Influence of Time since Fire and Micro-Habitat Availability on Terricolous Lichen Communities in Black Spruce (Picea mariana) Boreal Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analyses
2.3.1. Stand Characteristics
2.3.2. Cover and Richness Values
2.3.3. Species Composition
| Candidate Models |
|---|
| Age + Canopy cover + Ground |
| Age + Ground |
| Age + Canopy cover |
| Canopy cover + Ground |
| Age |
| Canopy cover |
| Ground |
| Coarse woody debris density |
| Moss cover |
| Plant cover |
| Organic layer depth |
| Intercept only |
3. Results
3.1. Stand Characteristics
| Variable | 50 Years | 90 Years | 130 Years | 180 Years | >280 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | F | p | |
| Organic layer depth | 60 | 22.9 ± 7.2b | 90 | 25.3 ± 10.6b | 60 | 34.6 ± 10.5ab | 90 | 35.7 ± 7.2a | 60 | 41.3 ± 8.9a | 7.11 | <0.001 |
| Canopy cover | 60 | 76.7 ± 14.7a | 90 | 71.9 ± 21.7a | 60 | 60.0 ± 26.9ab | 90 | 41.5 ± 18.6b | 60 | 43.3 ± 19.5b | 9.04 | <0.001 |
| Basal area | 60 | 26.0 ± 18.6ab | 90 | 33.6 ± 21.3a | 60 | 23.6 ± 16.6ab | 90 | 21.0 ± 17.7b | 60 | 14.5 ± 12.8b | 5.41 | 0.002 |
| Coarse woody debris density | 60 | 2.4 ± 2.6b | 89 | 3.6 ± 3.0b | 60 | 2.5 ± 2.6b | 90 | 6.6 ± 5.2a | 60 | 3.6 ± 3.8ab | 4.54 | 0.005 |
3.2. Cover and Richness Values
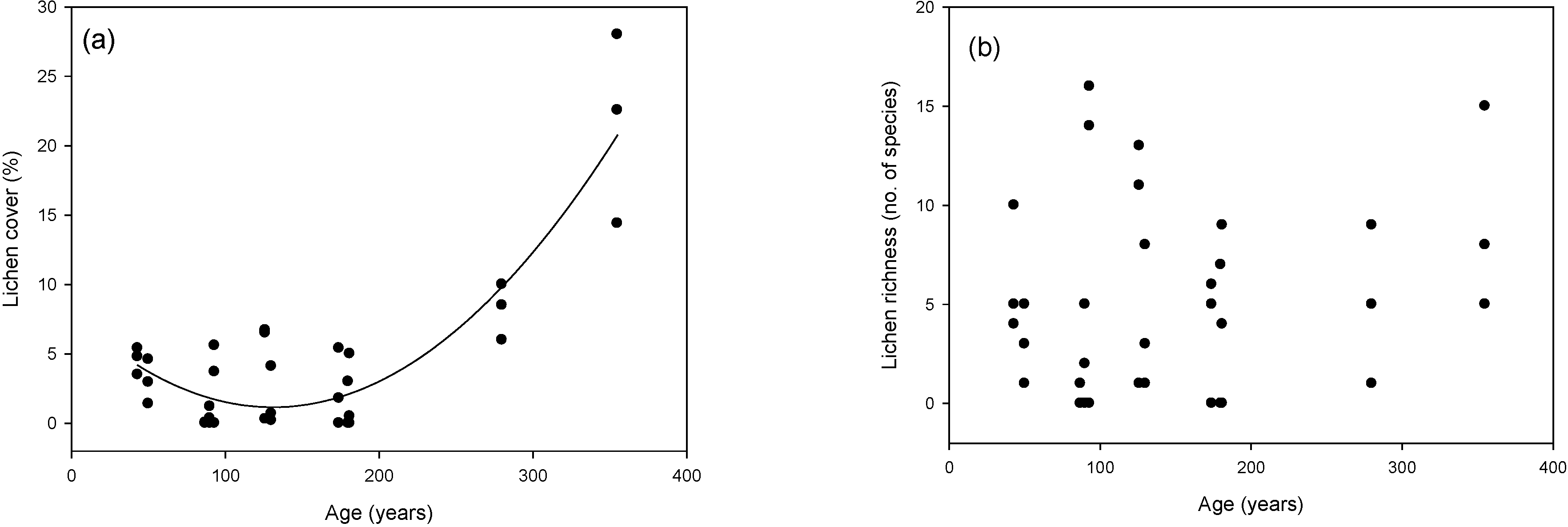
| Model | K | AICc | ∆AICc | W |
|---|---|---|---|---|
| Lichen richness | ||||
| Organic layer depth | 3 | 213.57 | 0 | 0.37 |
| Ground | 6 | 214.73 | 1.16 | 0.21 |
| CWD | 3 | 215.36 | 1.79 | 0.15 |
| Canopy cover | 3 | 217.21 | 3.64 | 0.06 |
| Intercept only | 2 | 217.29 | 3.72 | 0.06 |
| Canopy cover + ground | 7 | 217.81 | 4.24 | 0.04 |
| Age + Canopy cover | 5 | 218.66 | 5.09 | 0.03 |
| Moss cover | 3 | 219.39 | 5.82 | 0.02 |
| Plant cover | 3 | 219.42 | 5.85 | 0.02 |
| Lichen cover | ||||
| Age | 4 | 184.09 | 0 | 0.43 |
| Age + Canopy cover | 5 | 185.09 | 0.96 | 0.26 |
| Age + Ground | 8 | 185.07 | 0.98 | 0.26 |
| Dependent variable | Independent variable | Estimate | SE | 95% CI |
|---|---|---|---|---|
| Richness | Age | −1.26 | 1.65 | (−4.45, 1.94) |
| Age2 | 1.66 | 1.26 | (−0.82, 4.14) | |
| Canopy cover | −0.91 | 1.28 | (−3.41, 1.60) | |
| Organic layer depth | 1.87 | 0.80 | (0.31, 3.43) | |
| Coarse woody debris density | −1.73 | 0.79 | (−3.27, −0.19) | |
| Moss cover | −0.96 | 0.80 | (−2.52, 0.60) | |
| Plant cover | −0.16 | 0.76 | (−1.65, 1.32) | |
| Intercept | 4.92 | 0.74 | (3.47, 6.36) | |
| Cover | Age | 0.64 | 1.03 | (−1.38, 2.67) |
| Age2 | 4.76 | 0.75 | (3.29, 6.22) | |
| Canopy cover | −0.69 | 0.80 | (−2.23, 0.88) | |
| Organic layer depth | 1.73 | 0.70 | (0.35, 3.11) | |
| Coarse woody debris density | −0.41 | 059 | (−1.56, 0.75) | |
| Moss cover | −0.77 | 0.56 | (−1.86, 0.32) | |
| Plant cover | −0.22 | 0.49 | (−1.17, 0.74) | |
| Intercept | 4.37 | 0.46 | (3.56, 5.28) |
3.3. Species Composition
