Clonal selection typically occurs when trees are approaching their mature phase, and so efficient clonal propagation relies on the ability to produce rejuvenated stock plants of the selected clones or to maintain clonal archives of juvenile stock plants throughout the period of clonal field testing [
9,
10]. One of the most common strategies to maintain juvenility and delay maturation is to repeatedly prune the stock plants, or serially propagate cuttings from the stock plants, at a low height [
1,
11,
12]. Even then, propagation ability within clones can vary because of maturation- or morphologically-related topophysic effects;
i.e., positional effects within the stock plant on the subsequent rooting and growth of cuttings [
2,
5]. Topophysic effects can occur at very fine scales; e.g., rooting capacity of cuttings varies from the first to the fifteenth node of
Eucalyptus grandis W.Hill seedlings [
13] and rooting capacity of
in vitro shoots varies from the first to the fifth node of
Corymbia torelliana (F.Muell.) K.D.Hill & L.A.S.Johnson ×
C. citriodora (Hook.) K.D.Hill & L.A.S.Johnson seedlings [
14].
The hormone, indole-3-acetic acid (IAA), is involved in regulating many aspects of plant development from embryogenesis to senescence, and is one of the most important hormones involved in root initiation [
15,
16]. Another hormone, abscisic acid (ABA), affects root elongation, lateral root formation [
17] and the biosynthesis and activity of IAA [
18]. IAA and ABA may interact in regulating rooting potential [
19]. However, we have demonstrated recently that topophysic effects during the rooting and vigour of
C. torelliana ×
C. citriodora cuttings are related to the degree of lignification and sclerification of the stem rather than the concentrations of IAA or ABA in the cuttings [
20]. That research compared dual-node cuttings from the first 10 nodes of juvenile seedlings (
i.e., ortets). However, cuttings are normally harvested from larger stock plants that have been raised from cuttings (
i.e., ramets). Stock plants of
Eucalyptus and
Corymbia in Australia are often maintained by pruning at ~30-cm height [
21,
22,
23,
24] whereas eucalypt nurseries in Brazil use shorter stock plants to maintain clonal juvenility [
1,
2,
25,
26,
27,
28].
In this study, we compared the production, rooting and vigour of cuttings from C. torelliana × C. citriodora ramet stock plants that were maintained at two different heights (15 cm and 30 cm). We assessed whether differences in propagation potential were related to differences in IAA and ABA concentrations or stem anatomy. An understanding of the extent of maturation effects in eucalypt stock plants, and their underlying hormonal or anatomical bases, will assist in developing efficient propagation systems for establishing clonal plantations.
2. Experimental Section
2.1. Stock Plant and Cutting Production
Six clones of
C. torelliana ×
C. citriodora subsp.
variegata with varying rooting capacity were selected from our previous micropropagation and stock plant studies [
14,
29,
30]. Briefly, the clones were produced by germinating seeds of a full-sibling family (1CT2-013 × 1CV2-109)
in vitro and proliferating shoots in media containing half- or full-strength Murashige and Skoog medium [
14,
29]. Shoots were converted into plantlets after 18 months, and these were maintained as
ex vitro stock plants in 1.6-L pots for 9 months by pruning at a height of ~30 cm. Fresh rooted cuttings were then produced from the
ex vitro stock plants and these were raised as nursery stock plants in 1.6-L pots for a further 9 months [
30]. The stock plants of each clone were then divided into two treatments by pruning at one of two different heights: “Tall” (pruned at ~30 cm) or “Short” (pruned at ~15 cm). The experiment comprised eight stock plants per treatment per clone, spaced at 26 plants per m
2 on a glasshouse bench at the University of the Sunshine Coast, Australia (26°43′01″ S 153°03′44″ E). The potting mixture was the eucalypt seedling mix described previously [
22,
23]. Each stock plant received a weekly application of 150 mL of Flowfeed BM7 foliar fertilizer (Growforce, Acacia Ridge, Australia) at a rate of 10 g fertilizer L
−1.
Cuttings were harvested from each stock plant on 12 occasions over the following 6 months. Three-node cuttings were prepared by removing the shoot apex, dissecting the shoot into cuttings of 3–5 cm length, and pruning 50% of the length of each leaf from each cutting. The cuttings were not treated with auxin. The cuttings were set in 70 mL plastic Hyco tubes containing a 75/25 (v/v) mixture of perlite and shredded pine bark, supplemented with 3 kg of 8–9 month slow-release Osmocote
TM fertilizer and 1 kg of gypsum per m
3 [
22,
23]. The cuttings were placed in a translucent white polyethylene chamber and misted for 10 s every 10 min from 0600–1800 h. The cuttings remained under mist irrigation for up to 60 days, depending on the season.
