Transformation of a Degraded Pinus massoniana Plantation into a Mixed-Species Irregular Forest: Impacts on Stand Structure and Growth in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Plot Establishment
| Treatment | Target Forest | Enrichment Species | Enrichment Density (n∙ha−1) | Spacing (m) |
|---|---|---|---|---|
| A1 | Pinus massoniana with 2 broad-leaved trees | Castanopsis hystrix | 350 | 3.5 × 4 |
| Magnoliaceae glanca | 350 | |||
| A2 | Pinus massoniana with 2 broad-leaved trees | Erythrophleum fordii | 406 | 3 × 4 |
| Quercus griffithii | 406 | |||
| A3 | Pinus massoniana with 3 broad-leaved trees | Quercus griffithii | 219 | 3 × 4 |
| Castanopsis hystrix | 375 | |||
| Magnoliaceae glanca | 219 | |||
| A4 | Pinus massoniana with 4 broad-leaved trees | Castanopsis hystrix | 163 | 3 × 5 |
| Magnoliaceae glanca | 163 | |||
| Erythrophleum fordii | 163 | |||
| Quercus griffithii | 163 | |||
| A5 (Control) | Pure Pinus massoniana plantation | 0 |
2.3. Analysis
2.3.1. Species Composition and Forest Structure
2.3.2. Growth Pattern
2.3.3. Statistical Analysis
3. Results
3.1. Species Composition
3.1.1. Overstory Species Composition
| Treatment | Species Category | Number of Tree Species | Number of Stems (n∙ha−1) | Proportion (%) | Basal Area (m2∙ha−1) | Proportion (%) |
|---|---|---|---|---|---|---|
| A1 | Pinus massoniana | 18 | 375 | 43.5 | 22.6 | 91.2 |
| Castanopsis hystrix | 188 | 21.7 | 0.7 | 3.0 | ||
| Magnoliaceae glanca | 75 | 8.7 | 0.5 | 1.8 | ||
| Others (15 from natural regeneration) | 225 | 26.1 | 1.0 | 4.0 | ||
| A2 | Quercus griffithii | 15 | 281 | 40.2 | 3.3 | 14.4 |
| Pinus massoniana | 275 | 39.3 | 18.9 | 81.6 | ||
| Erythrophleum fordii | 25 | 3.6 | 0.1 | 0.3 | ||
| Others (12 from natural regeneration) | 119 | 17.0 | 0.8 | 3.6 | ||
| A3 | Pinus massoniana | 17 | 331 | 41.1 | 22.2 | 87.1 |
| Magnoliaceae glanca | 231 | 28.7 | 1.4 | 5.6 | ||
| Quercus griffithii | 69 | 8.5 | 0.8 | 3.0 | ||
| Castanopsis hystrix | 44 | 5.4 | 0.2 | 0.7 | ||
| Others (13 from natural regeneration) | 131 | 16.3 | 0.9 | 3.7 | ||
| A4 | Pinus massoniana | 17 | 344 | 45.5 | 22.9 | 89.0 |
| Quercus griffithii | 138 | 18.2 | 1.3 | 4.9 | ||
| Castanopsis hystrix | 69 | 9.1 | 0.3 | 1.1 | ||
| Magnoliaceae glanca | 63 | 8.3 | 0.4 | 1.7 | ||
| Erythrophleum fordii | 13 | 1.7 | 0.0 | 0.1 | ||
| Others (12 from natural regeneration) | 131 | 17.4 | 0.8 | 3.2 | ||
| A5 (Control) | Pinus massoniana | 3 | 1175 | 98.4 | 44.3 | 99.9 |
| Schefflera actinophylla | 13 | 1.1 | 0.0 | 0.1 | ||
| Evodia lepta | 6 | 0.5 | 0.0 | 0.0 |
3.1.2. Understory Species Composition
| Treatment | Species Category | Number of Tree Species | Number of Stems (n∙ha−1) | Proportion (%) | Basal Area (m2∙ha−1) | Proportion (%) |
|---|---|---|---|---|---|---|
| A1 | Castanopsis hystrix | 11 | 63 | 30.0 | 0.1 | 32.9 |
| Magnoliaceae glanca | 44 | 21.2 | 0.1 | 24.3 | ||
| Others (9 from natural regeneration) | 100 | 48.5 | 0.1 | 42.8 | ||
| A2 | Quercus griffithii | 8 | 6 | 3.6 | 0.0 | 6.2 |
| Erythrophleum fordii | 81 | 46.4 | 0.1 | 39.1 | ||
| Others (6 from natural regeneration) | 88 | 50.0 | 0.1 | 54.7 | ||
| A3 | Magnoliaceae glanca | 10 | 63 | 25.6 | 0.1 | 32.9 |
| Quercus griffithii | 6 | 2.6 | 0.0 | 3.2 | ||
| Erythrophleum fordii | 13 | 5.1 | 0.0 | 6.6 | ||