| Age class | 90 Years | 130 Years | 180 Years | >280 Years | ||||
|---|---|---|---|---|---|---|---|---|
| A | p | A | p | A | p | A | P | |
| 50 years | 0.206 | 0.010 | 0.162 | 0.007 | 0.112 | 0.050 | 0.263 | 0.007 |
| 90 years | - | - | 0.054 | 0.146 | 0.045 | 0.160 | 0.316 | 0.001 |
| 130 years | - | - | - | - | 0.140 | 0.033 | 0.254 | <0.001 |
| 180 years | - | - | - | - | - | - | 0.195 | 0.012 |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Species | 50 Years | 90 Years | 130 Years | 180 Years | >280 Years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | N | % | |
| Cetraria islandica | 0 | 0 | 6 | 0.1 | 9 * | 0.35 | 0 | 0 | 0 | 0 |
| Cladonia acuminata | 0 | 0 | 3 | 0.03 | 2 | 0.05 | 0 | 0 | 0 | 0 |
| Cladonia arbuscula | 9 * | 0.43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladonia bellidiflora | 2 | 0.03 | 0 | 0 | 0 | 0 | 3 | 0.03 | 0 | 0 |
| Cladonia bacilliformis | 1 | 0.02 | 0 | 0 | 1 | 0.02 | 1 | 0.01 | 0 | 0 |
| Cladonia borealis | 0 | 0 | 0 | 0 | 3 | 0.05 | 2 | 0.02 | 2 | 0.05 |
| Cladonia botrytes | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.03 | 2 | 0.03 |
| Cladonia caespiticia | 1 | 0.02 | 1 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladonia cariosa | 0 | 0 | 2 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladonia carneola | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 | 0 | 0 | 0 | 0 |
| Cladonia cenotea | 1 | 0.03 | 0 | 0 | 1 | 0.03 | 1 | 0.01 | 1 | 0.13 |
| Cladonia chlorophaea | 3 | 0.08 | 1 | 0.01 | 0 | 0 | 4 | 0.11 | 5 | 0.15 |
| Cladonia coniocraea | 0 | 0 | 1 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladonia cornuta ssp. cornuta | 0 | 0 | 1 | 0.02 | 2 | 0.05 | 2 | 0.03 | 1 | 0.02 |
| Cladonia cristatella | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.03 |
| Cladonia cyanipes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.11 |
| Cladonia digitata | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.01 | 1 | 0.02 |
| Cladonia ecmocyna | 0 | 0 | 1 | 0.01 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| Cladonia fimbriata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.05 |
| Cladonia gracilis ssp. turbinata | 1 | 0.02 | 0 | 0 | 0 | 0 | 1 | 0.13 | 0 | 0 |
| Cladonia macilenta | 3 | 0.05 | 0 | 0 | 2 | 0.03 | 0 | 0 | 0 | 0 |
| Cladonia mitis | 5 | 0.22 | 4 | 0.1 | 2 | 0.25 | 2 | 0.08 | 1 | 0.22 |
| Cladonia multiformis | 0 | 0 | 2 | 0.02 | 0 | 0 | 1 | 0.01 | 1 | 0.02 |
| Cladonia ochrochlora | 1 | 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladonia phyllophora | 1 | 0.01 | 1 | 0.01 | 0 | 0 | 0 | 0 | 1 | 0.01 |
| Cladonia pleurota | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 * | 0.08 |
| Cladonia ramulosa | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.02 | 3 | 0.07 |
| Cladonia rangiferina | 12 | 1.05 | 7 | 0.26 | 6 | 0.63 | 10 | 0.84 | 13 | 3.7 |
| Cladonia rei | 0 | 0 | 1 | 0.01 | 0 | 0 | 1 | 0.01 | 0 | 0 |