Cuttings from harvest 2 (setting 1: “S1”, 10 July 2012) and harvest 12 (setting 2: “S2”, 20 December 2012) were used to assess root protrusion from the propagation tubes, percentage of cuttings that formed roots, and the growth of rooted cuttings. The percentage of tubes with protruding roots was recorded every 4 days from 36–60 days post-setting in S1 and from 16–36 days post-setting in S2. The percentage of cuttings with roots, as well as the root dry mass, shoot dry mass, root:shoot ratio and height of rooted cuttings, were measured at 60 days post-setting in S1 and 40 days post-setting in S2. The number of cuttings produced per stock plant (stock plant productivity) was recorded at each of the 12 harvests and converted into cutting production per m2 per month for comparison with other studies. Plant multiplication rate for S1 and S2 was determined by multiplying stock plant productivity × the percentage of cuttings that formed roots.
2.2. Hormone Analyses
A sample of cuttings of the same morphology was harvested concurrently in both S1 and S2 for hormone analysis. Three replicates of four cuttings per clone were snap-frozen in liquid nitrogen and then freeze dried for 36 h prior to storage at −80 °C. Plant material was then ground to a powder using a Mixer Mill MM200 tissue homogenizer (Retsch, Haan, Germany) and weighed into a 5 mL vial. Extraction and purification was adapted from a previous method [
31]. Ground material was suspended in 70% (v/v) methanol (15 mL·g
−1) with 0.1 mg·L
−1 butylated hydroxytoluene (BHT). Then, 10 µL of 10
−5 mol per 10 mL IAA D7 (indole-2,4,5,6,7-d5-3-acetic-2,2-d2 acid) (Sigma-Aldrich, Sydney, Australia) was added as a deuterated internal standard to a final quantity of 10
−8 mol to corroborate chromatographic retention times and quantification.
The vials were flushed with N2 gas and stored overnight at 4 °C. The supernatant was transferred into a test tube, centrifuged for 5 min, transferred to a 25 mL round bottom flask and evaporated under vacuum to an aqueous residue. The solution pH was adjusted to 8–9 with 0.5 M sodium hydroxide and the water layer was extracted twice with 1 mL ethyl acetate. The pH was readjusted to 2.5 with 0.5 M hydrochloric acid, and the solution was partitioned three times into 1 mL diethyl ether with 0.1 mg·mL−1 BHT, passed through anhydrous sodium sulfate, and evaporated under N2 gas to dryness. The residue was dissolved in 300 µL dichloromethane, 15 µL of derivatising agent [N–(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide, with 1% TBDMSCl] (Sigma-Aldrich, Sydney, Australia) was added, and the sample was stored for 1 h at 65 °C.
The sample was analyzed by GC-MS (Clarus 580 and Clarus SQ8S, Perkin Elmer) and the hormone concentrations calculated in ng·g−1 dry mass. The compound signals were monitored at m/z 239, 232 and 190 with retention times around 14.47, 14.55 and 19.57 min for IAA D7, natural IAA and ABA, respectively. Peak identification was based on retention time and mass spectra. Spiking of the sample with the standard solution of IAA D7 was used to determine the peak area ratio for each hormone and to calculate the natural hormone concentrations in the samples.
2.3. Stem Anatomy
An additional sample of cuttings of the same morphology was harvested concurrently in both S1 and S2 for anatomical observation. The cuttings were processed using a method adapted from our previous research [
32]. Transverse stem sections of 5 mm length were collected from the base of cuttings, fixed in a solution of 3% glutaraldehyde and 0.1 M phosphate buffer, and stored at 4 °C. The samples were then washed in 0.1 M phosphate buffer and processed under vacuum in a Shandon Excelsior ES Tissue Processor (Thermo Electron Corp., Marietta, OH, USA). Dehydration in alcohol was in six stages of 1 h each, followed by clearing in xylene in three 1-h stages, and infiltration with wax for 2 h. The samples were transverse sectioned at 8 µm using a UYD-335 Automated Microtome (ProSciTech, Thuringowa, Australia), stained with safranin and fast green [
33], and mounted with Permount medium (ProSciTech). Sections were examined with an Eclipse E200 light microscope (Nikon, Sydney, Australia).
2.4. Experimental Design and Statistical Analyses
Ten cuttings were allocated randomly to each of five replicates per treatment per clone in both settings (S1 and S2). Data were analyzed by 1-way ANOVA within treatments and t-test within clones because extensive interactions between clone and treatment were detected by 2-way ANOVA. Protruding roots and dry mass data were log transformed and rooting data was square root–log transformed when variance was heterogeneous. Post-hoc Tukey’s Honestly Significant Difference (HSD) tests were performed only when significant differences (p < 0.05) among clones were detected by ANOVA. Pearson’s correlations were also calculated between all variables in each setting.