| Others (7 from natural regeneration) | 160 | 66.7 | 0.1 | 57.4 | ||
| A4 | Quercus griffithii | 12 | 13 | 5.7 | 0.0 | 4.5 |
| Erythrophleum fordii | 31 | 14.3 | 0.0 | 20.8 | ||
| Magnoliaceae glanca | 25 | 11.4 | 0.0 | 9.7 | ||
| Erythrophleum fordii | 44 | 20.0 | 0.0 | 13.8 | ||
| Others (8 from natural regeneration) | 106 | 48.6 | 0.1 | 51.3 | ||
| A5 (Control) | Aluerites fordii | 3 | 7 | 15.6 | 0.0 | 13.9 |
| Schefflera actinophylla | 30 | 66.7 | 0.0 | 53.2 | ||
| Evodia lepta | 8 | 17.8 | 0.0 | 32.9 |
3.2. Forest Structure
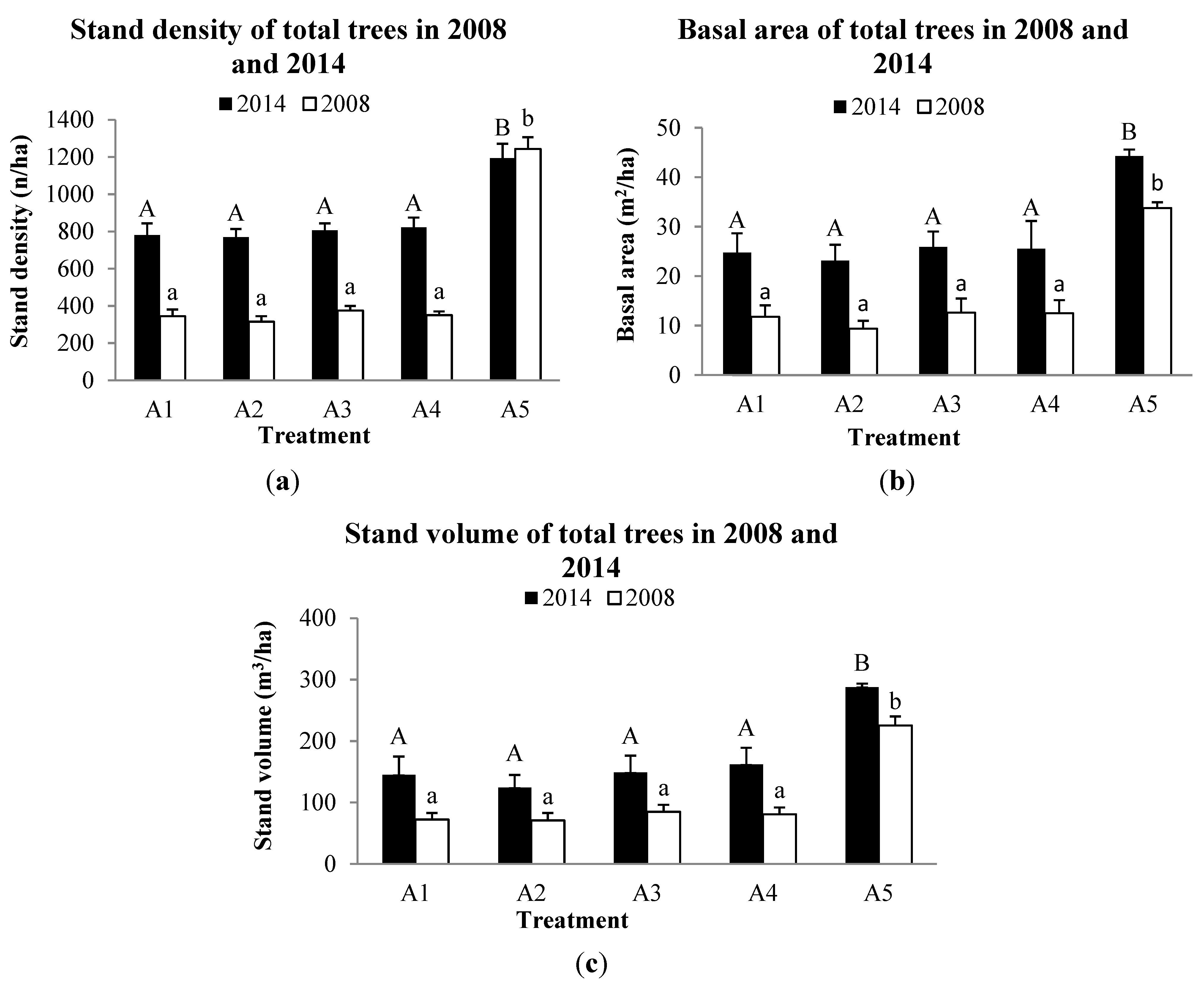
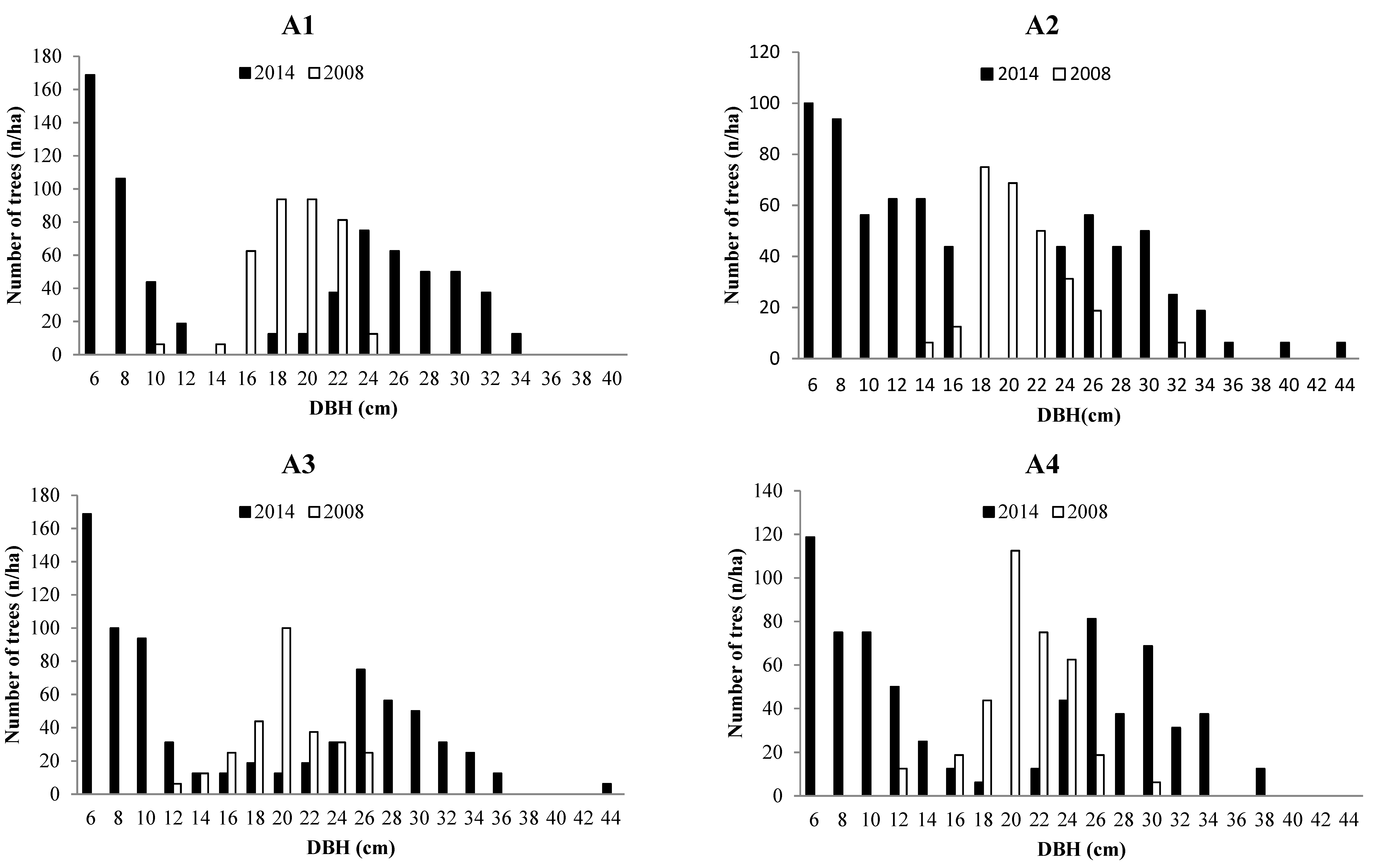
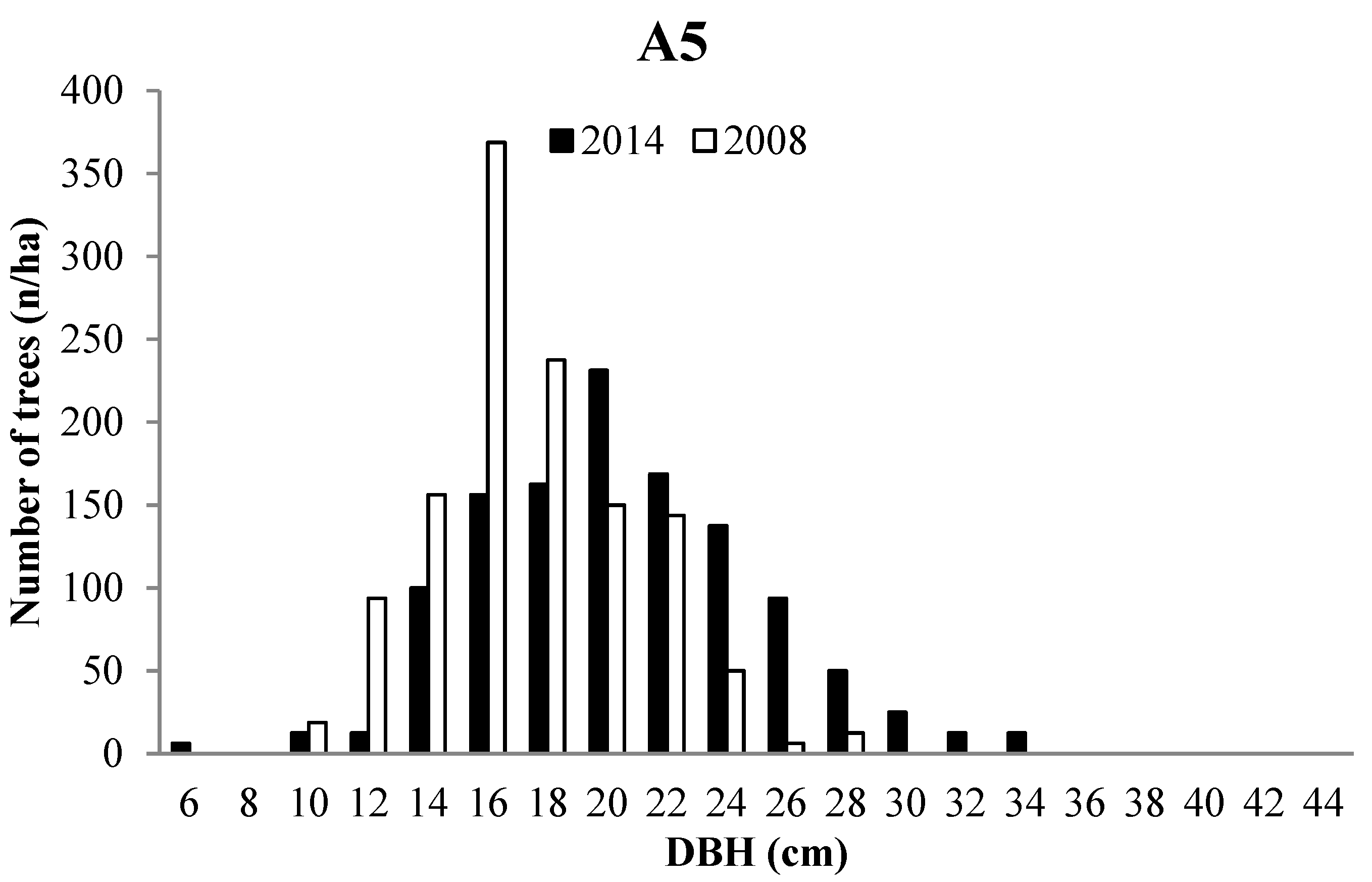
3.3. Tree growth
3.3.1. Survival Growth
| Species | Diameter Growth (cm/year/n) | Basal Area Growth (cm2/year/n) | Volume Growth (cm3/year/n) |
|---|---|---|---|
| Enrichment Planting | |||
| Magnoliaceae glance | 1.2 | 23.4 | 6327.1 |
| Castanopsis hystrix | 0.9 | 8.2 | 5143.2 |
| Quercus griffithii | 1.5 | 29.7 | 10321.4 |
| Natural Regeneration | |||
| Styrax serrulatus | 1.8 | 28.2 | 8923.5 |
| Ficus simplicissima | 0.7 | 8.4 | 1309.7 |
| Mallotus philippensis | 0.7 | 7.2 | 1802.6 |
| Trema orientalis | 1.0 | 14.8 | 6386.6 |
| Schefflera actinophylla | 0.7 | 9.7 | 3513.0 |
3.3.2 Ingrowth
| Treatment | Species | Number of Stems (n/ha) | Average DBH (cm) | Basal Area (m2/ha) | Volume (m3/ha) |
|---|---|---|---|---|---|
| A1 | Castanopsis hystrix | 188 | 6.9 | 0.7 | 1.6 |
| Magnoliaceae glance | 75 | 8.5 | 0.5 | 0.9 | |
| A2 | Quercus griffithii | 281 | 11.8 | 3.3 | 10.4 |
| Erythrophleum fordii | 25 | 6.2 | 0.1 | 0.1 | |
| A3 | Quercus griffithii | 69 | 11.5 | 0.8 | 2.5 |
| Castanopsis hystrix | 44 | 7.3 | 0.2 | 0.4 | |
| Magnoliaceae glance | 231 | 8.5 | 1.4 | 2.6 | |
| A4 | Quercus griffithii | 138 | 10.5 | 1.3 | 3.3 |
| Erythrophleum fordii | 13 | 5.4 | 0.0 | 0.0 | |
| Castanopsis hystrix | 69 | 7.1 | 0.3 | 1.2 | |
| Magnoliaceae glance | 63 | 9.1 | 0.4 | 0.9 |
3.3.3. Mortality
| Treatment | Species | Number of Individuals in 2008 (n/ha) | Number of Mortality Trees (n/ha) | Mortality Rate (%) | Overall Mortality Rate (%) |
|---|---|---|---|---|---|
| A1 | Castanopsis hystrix | 350 | 138 | 39.3 | 35.7 |
| Magnoliaceae glanca | 350 | 113 | 32.1 | ||
| A2 | Quercus griffithii | 406 | 81 | 20.0 | 43.9 |
| Erythrophleum fordii | 406 | 275 | 67.7 | ||
| A3 | Quercus griffithii | 219 | 50 | 22.9 | 31.5 |
| Castanopsis hystrix | 375 | 125 | 33.3 | ||
| Magnoliaceae glanca | 219 | 81 | 37.1 | ||
| A4 | Quercus griffithii | 163 | 31 | 19.2 | 26.9 |
| Erythrophleum fordii | 163 | 69 | 42.3 | ||
| Castanopsis hystrix | 163 | 38 | 23.1 | ||
| Magnoliaceae glanca | 163 | 38 | 23.1 |
4. Discussion
4.1. Species Composition and Forest Structure
4.2. Growth Pattern and Determination of Optimal Transformation Treatment
5. Conclusions and Future Prospects for Sustainable Forest Management
5.1. Conclusions
5.2. Future Prospects for Sustainable Forest Management
Acknowledgments
Author Contributions
Appendix
| Data | Measure | Source | df | Sum sq | F-Value | P > F | Paired Comparisons |
|---|---|---|---|---|---|---|---|
| Total Trees 2014 | Stand density( n/ha) | Treatment | 4 | 582812 | 10.95 | 0.000565 | A21 < A12 < A33 < A44 < A51–5 |