| Cladonia scabriuscula | 0 | 0 | 1 | 0.02 | 4 | 0.05 | 0 | 0 | 0 | 0 |
| Cladonia stellaris | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.26 | 21 * | 5.3 |
| Cladonia squamosa | 0 | 0 | 0 | 0 | 2 | 0.03 | 0 | 0 | 3 | 0.12 |
| Cladonia sulphurina | 0 | 0 | 0 | 0 | 2 | 0.07 | 0 | 0 | 3 | 0.1 |
| Cladonia uncialis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.02 |
| Flavocetraria cucullata | 0 | 0 | 12 | 0.24 | 13 * | 0.53 | 0 | 0 | 1 | 0.02 |
| Flavocetraria nivalis | 3 | 0.07 | 7 | 0.13 | 11 * | 0.35 | 0 | 0 | 3 | 0.05 |
| Peltigera aphtosa | 0 | 0 | 4 | 0.09 | 2 | 0.08 | 0 | 0 | 0 | 0 |
| Peltigera rufescens | 0 | 0 | 3 | 0.03 | 1 | 0.02 | 1 | 0.01 | 0 | 0 |
| Peltigera scabrosa | 0 | 0 | 2 | 0.02 | 2 | 0.05 | 0 | 0 | 0 | 0 |
| No. of lichens species | 15 | 21 | 18 | 16 | 23 | |||||
References
- Foster, D.R. Vegetation development following fire in Picea mariana (black spruce)-Pleurozium forests of south-eastern Labrador, Canada. J. Ecol. 1985, 73, 517–534. [Google Scholar] [CrossRef]
- Taylor, S.J.; Carleton, T.J.; Adams, P. Understory vegetation change in a chronosequence. Vegetatio 1987, 73, 63–72. [Google Scholar] [CrossRef]
- Carleton, T.J.; Maycock, P.J. Vegetation of the boreal forests south of James Bay: Non-centered component analysis of the vascular flora. Ecology 1980, 61, 1199–1212. [Google Scholar] [CrossRef]
- Esseen, P.A.; Ehnstrôm, B.; Ericson, L.; Sjôberg, K. Boreal forests. Ecol. Bull. 1997, 46, 16–47. [Google Scholar]
- Coxson, D.S.; Marsh, J. Lichen chronosequences (postfire and postharvest) in lodgepole pine (Pinus contorta) forest of northern interior British Columbia. Can. J. Bot. 2001, 79, 1449–1464. [Google Scholar]
- Fenton, N.; Lecomte, N.; Légaré, S.; Bergeron, Y. Paludification in black spruce (Picea mariana) forests of eastern Canada: Potential factors and management implications. For. Ecol. Manag. 2005, 213, 151–159. [Google Scholar] [CrossRef]
- Rheault, H.; Bélanger, L.; Grondin, P.; Ouimet, R.; Hébert, C.; Dussault, C. Stand composition and structure as indicators of epixylic diversity in old-growth boreal forests. Écoscience 2009, 16, 183–196. [Google Scholar] [CrossRef]
- Desponts, M.; Desrochers, A.; Bélanger, L.; Huot, J. Structure de sapinières aménagées et anciennes du massif des Laurentides (Québec) et diversité des plantes invasculaires. Can. J. For. Res. 2002, 32, 2077–2093. [Google Scholar] [CrossRef]
- Kruys, N.; Jonsson, B.G. Fine woody debris is important for species richness on logs in managed boreal spruce forests of northern Sweden. Can. J. For. Res. 1999, 29, 1295–1299. [Google Scholar] [CrossRef]
- Dettki, H.; Esseen, P.A. Modelling long-term effects of forest management on epiphytic lichens in northern Sweden. For. Ecol. Manag. 2003, 175, 223–238. [Google Scholar] [CrossRef]
- Nordén, B.; Appelqvist, T. Conceptual problems of ecological continuity and its bioindicators. Biodiv. Conserv. 2001, 10, 779–791. [Google Scholar] [CrossRef]