3. Results
Cuttings from short stock plants often had faster root protrusion from the propagation tubes than cuttings from tall stock plants (
Table 1). Cuttings from short stock plants also often produced adventitious roots at a higher frequency than cuttings from tall stock plants (
Table 2). Mean rooting percentages (across all clones) from short stock plants (53.3% and 52.3% for S1 and S2, respectively) were higher than from tall stock plants (30.0% and 30.5% for S1 and S2, respectively) (
Table 2). Pruning stock plants at the low height accelerated root protrusion and increased rooting percentages in low-, medium-, and high-rooting clones (
Table 1 and
Table 2). The correlations between root protrusion and final rooting percentage were significant (
p < 0.05) at all evaluation times in both settings;
i.e., from 36–60 days and from 16–36 days post-setting in S1 and S2, respectively.
Table 1.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on the percentage of Corymbia torelliana × C. citriodora cuttings with roots protruding from the base of the propagation tube in setting 1 and setting 2. Means with different letters within a time point are significantly different (t-test; p < 0.05; n = 5).
Table 1.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on the percentage of Corymbia torelliana × C. citriodora cuttings with roots protruding from the base of the propagation tube in setting 1 and setting 2. Means with different letters within a time point are significantly different (t-test; p < 0.05; n = 5).
| Clone | Pruning Height | Setting 1 | Setting 2 |
|---|
| Time after Setting (days) |
|---|
| 36 | 40 | 44 | 48 | 52 | 56 | 60 | 16 | 20 | 24 | 28 | 32 | 36 | |
|---|
| 29 | Tall | 0 a | 0 a | 0 a | 0 b | 4 b | 4 b | 6 b | 0 a | 2 a | 2 a | 4 a | 4 a | 4 b | |
| Short | 0 a | 8 a | 12 a | 18 a | 24 a | 24 a | 26 a | 2 a | 4 a | 8 a | 10 a | 16 a | 22 a | |
| 67 | Tall | 2 a | 8 a | 10 a | 12 b | 16 b | 16 b | 16 b | 12 a | 14 a | 18 b | 26 a | 34 a | 36 a | |
| Short | 0 a | 10 a | 16 a | 26 a | 32 a | 32 a | 34 a | 20 a | 34 a | 42 a | 42 a | 42 a | 42 a | |
| 83 | Tall | 0 a | 2 a | 4 a | 6 a | 14 a | 14 a | 14 a | 0 b | 0 b | 14 a | 16 a | 22 a | 22 a | |
| Short | 0 a | 0 a | 6 a | 12 a | 22 a | 22 a | 26 a | 20 a | 22 a | 22 a | 26 a | 26 a | 26 a | |
| 156 | Tall | 4 a | 6 a | 22 a | 40 a | 50 a | 50 a | 54 a | 0 b | 2 b | 12 b | 16 b | 26 b | 32 b | |
| Short | 6 a | 12 a | 22 a | 30 a | 36 a | 36 a | 42 a | 30 a | 58 a | 68 a | 72 a | 74 a | 74 a | |
| 160 | Tall | 0 a | 0 a | 0 b | 0 b | 4 b | 4 b | 4 b | 4 b | 4 b | 10 b | 10 b | 10 b | 18 b | |
| Short | 6 a | 10 a | 14 a | 22 a | 28 a | 28 a | 28 a | 20 a | 40 a | 40 a | 44 a | 50 a | 50 a | |
| 163 | Tall | 4 a | 12 a | 26 a | 28 a | 40 a | 40 a | 40 a | 18 a | 28 b | 30 b | 34 b | 34 b | 34 a | |
| Short | 8 a | 16 a | 30 a | 40 a | 58 a | 58 a | 60 a | 34 a | 48 a | 48 a | 52 a | 54 a | 54 a | |
In most cases, the pruning height of stock plants did not affect the shoot dry mass, root dry mass or height of rooted cuttings (data not presented). However, short stock plants of clone 29 produced rooted cuttings with higher root dry mass than did tall stock plants in S1, short stock plants of clones 29, 163 and 160 produced rooted cuttings with higher shoot dry mass than did tall stock plants in S1, and short stock plants of clone 83 produced taller rooted cuttings than did tall stock plants in S2. The pruning height of stock plants generally did not affect the root:shoot ratio (RSR) of rooted cuttings, although cuttings from short stock plants of clones 29 and 160 in S1 had lower RSR than cuttings from tall stock plants (
Table 3).
Table 2.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on the percentage of Corymbia torelliana × C. citriodora cuttings with adventitious roots in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 5).