| Block | 3 | 76094 | 1.91 | 0.182437 | |||
| Stand volume (m3/ha) | Treatment | 4 | 68170 | 24.58 | 1.05 × 10−5 | A21 < A12 < A33 < A44 < A51–5 | |
| Block | 3 | 5588 | 2.69 | 0.0936 | |||
| Stand basal area (m2/ha) | Treatment | 4 | 1231.8 | 22.73 | 1.58 × 10−5 | A21 < A12 < A44 < A33 < A51–4 | |
| Block | 3 | 44.1 | 1.09 | 0.393 | |||
| Mean volume (m3) | Treatment | 4 | 0.012 | 2.50 | 0.0983 | A1 < A2 < A3 < A4 < A5 | |
| Block | 3 | 0.011 | 3.13 | 0.0659 | |||
| Mean basal area (m2) | Treatment | 4 | 0 | 1.38 | 0.3 | A1 < A3 < A4 < A2 < A5 | |
| Block | 3 | 0 | 2.00 | 0.17 | |||
| Mean diameter (cm) | Treatment | 4 | 55.62 | 4.94 | 0.0138 | A11 < A23 < A4 < A2 < A51,2 | |
| Block | 3 | 23.38 | 2.77 | 0.0877 | |||
| Mean height (m) | Treatment | 4 | 56.52 | 4.68 | 0.0166 | A2 < A3 < A4 < A1 < A5 | |
| Block | 3 | 25.88 | 2.86 | 0.082 | |||
| Total Trees 2008 | Stand density(n/ha) | Treatment | 4 | 2659188 | 142.94 | 5.19 × 10−10 | A21 < A12 < A44 < A33 < A51–5 |
| Block | 3 | 82625 | 5.92 | 0.0102 | |||
| Stand volume (m3/ha) | Treatment | 4 | 73437 | 32.17 | 5.16 × 10−6 | A21 < A12 < A34 < A43 < A51–5 | |
| Block | 3 | 4257 | 2.49 | 0.115 | |||
| Stand basal area (m2/ha) | Treatment | 4 | 1591 | 49.00 | 6.06 × 10−7 | A21 < A12 < A44 < A33 < A51–5 | |
| Block | 3 | 87.6 | 3.60 | 0.0497 | |||
| Mean volume (m3) | Treatment | 4 | 0.00602 | 0.97 | 0.464 | A5 < A1 < A3 < A2 < A4 | |
| Block | 3 | 0.00744 | 1.60 | 0.246 | |||
| Mean basal area (m2) | Treatment | 4 | 0.00015 | 1.33 | 0.319 | A5 < A1 < A3 < A2 < A4 | |
| Block | 3 | 0.00017 | 1.90 | 0.188 | |||
| Mean diameter (cm) | Treatment | 4 | 10.7 | 0.42 | 0.791 | A5 < A3 < A4 < A1 < A2 | |
| Block | 3 | 40.05 | 2.10 | 0.159 | |||
| Mean height (m) | Treatment | 4 | 2.668 | 1.24 | 0.35 | A1 < A3 < A2 < A5 < A4 | |
| Block | 3 | 1.637 | 1.01 | 0.424 | |||
| Residual Trees of Pinus massoniana 2014 | Stand volume (m3/ha) | Treatment | 4 | 76719 | 27.32 | 6.01 × 10−6 | A21 < A12 < A33 < A44 < A51–4 |
| Block | 3 | 6743 | 3.20 | 0.0622 | |||
| Basal area (m2/ha) | Treatment | 4 | 1677.3 | 30.31 | 3.45 × 10−6 | A21 < A32 < A13 < A44 < A51–4 | |
| Block | 3 | 93.8 | 2.26 | 0.134 | |||
| Mean volume (m3) | Treatment | 4 | 0.1003 | 9.32 | 0.00115 | A51,2,3,4 < A11 < A22 < A33 < A44 | |
| Block | 3 | 0.03674 | 4.55 | 0.02372 | |||
| Mean basal area (m2/ha) | Treatment | 4 | 0.00260 | 12.92 | 0.000263 | A51–4 < A11 < A32 < A43 < A24 | |
| Block | 3 | 0.00092 | 6.12 | 0.139 | |||
| Mean diameter (cm) | Treatment | 4 | 156.6 | 8.60 | 0.00163 | A51–4 < A21 < A12 < A43 < A34 | |
| Block | 3 | 61.17 | 4.48 | 0.127 | |||
| Mean height (m) | Treatment | 4 | 11.69 | 1.60 | 0.243 | A2 < A3 < A5 < A4 < A1 | |
| Block | 3 | 20.71 | 3.78 | 0.0439 | |||
| Growth of Residual Trees of Pinus massoniana | Volume (m3/year/n) | Treatment | 4 | 0.002 | 7.76 | 0.00318 | A51–4 < A21 < A42 < A13 < A34 |
| Block | 3 | 0 | 0.72 | 0.559 | |||
| Basal area(m2/year/n) | Treatment | 4 | 4.85E-06 | 11.93 | 0.00055 | A51–4 < A41 < A12 < A33 < A24 | |
| Block | 3 | 4.85E-06 | 1.701 | 0.22413 |
| Treatment | Measure | Residual Trees of Pinus massoniana | Total Trees | |||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2014 | p | 2008 | 2014 | p | |||
| A1 | Stand density(n/ha) | 375 ± 117 | 375 ± 117 | 375 ± 117 | 862 ± 198 | 0.021 | ||