- Hilmo, O.; Såstad, S. Colonization of old-forest lichens in a young and an old boreal Picea abies forest: An experimental approach. Biol. Conserv. 2001, 102, 251–259. [Google Scholar] [CrossRef]
- Keon, D.B.; Muir, P. Growth of Usnea longissima across a variety of habitats in the Oregon coast range. Bryologist 2002, 105, 233–242. [Google Scholar] [CrossRef]
- Lättman, H.; Lindblomc, L.; Mattsson, J.E.; Milberg, P.; Skage, M.; Ekmanc, S. Estimating the dispersal capacity of the rare lichen Cliostomum corrugatum. Biol. Conserv. 2009, 142, 1870–1878. [Google Scholar] [CrossRef]
- Söderström, L. The occurrence of epixylic bryophyte and lichen species in an old natural and a managed forest stand in Northeast Sweden. Biol. Conserv. 1998, 45, 169–178. [Google Scholar] [CrossRef]
- Harper, K.A.; Bergeron, Y.; Drapeau, P.; Gauthier, S.; DeGrandpré, L. Structural development following fire in black spruce boreal forest. For. Ecol. Manag. 2005, 206, 293–306. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest productivity decline caused by successional paludification of boreal soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Fenton, N.J.; Bergeron, Y. Does time or habitat make old-growth forests species rich? Bryophyte richness in boreal Picea mariana forests. Biol. Conserv. 2008, 141, 1389–1399. [Google Scholar] [CrossRef]
- Boudreault, C.; Bergeron, Y.; Coxson, D.S. Factors controlling epiphytic lichen biomass accumulation during postfire succession in black spruce boreal forests. Can. J. For. Res. 2009, 39, 2168–2179. [Google Scholar] [CrossRef]
- Morneau, C.; Payette, S. Postfire lichen-spruce woodland recovery at the limit of the boreal forest in northern Québec. Can. J. Bot. 1989, 60, 2770–2782. [Google Scholar] [CrossRef]
- Boudreault, C.; Bergeron, Y.; Gauthier, S.; Drapeau, P. Bryophyte and lichen communities in mature to old-growth stands in eastern boreal forests in Canada. Can. J. For. Res. 2002, 32, 1080–1093. [Google Scholar] [CrossRef]
- Kelsall, J.P. COSEWIC Status Report on the Woodland Caribou Rangifer tarandus caribou in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 1984. [Google Scholar]
- Webb, E.T. Survival, persistence, and regeneration of the reindeer lichens, Cladina stellaris, C. rangiferina, and C. mitis following clear-cut logging and forest fire in Northwestern Ontario. Rangifer 1998, 10, 41–47. [Google Scholar] [CrossRef]
- Vincent, J.S.; Hardy, L. L’évolution et l’extinction des lacs glaciaires Barlow et Ojibway en territoire québécois. Geogr. Phys. Quatern. 1977, 31, 357–372. [Google Scholar]
- Rowe, J.S. Forest Regions of Canada; Environment Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Environment Canada. Canadian Climate Normals or Averages 1971–2000. Available online: http://www.climate.weatheroffice.ec.gc.ca/climate_normals/results_e.html (accessed on 10 October 2014).