Table 2.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on the percentage of Corymbia torelliana × C. citriodora cuttings with adventitious roots in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 5).
| Clone | Pruning Height | Setting 1 | Setting 2 |
|---|
| 29 | Tall | 6 ± 2 Db | 8 ± 4 Db |
| Short | 26 ± 5 Ca | 22 ± 4 Ca |
| 67 | Tall | 20 ± 4 ABb | 40 ± 3 ABb |
| Short | 54 ± 9 Ba | 54 ± 2 Ba |
| 83 | Tall | 20 ± 3 BCa | 28 ± 5 BCa |
| Short | 34 ± 7 Ca | 36 ± 2 Ca |
| 156 | Tall | 74 ± 5 Ab | 54 ± 7 Ab |
| Short | 82 ± 4 Aa | 86 ± 2 Aa |
| 160 | Tall | 6 ± 2 CDb | 17 ± 1 CDb |
| Short | 50 ± 4 Ba | 60 ± 3 Ba |
| 163 | Tall | 54 ± 7 Bb | 36 ± 2 Bb |
| Short | 74 ± 6 Ba | 56 ± 5 Ba |
| Mean | Tall | 30.0 | 30.5 |
| Short | 53.3 | 52.3 |
Table 3.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on root:shoot dry mass ratio of Corymbia torelliana × C. citriodora rooted cuttings in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 5).
Table 3.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on root:shoot dry mass ratio of Corymbia torelliana × C. citriodora rooted cuttings in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 5).
| Clone | Pruning Height | Setting 1 | Setting 2 |
|---|
| 29 | Tall | 0.30 ± 0.01 Aa | 0.16 ± 0.00 Ba |
| Short | 0.24 ± 0.02 Ab | 0.15 ± 0.01 Aa |
| 67 | Tall | 0.22 ± 0.04 Aa | 0.17 ± 0.01 Ba |
| Short | 0.21 ± 0.02 Aa | 0.20 ± 0.04 Aa |
| 83 | Tall | 0.24 ± 0.02 Aa | 0.24 ± 0.03Aba |
| Short | 0.23 ± 0.03 Aa | 0.22 ± 0.03 Aa |
| 156 | Tall | 0.24 ± 0.03 Aa | 0.19 ± 0.02 Ba |
| Short | 0.23 ± 0.02 Aa | 0.24 ± 0.04 Aa |
| 160 | Tall | 0.31 ± 0.03 Aa | 0.30 ± 0.03 Aa |
| Short | 0.18 ± 0.03 Ab | 0.28 ± 0.04 Aa |
| 163 | Tall | 0.23 ± 0.02 Aa | 0.20 ± 0.02 Ba |
| Short | 0.20 ± 0.02 Aa | 0.19 ± 0.00 Aa |
| Mean | Tall | 0.26 | 0.21 |
| Short | 0.21 | 0.21 |
Stock plant productivity ranged from 141–1433 cuttings·m
−2·month
−1 depending on the clone, pruning height and harvest time, with productivity increasing across the six-month experimental period (
Table 4). Short stock plants of clones 163, 156 and 67 had lower productivity than tall stock plants, whereas short stock plants of clone 160 had higher productivity than tall stock plants, at several harvest times (
Table 4). Short stock plants of clones 29 and 83 sometimes had lower or higher productivity than tall stock plants, depending on the harvest time. Stock plant productivity was correlated (
p < 0.05) with ABA concentration at the second harvest time (
i.e., S1) and with IAA concentration at the twelfth harvest time (
i.e., S2), but it was not correlated significantly with other variables.
Table 4.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on Corymbia torelliana × C. citriodora stock plant productivity (cuttings·m−2·month−1). Means with different letters within each collection are significantly different (t-test; p < 0.05; n = 8 stock plants).
Table 4.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on Corymbia torelliana × C. citriodora stock plant productivity (cuttings·m−2·month−1). Means with different letters within each collection are significantly different (t-test; p < 0.05; n = 8 stock plants).