| Stand volume (m3/ha) | 74.9 ± 22.2 | 140.7 ± 31.2 | 0.001 | 74.9 ± 22.2 | 144.9 ± 29.9 | 0.001 | ||
| Stand basal area (m2/ha) | 11.8 ± 3.1 | 22.6 ± 4.7 | 0.001 | 11.8 ± 3.1 | 24.8 ± 4.0 | 0.001 | ||
| Mean volume (m3) | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.007 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.481 | ||
| Mean basal area (m2) | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.005 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.441 | ||
| Mean height (m) | 13.3 ± 0.6 | 15.4 ± 3.3 | 0.120 | 13.3 ± 0.6 | 11.6 ± 3.8 | 0.802 | ||
| Mean diameter (cm) | 20.1 ± 1.8 | 28.0 ±3.3 | 0.002 | 20.1 ± 1.8 | 16.5 ± 2.9 | 0.836 | ||
| A2 | Stand density | 275 ± 46 | 275 ± 46 | 275 ± 46 | 700 ± 96 | 0.001 | ||
| Stand volume (m3/ha) | 59.1 ± 10.0 | 111.5 ± 21.0 | 0.004 | 59.1 ± 10.0 | 124.4 ± 20.4 | 0.004 | ||
| Stand basal area (m2/ha) | 9.4 ± 1.6 | 18.9 ± 2.9 | 0.001 | 9.4 ± 1.6 | 23.1 ± 3.2 | 0.000 | ||
| Mean volume (m3) | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.002 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.996 | ||
| Mean basal area (m2) | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.000 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.995 | ||
| Mean height (m) | 13.4 ± 0.2 | 13.2 ± 0.8 | 0.705 | 13.4 ± 0.2 | 9.1 ± 1.6 | 0.994 | ||
| Mean diameter (cm) | 20.3 ± 2.3 | 27.6 ± 4.8 | 0.000 | 20.3 ± 2.3 | 18.0 ± 2.1 | 0.999 | ||
| A3 | Stand density | 343 ± 97 | 331 ± 94 | 343 ± 97 | 806 ± 38 | 0.029 | ||
| Stand volume (m3/ha) | 80.8 ± 36.0 | 141.8 ± 29.4 | 0.005 | 80.8 ± 35.9 | 149.0 ± 27.2 | 0.004 | ||
| Stand basal area (m2/ha) | 12.6 ± 5.0 | 22.2 ± 4.3 | 0.003 | 12.6 ± 5.0 | 25.5 ± 3.2 | 0.002 | ||
| Mean volume (m3) | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.002 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.705 | ||
| Mean basal area (m2) | 0.01 ± 0.01 | 0.07 ± 0.01 | 0.004 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.710 | ||
| Mean height (m) | 13.3 ± 0.9 | 13.9 ± 0.8 | 0.004 | 13.3 ± 0.9 | 9.6 ± 1.1 | 0.996 | ||
| Mean diameter (cm) | 19.1 ± 3.6 | 29.2 ±1.7 | 0.004 | 19.1 ± 3.6 | 17.1 ± 1.3 | 0.937 | ||
| A4 | Stand density | 350 ± 94 | 343 ± 103 | 350 ± 94 | 781 ± 103 | 0.000 | ||
| Stand volume (m3/ha) | 84.7 ± 42.9 | 153.5 ± 52.4 | 0.000 | 84.7 ± 42.9 | 161.9 ± 50.6 | 0.000 | ||
| Stand basal area (m2/ha) | 12.5 ± 5.4 | 22.9 ± 6.0 | 0.000 | 12.5 ± 5.4 | 25.9 ± 5.6 | 0.000 | ||
| Mean volume (m3) | 0.2 ± 0.1 | 0.4 ± 0.0 | 0.000 | 0.2 ± 0.1 | 0.2 ± 0.1 | 1.000 | ||
| Mean basal area (m2) | 0.04 ±0.01 | 0.07 ±0.00 | 0.002 | 0.04 ±0.01 | 0.03 ± 0.01 | 1.000 | ||
| Mean height (m) | 14.2 ± 1.1 | 14.7 ± 1.2 | 0.009 | 14.2 ± 1.1 | 10.0 ± 1.5 | 1.000 | ||
| Mean diameter (cm) | 20.0 ± 4.4 | 29.0 ± 0.6 | 0.002 | 19.9 ± 4.4 | 17.5 ± 1.9 | 1.000 | ||
| A5 | Stand density | 1244 ± 111 | 1175 ± 151 | 0.196 | 1244 ± 111 | 1194 ± 134 | 0.196 | |
| Stand volume (m3/ha) | 225.4 ± 14.7 | 287.8 ± 5.7 | 0.011 | 225.4 ± 14.7 | 287.9 ± 5.7 | 0.011 | ||
| Stand basal area (m2/ha) | 33.8 ± 1.2 | 44.3 ±1.3 | 0.000 | 33.8 ± 1.2 | 44.3 ± 1.3 | 0.000 | ||
| Mean volume (m3) | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.015 | 0.2 ±0.0 | 0.2 ±0.0 | 0.019 | ||