- Bergeron, Y.; Gauthier, S.; Flannigan, M.; Kafka, V. Fire regimes at the transition between mixedwoods and coniferous boreal forests in Northwestern Québec. Ecology 2004, 85, 1916–1932. [Google Scholar] [CrossRef]
- Lecomte, N.; Simard, M.; Bergeron, Y. Effects of fire severity and initial tree composition on stand structural development in the coniferous boreal forest of northwestern Québec, Canada. Écoscience 2006, 13, 152–163. [Google Scholar] [CrossRef]
- Lecomte, N.; Simard, M.; Bergeron, Y.; Larouche, A.; Asnong, H.; Richard, P.J.H. Effects of fire severity and initial tree composition on understorey vegetation dynamics in a boreal landscape inferred from chronosequence and paleoecological data. J. Veg. Sci. 2005, 16, 665–674. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Mazerolle, M.J. Package AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c) Ver. 1.24. Available online: cran.r-project.org/web/packages/AICcmodavg/index.htm (accessed on 10 October 2014).
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Henry, M.; Stevens, H.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 1.13–1. Available online: http://vegan.r-forge.r-project.org/ (accessed on 10 October 2014).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monog. 1997, 67, 345–366. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. In Multivariate Analysis of Ecological Data; Version 4; MjM Software Design: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R. Use R! Series; Springer: New York, NY, USA, 2011. [Google Scholar]
- Brodo, I.M.; Sharnoff, S.D.; Sharnoff, S. Lichens of North America; Yale University Press: New Haven, CT, USA, 2001. [Google Scholar]
- Mistry, J. Corticolous lichens as potential bioindicators of fire history: A study in the cerrado of the Distrito Federal, central Brazil. J. Biogeogr. 1998, 25, 409–441. [Google Scholar] [CrossRef]
- Hedenås, H.; Ericson, L. Epiphytic macrolichens as conservation indicators: Successional sequence in Populus tremula stands. Biol. Conserv. 2000, 93, 43–53. [Google Scholar] [CrossRef]
- Johansson, P.; Wetmore, C.M.; Carlson, D.J.; Reich, P.B.; Göran, T. Habitat preference, growth form, vegetative dispersal and population size of lichens along a wildfire severity gradient. Bryologist 2006, 109, 527–540. [Google Scholar] [CrossRef]
- Rogers, R.W. Ecological strategies of lichens. Lichenologist 1990, 22, 149–162. [Google Scholar] [CrossRef]
- Crittenden, P.D. Aspects of the ecology of mat-forming lichens. Rangifer 2000, 20, 127–139. [Google Scholar] [CrossRef]
- Yahr, R. Ecology and post-fire recovery of Cladonia perforata, an endangered Florida-scrub lichen. For. Snow Landsc. Res. 2000, 75, 339–356. [Google Scholar]
- Roturier, S.; Bäcklund, S.; Sundén, M.; Bergsten, U. Influence of ground substrate on establishment of reindeer lichen after artificial dispersal. Silva Fenn. 2007, 41, 269–280. [Google Scholar] [CrossRef]
- Roturier, S.; Bergsten, U. Establishment of Cladonia stellaris after artificial dispersal in an unfenced forest in northern Sweden. Rangifer 2009, 29, 39–49. [Google Scholar] [CrossRef]
- Heinken, T. Dispersal patterns of terricolous lichens by thallus fragments. Lichenologist 1999, 31, 603–612. [Google Scholar] [CrossRef]
- Hilmo, O.; Ott, S. Juvenile development of the cyanolichen Lobaria scrobiculata and the green algal lichens Platismatia glauca and Platismatia norvegica in a boreal Picea abies forest. Plant Biol. 2002, 4, 273–280. [Google Scholar] [CrossRef]
- Scheidegger, C. Early development of transplanted isidioid soredia of Lobaria pulmonaria in an endangered population. Lichenologist 1995, 27, 361–374. [Google Scholar]
- Kalwij, J.M.; Wagner, H.H.; Scheidegger, C. Effects of stand-level disturbances on the spatial distribution of a lichen indicator. Ecol. Appl. 2005, 15, 2015–2024. [Google Scholar] [CrossRef]