| Clone | Pruning Height | Collection |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Mean |
|---|
| 29 | Tall | 243 a | 245 a | 298 a | 354 b | 658 a | 548 b | 919 a | 589 a | 897 a | 851 a | 858 a | 1,170 a | 560 |
| Short | 177 b | 226 a | 201 b | 533 a | 423 b | 699 a | 533 b | 741 a | 780 a | 824 a | 692 b | 1,160 a | 514 |
| 67 | Tall | 259 a | 440 a | 255 a | 642 a | 580 a | 733 a | 793 a | 605 a | 917 a | 1,081 a | 1,190 a | 1,277 a | 631 |
| Short | 141 b | 209 b | 154 b | 488 b | 474 b | 499 b | 676 a | 565 a | 680 b | 648 b | 903 b | 1,070 b | 453 |
| 83 | Tall | 141 b | 412 a | 252 a | 660 a | 557 a | 627 a | 867 a | 676 a | 728 a | 922 a | 1,248 a | 1,307 a | 584 |
| Short | 185 a | 245 b | 217 a | 631 a | 460 a | 695 a | 559 b | 741 a | 780 a | 1,010 a | 917 b | 936 b | 552 |
| 156 | Tall | 262 a | 348 a | 293 a | 677 a | 571 a | 790 a | 787 a | 646 a | 713 a | 891 a | 925 a | 1,426 a | 598 |
| Short | 280 a | 256 a | 192 b | 568 a | 562 a | 483 b | 650 a | 566 a | 614 a | 895 a | 644 b | 1,151 b | 507 |
| 160 | Tall | 151 b | 226 a | 142 b | 373 a | 418 a | 421 a | 426 b | 478 a | 577 a | 425 b | 686 a | 655 b | 364 |
| Short | 190 a | 213 a | 226 a | 433 a | 324 a | 494 a | 594 a | 468 a | 669 a | 770 a | 657 a | 869 a | 438 |
| 163 | Tall | 277 a | 323 a | 396 a | 505 a | 761 a | 916 a | 923 a | 813 a | 1,073 a | 1,108 a | 1,112 a | 1,433 a | 709 |
| Short | 151 b | 153 b | 230 b | 459 a | 450b | 431 b | 713 a | 646 a | 992 a | 800 b | 1,125 a | 1,237 a | 503 |
| Mean | Tall | 222 | 332 | 273 | 535 | 591 | 672 | 786 | 635 | 817 | 880 | 1,003 | 1,211 | 574 |
| Short | 187 | 217 | 203 | 518 | 449 | 550 | 621 | 621 | 752 | 825 | 823 | 1,070 | 494 |
Multiplication rate (
i.e., stock plant productivity × rooting percentage) generally did not differ significantly between short and tall stock plants (
Table 5). However, short stock plants of clone 29 provided a higher multiplication rate than tall stock plants in S1, and short stock plants of clone 160 provided a higher multiplication rate than tall stock plants in both S1 and S2. Multiplication rates ranged from 15–246 rooted cuttings m
−2·month
−1 in S1 and from 106–885 rooted cuttings m
−2·month
−1 in S2, depending on the clone. Multiplication rate was correlated (
p < 0.05) with ABA concentration at the second harvest time (
i.e., S1), with root dry mass at the twelfth harvest time (
i.e., S2) and with root protrusion at both harvest times (S1 and S2), but it was not correlated significantly with other variables.
The pruning height of stock plants often affected the IAA or ABA concentration of
C. torelliana ×
C. citriodora cuttings, although the effects varied between clones and settings (
Table 6). Cuttings from short stock plants of clones 29 and 83 had lower IAA concentrations than cuttings from tall stock plants in S1, whereas cuttings from short stock plants of clones 163, 83 and 156 had higher IAA concentrations than cuttings from tall stock plants in S2. Mean IAA concentrations ranged from 331–771 ng·g
−1 dry mass in S1 and from 224–445 ng·g
−1 dry mass in S2. Cuttings from short stock plants of clones 83 and 67 in S1 and clone 67 in S2 had lower ABA concentrations than cuttings from tall stock plants, whereas cuttings from short stock plants of clone 160 in S1 and clones 163 and 156 in S2 had higher ABA concentrations than cuttings from tall stock plants. Mean ABA concentrations ranged from 88–345 ng·g
−1 dry mass in S1 and from 99–329 ng·g
−1 dry mass in S2 (
Table 6). IAA and ABA concentrations were correlated significantly (
p < 0.05) with each other in S1 but not in S2. Rooting characteristics and endogenous hormone concentrations were not correlated with each other in either setting (S1 or S2).
Table 5.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on multiplication rate (rooted cuttings m−2·month−1) of Corymbia torelliana × C. citriodora clones in setting 1 and setting 2. Means with different letters within each clone are significantly different (t-test; p < 0.05).
Table 5.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on multiplication rate (rooted cuttings m−2·month−1) of Corymbia torelliana × C. citriodora clones in setting 1 and setting 2. Means with different letters within each clone are significantly different (t-test; p < 0.05).
| Clone | Pruning Height | Setting 1 | Setting 2 |
|---|
| 29 | Tall | 14.7 b | 93.6 a |
| Short | 58.7 a | 255.3 a |
| 67 | Tall | 88.0 a | 510.9 a |
| Short | 113.4 a | 427.9 a |
| 83 | Tall | 82.5 a | 365.8 a |
| Short | 83.3 a | 337.0 a |
| 156 | Tall | 257.6 a | 770.2 a |
| Short | 210.1 a | 989.4 a |
| 160 | Tall | 13.5 b | 112.0 b |
| Short | 106.6 a | 521.5 a |
| 163 | Tall | 174.5 a | 516.0 a |
| Short | 113.4 a | 692.6 a |
| Mean | Tall | 105.1 | 394.7 |
| Short | 114.2 | 537.2 |
Table 6.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on free indole-3-acetic acid (IAA) and abscisic acid (ABA) concentrations in Corymbia torelliana × C. citriodora cuttings in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 3).
