Stability of Slash Pine Families with Major Gene and Partial Resistance to Single-Gall and Mixed-Gall Inocula of Cronartium quercuum fusiforme in Greenhouse Studies
Abstract
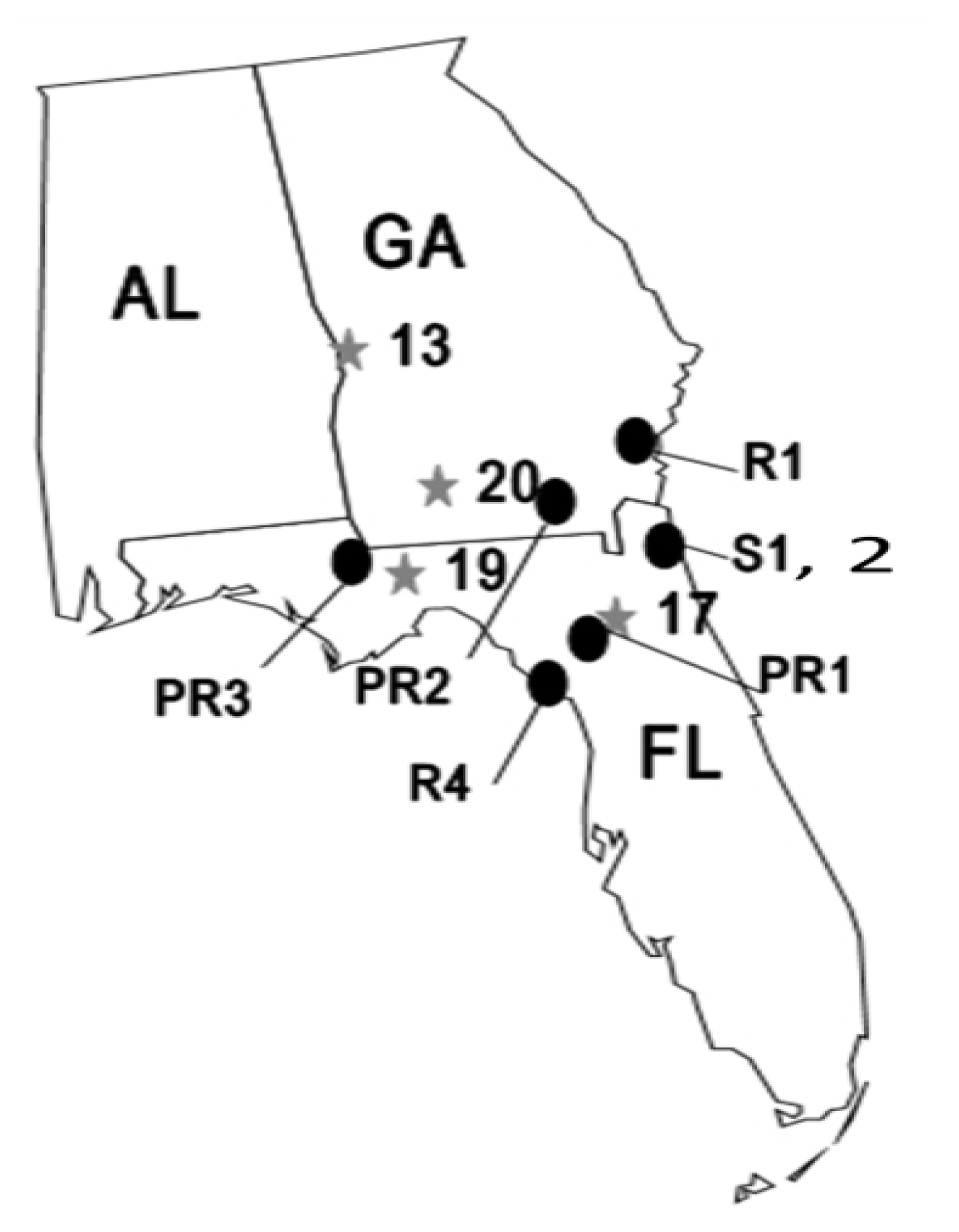
:1. Introduction
2. Methods and Materials
2.1. Methods Common to All Experiments
| Family c | Field progeny tests | ||||
|---|---|---|---|---|---|
| CFGRP a | FBRC b | ||||
| No. of tests d | R50 e | No. of OP tests | % f | ||
| OP | CP | ||||
| PR3 | 10 | 2 | −15.5 | -- | -- |
| PR1 | 26 | 16 | 04.4 | 48 | 15.5 |
| R1 | 17 | 3 | 04.8 | 39 | 20.0 |
| R4 | 22 | 13 | 19.9 | 48 | 22.3 |
| PR2 | 5 | 2 | 26.6 | 46 | 17.4 |
| S1 | 8 | 0 | 83.4 | 36 | 74.5 |
| S2 | 8 | 10 | 81.9 | 44 | 74.5 |
2.2. Temporal Stability
2.3. Spatial Stability
3. Results
3.1. Temporal Stability
| Test No. | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Inocula sources a | ||||
| Temporal stability | Spatial stability | |||
| Mixed-gall from sequential plantings | Family-specific and mixed galls from sequential plantings | Family-specific and location-specific, single-gall | Mixed-gall and family-specific single-gall | |
| No. of families | 5 | 5 | 4 | 6 |
| No. of inocula | 8 | 12 | 16 | 8 |
| ---------------------------------------------------Analysis of variance------------------------------------------------ | ||||
| Source of variation | Statistical significance b | |||
| Family (F) | *** | *** | *** | *** |
| Inocula (I) | NS | *** | * | *** |
| Family inocula (FI) | *** | NS | ||
| Location inocula (LI) | NS | |||
| Sequential inocula (SI) | NS | NS | ||
| F × I | ** | *** | ** | *** |
| F × FI | *** | NS | ||
| F × LI | NS | NS | ||
| F × SI | *** | NS | ||
| F1 × SI | NS | NS | ||
| L1 × SI | NS | |||
| F × LI × SI | NS | |||