| Mean basal area (m2) | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.000 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.000 | ||
| Mean height (m) | 13.9 ± 0.7 | 13.7 ± 0.7 | 0.604 | 13.9 ± 0.7 | 13.7 ± 0.7 | 0.622 | ||
| Mean diameter (cm) | 18.3 ± 0.9 | 21.6 ±1.3 | 0.000 | 18.3 ± 0.9 | 21.3 ± 1.0 | 0.000 | ||
Conflicts of Interest
References
- Department of Forest Resource Management. China’s forest resource status and dynamic change-based on analysis of the seventh national forest resource inventory results. For. Econ. 2010, 7, 42–48. [Google Scholar]
- Gärtner, S.; Reif, A. The impact of forest transformation on stand structure and ground vegetation in the southern Black Forest, Germany. Plant Soil 2004, 264, 35–51. [Google Scholar] [CrossRef]
- Felton, A.; Lindbladh, M.; Brunet, J.; Fritz, Ö. Replacing coniferous monocultures with mixed-species production stands: An assessment of the potential benefits for forest biodiversity in northern Europe. For. Ecol. Manag. 2010, 260, 939–947. [Google Scholar]
- Li, W. Degradation and restoration of forest ecosystems in China. For. Ecol. Manag. 2004, 201, 33–41. [Google Scholar] [CrossRef]
- Kelty, M.J. The role of species mixtures in plantation forestry. For. Ecol. Manag. 2006, 233, 195–204. [Google Scholar] [CrossRef]
- Kerr, G. The use of silvicultural systems to enhance the biological diversity of plantation forests in Britain. Forestry 1999, 72, 191–205. [Google Scholar] [CrossRef]
- Liu, H.; Li, J. The study of the ecological problems of eucalyptus plantation and sustainable development in Maoming Xiaoliang. J. Sustain. Dev. 2010, 3, 197. [Google Scholar]
- Pommerening, A.; Murphy, S.T. A review of the history, definitions and methods of continuous cover forestry with special attention to afforestation and restocking. Forestry 2004, 77, 27–44. [Google Scholar] [CrossRef]
- Malkinson, D.; Kadmon, R.; Cohen, D. Pattern analysis in successional communities—An approach for studying shifts in ecological interactions. Forestry 2003, 14, 213–222. [Google Scholar] [CrossRef]
- Harmer, R.; Kiewitt, A.; Morgan, G. Effects of overstorey retention on ash regeneration and bramble growth during conversion of a pine plantation to native broadleaved woodland. Eur. J For. Res. 2012, 131, 1833–1843. [Google Scholar] [CrossRef]
- Nabuurs, G.J.; Paivinen, R.; Schelhaas, M.J.; Pussinen, A.; Verkaik, E.; Lioubimow, A.; Mohren, F. Nature-oriented forest management in Europe: modeling the long-term effects. J. For. 2001, 99, 28–33. [Google Scholar]
- Chauvat, M.; Titsch, D.; Zaytsev, A.S.; Wolters, V. Changes in soil faunal assemblages during conversion from pure to mixed forest stands. For. Ecol. Manag. 2011, 262, 317–324. [Google Scholar] [CrossRef]
- Démurger, S.; Hou, Y.Z.; Yang, W.Z. Forest management policies and resource balance in China: An assessment of the current situation. J. Environ. Dev. 2009, 18, 17–41. [Google Scholar] [CrossRef]
- Shao, Q. An elucidation to nature-approximating forestry and forestry target. World For. Res. 2003, 16, 1–5. [Google Scholar]
- Lu, Y.; Zhang, S.; Lei, X.; Ning, J.; Wang, Y. Theoretical basis and implementation techniques on close-to-nature transformation of plantations. World For. Res. 2009, 22, 20–27. [Google Scholar]