Table 6.
Effect of stock plant pruning height (Tall, 30 cm; Short, 15 cm) on free indole-3-acetic acid (IAA) and abscisic acid (ABA) concentrations in Corymbia torelliana × C. citriodora cuttings in setting 1 and setting 2. Means (±SE) with different capital letters within each pruning treatment, and different lower case letters within each clone, are significantly different (ANOVA and HSD test, or t-test; p < 0.05; n = 3).
| Clone | Pruning Height | Setting 1 | Setting 2 |
|---|
| Free IAA (ng·g−1 DM) | ABA (ng·g−1 DM) | Free IAA (ng·g−1 DM) | ABA (ng·g−1 DM) |
|---|
| 29 | Tall | 657 ± 31 ABa | 153 ± 3 CDa | 305 ± 9 BCa | 130 ± 8 BCa |
| Short | 414 ± 11 Cb | 151 ± 5 Ba | 294 ± 9 Da | 168 ± 24 Da |
| 67 | Tall | 605 ± 28 Ba | 342 ± 24 Aa | 369 ± 27 ABa | 329 ± 12 ABa |
| Short | 624 ± 27 ABa | 195 ± 23 Ba | 445 ± 18 Aa | 225 ± 27 Ab |
| 83 | Tall | 771 ± 26 Aa | 282 ± 26 ABa | 243 ± 11 CDb | 241 ± 24 CDa |
| Short | 599 ± 13 ABb | 149 ± 6 Bb | 396 ± 10 BCa | 166 ± 15 BCa |
| 156 | Tall | 741 ± 5 ABa | 330 ± 30 Aa | 247 ± 16 CDb | 99 ± 8 CDb |
| Short | 668 ± 22 Ab | 345 ± 41 Aa | 360 ± 2 Ca | 267 ± 29 Ca |
| 160 | Tall | 331 ± 40 Ca | 88 ± 4 Db | 400 ± 10 Aa | 169 ± 20 Aa |
| Short | 361 ± 38 Ca | 130 ± 3 Ba | 388 ± 5 BCa | 138 ± 9 BCa |
| 163 | Tall | 448 ± 32 Ca | 197 ± 11 BCa | 224 ± 14 Db | 134 ± 2 Db |
| Short | 495 ± 45 BCa | 208 ± 5 Ba | 413 ± 9 ABa | 257 ± 4 ABa |
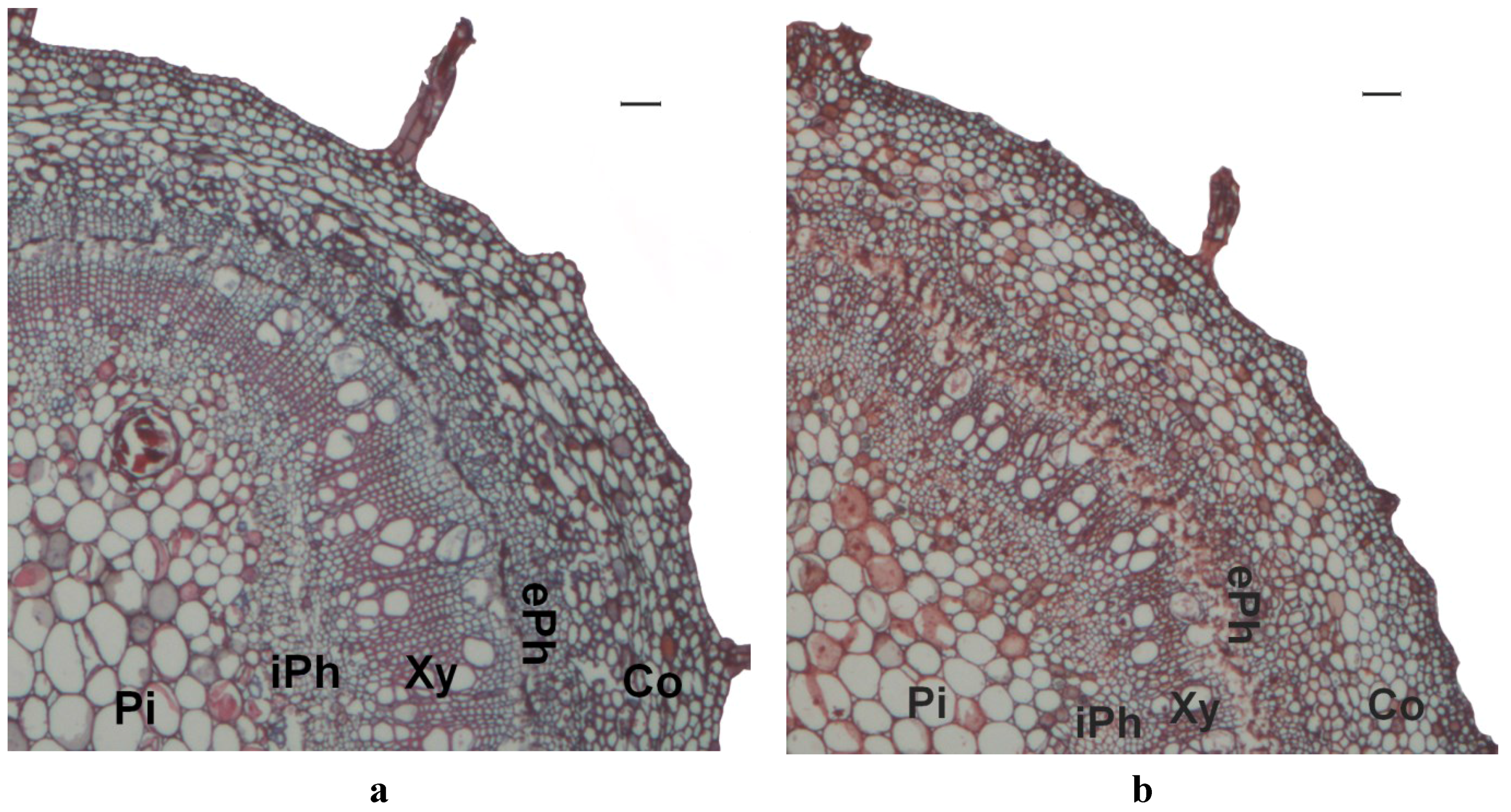
The stems of cuttings from short and tall
C. torelliana ×
C. citriodora stock plants exhibited similar anatomy, irrespective of the clone and setting. Stems had a central pith region, surrounded by vascular tissue containing internal phloem, xylem, cambium and external phloem arranged in a circular pattern. This was surrounded by the cortical tissue and epidermis (