- Ahti, T. Taxonomic studies on reindeer lichens (Cladonia, subgenus Cladina). Ann. Bot. Soc. 1961, 32, 1–160. [Google Scholar]
- Helle, T.; Aspi, J. Effects of winter grazing by reindeer on the vegetation. Oikos 1983, 40, 337–343. [Google Scholar] [CrossRef]
- Scotter, G.W. Growth rates of Cladonia alpestris, C. mitis, and C. rangiferina in the Taltson River region Northwest Territories. Can. J. Bot. 1963, 41, 1199–1202. [Google Scholar] [CrossRef]
- Den Herder, M.; Kytöviita, M.M.; Niemela, P. Growth of reindeer lichens and effects of reindeer grazing on ground cover vegetation in a Scots pine forest and a subarctic heathland in Finnish Lapland. Ecography 2003, 26, 3–12. [Google Scholar] [CrossRef]
- Boudreault, C.; Zouaoui, S.; Drapeau, P.; Bergeron, Y.; Stevenson, S. Canopy openings created by partial cutting increase growth rates and maintain the cover of three Cladonia species in the Canadian boreal Forest. For. Ecol. Manag. 2013, 304, 473–481. [Google Scholar] [CrossRef]
- Södeström, L.; Jonsson, B. Spatial pattern and dispersal in the leafy hepatic Ptilidium pulcherrimum. J. Bryol. 1989, 15, 793–802. [Google Scholar] [CrossRef]
- Gustafsson, L.; Fiskesjö, A.; Hallingbäck, T.; Ingelög, T.; Pettersson, B. Semi-natural broadleaved woods in southern Sweden—Habitat factors of importance to some bryophyte species. Biol. Conserv. 1992, 59, 175–181. [Google Scholar] [CrossRef]
- Scheidegger, C.; Werth, S. Conservation strategies for lichens: Insights from population biology. Fungal Boil. Rev. 2009, 23, 55–66. [Google Scholar]
- Kershaw, K.A.; Field, G.F. Studies on lichen-dominated systems. XV. The temperature and humidity profiles in a Cladina alpestris mat. Can. J. Bot. 1975, 53, 2614–2620. [Google Scholar] [CrossRef]
- Jonsson, A.V.; Moen, J.; Palmqvist, K. Predicting lichen hydration using biophysical models. Oecologia 2008, 156, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, K.A.; Rouse, W.R. Studies on lichen-dominated systems. I. The water relations of Cladonia alpestris in spruce-lichen woodland in northern Ontario. Can. J. Bot. 1971, 49, 1389–1399. [Google Scholar] [CrossRef]
- Kershaw, K.A.; Rouse, W.R. Studies on lichen dominated systems. II. The growth pattern of Cladonia alpestris and Cladonia rangiferina. Can. J. Bot. 1971, 49, 1401–1410. [Google Scholar] [CrossRef]
- Sulyma, R.; Coxson, D.S. Microsite displacement of terricolous lichens by feather moss mats in late seral Pine-lichen woodlands of north-central British Columbia. Bryologist 2001, 104, 505–516. [Google Scholar] [CrossRef]
- Ahti, T.; Oksanen, J. Epigeic lichen communities of taiga and tundra regions. Vegetatio 1990, 86, 39–70. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouaoui, S.; Boudreault, C.; Drapeau, P.; Bergeron, Y. Influence of Time since Fire and Micro-Habitat Availability on Terricolous Lichen Communities in Black Spruce (Picea mariana) Boreal Forests. Forests 2014, 5, 2793-2809. https://doi.org/10.3390/f5112793
Zouaoui S, Boudreault C, Drapeau P, Bergeron Y. Influence of Time since Fire and Micro-Habitat Availability on Terricolous Lichen Communities in Black Spruce (Picea mariana) Boreal Forests. Forests. 2014; 5(11):2793-2809. https://doi.org/10.3390/f5112793
Chicago/Turabian StyleZouaoui, Saliha, Catherine Boudreault, Pierre Drapeau, and Yves Bergeron. 2014. "Influence of Time since Fire and Micro-Habitat Availability on Terricolous Lichen Communities in Black Spruce (Picea mariana) Boreal Forests" Forests 5, no. 11: 2793-2809. https://doi.org/10.3390/f5112793
APA StyleZouaoui, S., Boudreault, C., Drapeau, P., & Bergeron, Y. (2014). Influence of Time since Fire and Micro-Habitat Availability on Terricolous Lichen Communities in Black Spruce (Picea mariana) Boreal Forests. Forests, 5(11), 2793-2809. https://doi.org/10.3390/f5112793