- Zhang, S.X.; Lei, R.D.; Chen, C.G.; Liu, J.J. “Close-to-nature forest”—“A prospect manmade natural forest”. J. Northwest. For. Uni. 1996, 11, 157–162. [Google Scholar]
- Wang, L.Y.; Wang, X.H.; Song, Y.C. Study on restoration of degraded secondary forest and transformation of plantation in Tiantong forest farm. For. Sci. Technol. 2000, 11, 4–6. [Google Scholar]
- Zhang, J.Y.; Lu, Y.C.; Cheng, K.W.; Zhong, K.P.; Huang, Y.X. Impact of near nature modification on community structure and species biodiversity of artificial Pinus yunnanensis forest. J. Agric. Univ. Hebei 2010, 33, 72–77. [Google Scholar]
- Cai, N.H.; Cai, G.Q.; Lu, Y.C. Discussion on the approaching—Nature forestry management of Pinus yuananensis pure forests. J. Northwest For. Univ. 2006, 21, 85–88. [Google Scholar]
- Wang, X.H.; Wang, Q.H. Effects of the close-to-nature cultivation of Larix olgensis and Fraxinus mandshurica on the soil physiochemical properties. Sci. Silvae Sin. 2008, 44, 21–27. [Google Scholar]
- Zeng, D.H.; Jiang, F.Q.; Hao, H.; Fan, Z.H.; Du, X.J. Transformation and assessment of Chinese pine pure plantations for soil and water conservation in western Liaoning province, P.R. China. J. For. Res. 2004, 15, 127–134. [Google Scholar]
- Ning, J.K.; Lu, Y.C.; Zhao, H.Y.; Liu, X.Z.; Ren, Y.Q.; Chen, J.Q. Assessment on close-to-nature transformation of Pinus tabulaeformis plantation to Xishan region, Beijing. J. Northeast For. Univ. 2009, 37, 42–44. [Google Scholar]
- Ding, Z.F.; Ma, Y.C.; Wu, Z.N.; Wei, Z.H.; Chu, G.Q. Comprehensive discussion on low-function ecological forest reconstruction technology of Cunninghamia lanceolata and Pinus massoniana in Anhui northern subtropics region. J. Anhui Agric. Sci. 2013, 2, 660–662. [Google Scholar]
- Wu, H.Z.; Guo, M.X.; Chen, C.G.; Wang, Y. Discussion on ecological reconstruction of pure Pinus massoniana forest around Dinghu Mountain in Zhaoqing City. Cent. South For. Inventar. Plant 2011, 30, 25–28. [Google Scholar]
- Stone, R. Nursing China’s ailing forests back to health. Science 2009, 352, 556–558. [Google Scholar] [CrossRef]
- Kang, B.; Liu, S.H.; Zhang, G.J.; Chang, J.G.; Wen, Y.G.; Ma, J.M.; Hao, W.F. Carbon accumulation and distribution in Pinus massoniana and Cunninghamia lanceolata mixed forest ecosystem in Daqingshan, Guangxi of China. Acta Ecol. Sin. 2006, 26, 1320–1329. [Google Scholar] [CrossRef]
- Cai, D.; Guo, W.; Jia, H.; Lu, L.; Li, j.; Chen, J. Sustainable management of plantations—Taking as an example in the implementation of ecological management approach in Experimental Center of Tropical Forestry. For. Resour. Manag. 2008, 2, 42–46. [Google Scholar]
- Lu, L.H.; Wang, B.G.; He, R.M. The effect of site and cultivation model on growth of Castanopsis hystrix. For. Res. 1999, 12, 519–523. [Google Scholar]
- Tang, J.X.; Bai, L.H.; Guo, W.F.; Zeng, J.; Cai, D.X. Preliminary study on growth regularity of Castanpsis hystrixplantation. J. Cent. South Univ. For. Technol. 2012, 32, 51–60. [Google Scholar]
- Zhu, P.; Wang, Z.H.; Ye, W.H.; Cao, H.L.; Saravanan, T. Preliminary studies on pollination and mating system of rare and endangered plant Erythrophleum fordii Oliv. J. Trop. Subtrop. Bot. 2012, 21, 38–44. [Google Scholar]
- Jiang, Y.; Wang, Y.H.; Qiu, F.Y.; Huang, R.L. Early selection for superior provenances of Castanopsis fisa. For. Res. 2011, 40, 1–7. [Google Scholar] [CrossRef]