Figure 1).
Figure 1.
Stem anatomy of cuttings from Corymbia torelliana × C. citriodora clone 160 after stock plants were pruned at tall (30 cm, a) or short (15 cm, b) height. Scale bars = 100 µm. Pi: Pith, iPh: internal phloem, Xy: xylem, ePh: external phloem, Co: cortex.
Figure 1.
Stem anatomy of cuttings from Corymbia torelliana × C. citriodora clone 160 after stock plants were pruned at tall (30 cm, a) or short (15 cm, b) height. Scale bars = 100 µm. Pi: Pith, iPh: internal phloem, Xy: xylem, ePh: external phloem, Co: cortex.
4. Discussion
Maintaining stock plants of
C. torelliana ×
C. citriodora at the lower height of 15 cm sometimes reduced the production of stem cuttings but it very often increased the ensuing percentage of cuttings that formed roots. As a result, short stock plants had a similar or higher multiplication rate than tall stock plants. Mean rooting percentages for cuttings from the tall stock plants (30%) were comparable to those from nursery stock plants of
C. torelliana ×
C. citriodora that were pruned at the same height (30 cm) in previous studies;
i.e., 30% [
32], 31% [
30], 34% [
34] and 46% [
21]. In contrast, mean rooting percentages for cuttings from the short stock plants (53%) were closer to those from cuttings harvested directly from 10-node seedlings (61%) [
20]. Clonal storage under minimal-growth conditions at 14 °C (when compared with conventional nursery archiving) also increases, from 31%–47%, the subsequent rooting frequency of cuttings from
C. torelliana ×
C. citriodora stock plants [
30]. These results indicate that maintaining stock plants at the lower height of 15 cm increased rooting by reducing the maturation of
C. torelliana ×
C. citriodora shoots.
Gradients in maturation within plants strongly influence propagation capacity in several species [
14,
35], with different cutting positions affecting the shoot morphology, rooting ability, and subsequent growth of rooted cuttings [
13,
35,
36,
37,
38]. Rooting ability of cuttings generally decreases from the basal to the apical part of trees [
9,
10,
39,
40,
41]. Small differences in shoot position (
i.e., ~15 cm), due to the different stock plant heights in the current study, affected both the speed of root protrusion from propagation tubes and the percentage of cuttings that forms roots. Root protrusion was used primarily to assess the rooting vigor of cuttings although it has also been used as a convenient and reliable predictor of final rooting percentages [
27]. The consistent correlations between root protrusion at all assessment dates and the final rooting percentage support the use of root protrusion as a forecasting tool in the production of
C. torelliana ×
C. citriodora rooted cuttings. In most cases, the positive effects of a lower stock plant height on root protrusion were not reflected in differences in the root dry mass, shoot dry mass or height of rooted cuttings. However, shorter stock plants did, occasionally, produce larger rooted cuttings than taller stock plants. This supports the conclusion that maturation can increase with increasing distance from the base of the plant and that maturation can affect the growth of rooted cuttings [
4,
7,
42,
43].
Maturation and topophysic effects have often been attributed to gradients in cellular activity, lignification, sclerification, or the concentrations of plant hormones or assimilates along the stem axis [
9,
44]. The effects on root formation in the current study were not the result of differences in IAA or ABA concentrations of cuttings from the stock plants of different heights. Endogenous auxin levels are often pivotal in the rooting process of plants [
16,
45,
46,
47] but rooting potential and cutting vigor were not correlated with IAA or ABA concentrations of
C. torelliana ×
C. citriodora cuttings. These results accord with findings from other woody plants including
Castanea sativa Mill.,
Syringa vulgaris L.,
Prunus persica L.,
P. cerasus L. ×
P. canescens Bois and
Eucalyptus globulus Labill. [
48,
49,
50]. They are also similar to recent results from the 10-node seedlings of
C. torelliana ×
C. citriodora, where varying rooting capacity among the nodes was poorly related to differences in IAA or ABA concentrations [
20]. Tissue response to auxin may be regulated by other factors such as the sensitivity of cells to the auxin signal and the concentration of rooting inhibitors in the base of cuttings [
48,
51].
Differences in the rooting capacity of
C. torelliana ×
C. citriodora cuttings in the current study were not associated with differences in stem anatomy. Sclerenchyma often forms outside the site of adventitious root formation as the stem develops [
33,
44], and rooting capacity sometimes declines as the continuity or number of cell layers of this sclerenchyma ring increases [
52,
53,
54]. The rooting capacity of cuttings from 10-node seedlings of
C.
torelliana ×
C.
citriodora is related to tissue lignification and sclerification, with cuttings from the lower-capacity basal nodes being more lignified and sclerified than cuttings from the higher-capacity apical nodes [
20]. In the present study, however, cuttings that arose from the pruned
C. torelliana ×
C. citriodora stock plants of different heights shared similar levels of lignification and displayed very little sclerification. This demonstrates that regular pruning (at approximately fortnightly intervals in this study) provided fresh shoots with suitable morphology for adventitious root formation.
The effectiveness of continuous pruning in maintaining the juvenility of stock plants has varied among previous studies, most of which have been conducted on conifer species. Juvenility can often be maintained in continuously pruned hedges [
11,
55,
56] while, in other cases, long-term pruning does little to alleviate maturation [
11,
57] and rooting declines with increasing stock plant age [
58,
59,
60]. Very few studies have evaluated the effectiveness of continuous pruning in maintaining the juvenility of hardwood species [
6,
30]. Shoot production, adventitious rooting capacity and root vigor are considered markers of juvenility [
7,
8,
28,
41,
46,
61,
62,
63,
64], and our results suggest that juvenility is maintained over a six-month period by continuous pruning of
C. torelliana ×
C. citriodora stock plants at 15-cm height.
The ability to increase the production of stem cuttings across sequential harvests from 15-cm or 30-cm stock plants indicates that this type of intensive nursery system can maintain adequate plant condition for shoot production. The low initial productivity (seen in the first three collections) is common and may be related to adaptation of the ramet to the stock plant management system [
61,
62] and to seasonal effects on shoot production [
22,
23,
28,
65,
66]. Stock plant productivity is also dependent on the growing system which, in the case of commercial
Eucalyptus propagation, is often semi-hydroponic [
1]. The same growing system as the present study (
i.e., pots and potting mix) has been used previously for
C. citriodora stock plants [
22], achieving similar stock plant productivity once allowances for differences in stock plant density are considered. The calculation of multiplication rates is important to verify the effectiveness of the adopted cloning system, combining both stem cutting production and rooting percentage to determine overall productivity. Lower pruning tended to provide fewer stem cuttings but higher rooting percentages and, consequently, there was a balance—or sometimes an increase—in the multiplication rate. The lower pruning system also has advantages in reducing the amount of labor and nursery space required for producing rooted cuttings, thus lowering production costs [
1].