- Chhetri, R.; Toomsan, B.; Kaewpradit, W.; Limpimmtana, V. Mass loss, nitrogen, phosphorus and potassium release patterns and non-additive interactions in a decomposition study of chir pine (Pinus roxburghii) and Oak (Quercus griffithii). Int. J. Agric. Res. 2012, 7, 332–344. [Google Scholar] [CrossRef]
- He, F.; Ma, Q.Y.; Liu, X.L.; Wang, J.X.; He, D.H. Characteristics of biology and geographical distribution of wild Magnoliaceae plants in Sichuan Province. Sci Soil Water Conserv. 2008, 6, 48–53. [Google Scholar]
- Roberts, M.R. Stand development and overstory-understory interactions in an aspen-northern hardwoods stand. For. Ecol. Manag. 1992, 54, 157–174. [Google Scholar] [CrossRef]
- Bruelheide, H.; Böhnke, M.; Both, S.; Fang, T.; Assmann, T.; Baruffol, M. Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 2011, 81, 25–41. [Google Scholar] [CrossRef]
- Hahn, K.; Thomsen, R.P. Ground flora in Suserup Skov: Characterized by forest continuity and natural gap dynamics or edge-effect and introduced species? Ecol. Bull. 2007, 52, 167–181. [Google Scholar]
- Degen, T.; Devillez, F.; Jacquemart, A.L. Gaps promote plant diversity in beech forests (Luzulo-Fagetum), North Vosges, France. Annu. For. Sci. 2005, 62, 429–440. [Google Scholar] [CrossRef]
- Madsen, P.; Hahn, K. Natural regeneration in a beech-dominated forest managed by close-to-nature principles: A gap cutting based experiment. Can. J. For. Res. 2008, 38, 1716–1729. [Google Scholar] [CrossRef]
- Perera, A.H.; Buse, L.J.; Weber, M.G. Emulating Natural Forest Landscape Disturbances: Concepts and Applications. Columbia University Press: New York, NY, USA, 2007; p. 247. [Google Scholar]
- Cameron, A.D. Importance of early selective thinning in the development of long-term stand stability and improved log quality: A review. Forestry 2002, 75, 25–35. [Google Scholar] [CrossRef]
- Blakemore, P.; Cown, D.; Dumbrell, I.; McKinley, R.; Lyon, A.; Barr, B.; Northway, R. Western Australian Softwood Resource Evaluation: A Survey of Key Characteristics of the Pinus radiate and Pinus pinaster Resources in Western Australia with Links to Product Performance of Trees Sampled from Each Resource, as Determined by a Processing; Western Australian Softwood Resource: Melbourne, VIC, Australia, February 2010. [Google Scholar]
- Angers, V.A.; Messier, C.; Beaudet, M.; Leduc, A. Comparing composition and structure in old-growth and harvested (selection and diameter-limit cuts) northern hardwood stands in Quebec. For. Ecol. Manag. 2005, 217, 275–293. [Google Scholar] [CrossRef]
- Droessler, L.; von Luepke, B. Canopy gaps in two virgin beech forest reserves in Slovakia. J. For. Sci. 2005, 51, 446–457. [Google Scholar]
- Zeibig, A.; Diaci, J.; Wagner, S. Gap disturbance patterns of a Fagus sylvatica virgin forest remnant in the mountain vegetation belt of Slovenia. For. Snow Landsc. Res. 2005, 79, 2. [Google Scholar]
- O’Hara, K.L.; Nesmith, J.C.B.; Leonard, L.; Porter, D.L. Restoration of old forest features in coast redwood forests using earlystage variable-density thinning. Restor. Ecol. 2010, 18, 125–135. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics. Wiley: New York, NY, USA, 1996; p. 520. [Google Scholar]
- Mellinger, M.V.; McNaughton, S.J. Structure and function of successional vascular plant communities in central New York. Ecol. Monogr. 1975, 45, 161–182. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- Schläpfer, F.; Schmid, B.; Seidl, I. Expert estimates about effects of biodiversity on ecosystem processes and services. Oikos 1999, 84, 346–352. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Buongiorno, J.; Monserud, R.A.; Kruger, E.L.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Piotto, D. A meta-analysis comparing tree growth in monocultures and mixed plantations. For. Ecol. Manag. 2008, 255, 781–786. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: Evidence on stand level and explanation on individual tree level. Eur. J. For. Res. 2009, 128, 183–204. [Google Scholar] [CrossRef]
- Wacker, L.; Baudois, O.; Eichenberger-Glinz, S.; Schmid, B. Effects of plant species richness on stand structure and productivity. J. Plant Ecol. 2009, 2, 95–106. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Binkley, D. Seven decades of stand development in mixed and pure stands of conifers and nitrogen-fixing red alder. Can. J. For. Res. 2003, 33, 2274–2279. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Emborg, J. Suppression and release during canopy recruitment in Fagus sylvatica and Fraxinus excelsior, a dendro-ecological study of natural growth patterns and competition. Ecol. Bull. 2007, 52, 53–67. [Google Scholar]
- Collet, C.; Chenost, C. Using competition and light estimates to predict diameter and height growth of naturally regenerated beech seedlings growing under changing canopy conditions. Forestry 2006, 79, 489–502. [Google Scholar] [CrossRef]
- Young, C.G.; Dale, M.R.T.; Henry, G.H.R. Spatial pattern of vegetation in high arctic sedge meadows. Ecoscience 1999, 6, 556–564. [Google Scholar]
- Hou, J.H.; Mi, X.C.; Liu, C.R.; Ma, K.P. Spatial patterns and associations in a Quercus-Betula forest in northern China. J. Veg. Sci. 2004, 15, 407–414. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Tang, M.P. Study on Spatial Structure Optimizing Model of Stand Selection Cutting. Sci. Silvae. Sin. 2004, 40, 25–31. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Lu, Y.; Zeng, J. Transformation of a Degraded Pinus massoniana Plantation into a Mixed-Species Irregular Forest: Impacts on Stand Structure and Growth in Southern China. Forests 2014, 5, 3199-3221. https://doi.org/10.3390/f5123199
Meng J, Lu Y, Zeng J. Transformation of a Degraded Pinus massoniana Plantation into a Mixed-Species Irregular Forest: Impacts on Stand Structure and Growth in Southern China. Forests. 2014; 5(12):3199-3221. https://doi.org/10.3390/f5123199
Chicago/Turabian StyleMeng, Jinghui, Yuanchang Lu, and Ji Zeng. 2014. "Transformation of a Degraded Pinus massoniana Plantation into a Mixed-Species Irregular Forest: Impacts on Stand Structure and Growth in Southern China" Forests 5, no. 12: 3199-3221. https://doi.org/10.3390/f5123199
APA StyleMeng, J., Lu, Y., & Zeng, J. (2014). Transformation of a Degraded Pinus massoniana Plantation into a Mixed-Species Irregular Forest: Impacts on Stand Structure and Growth in Southern China. Forests, 5(12), 3199-3221. https://doi.org/10.3390/f5123199