| Inocula source a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13 c | 17 | 19 | 20 | Average | ||||||
| Family b | T1 d | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 |
| R1 | 39 e,f | 60 | 41 | 50 | 53 | 66 | 42 | 77 | 44Bc | 63Ad |
| R4 | 74 c | 71 | 84 | 67 | 72 | 79 | 69 | 85 | 74Ab f | 75Ac |
| PR1 | 91 | 87 | 86 | 78 | 91 | 90 | 87 | 96 | 89Aa | 88Aab |
| PR2 | 92 | 83 | 93 | 83 | 89 | 88 | 97 | 85 | 93Aa | 85Bb |
| S2 | 95 | 94 | 94 | 98 | 91 | 97 | 96 | 91 | 94Aa | 95Aa |
| Average | 78 | 79 | 80 | 75 | 79 | 84 | 79 | 87 | 79A | 81A |
| Family a | Interaction b | |
|---|---|---|
| F × LI b | F × LI × SI | |
| -----------------------------%----------------------------- | ||
| R1 | 51.4 | 49.6 |
| PR1 | 14.7 | 5.5 |
| S2 | 9.3 | 5.4 |
| R4 | 7.4 | 9.9 |
| PR2 | 4.1 | 22.3 |
| Inocula a | |||||
|---|---|---|---|---|---|
| Family specific | Mixed | ||||
| Family b | T1 | T2 | T1 | T2 | Average |
| R1 | 50A c,d | 44A | 51A | 46A | 48c |
| R4 | 68A | 78A | 78A | 77A | 76b |
| PR1 | 76A | 58B | 85A | 81A | 75b |
| PR2 | 81A | 76A | 82A | 85A | 81b |
| S2 | 80A | 87A | 96A | 94A | 89a |
| Average | 71A | 69A | 78A | 77A | 74 |
| Grand average | 70B | 78A | |||
3.2. Spatial Stability
| Family b | Interactions a | ||
|---|---|---|---|
| F × FI | F × LI | F × I | |
| --------------------------------------%---------------------------------------- | |||
| R1 | 34.6 | 47.9 | 50.4 |
| R4 | 08.9 | 38.3 | 23.9 |
| PR2 | 28.4 | 2.8 | 10.2 |
| S2 | 28.0 | 11.0 | 15.5 |

| Inocula source a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Family-specific single gall | Mixed-gall | ||||||||
| Family b | PR3 | PR2 | PR1 | R1 | R4 | 21-1 | 20-2 | 13-1 | Ave. |
| R1 | 57Bcd | 62ABc | 55Bc | 72Abc | 40Cc | 53Bd | 72Ab | 52Bc | 58e |
| PR1 | 65BCbc | 79Ab | 84Aa | 75ABb | 76Aab | 62Cc | 78Ab | 82Aab | 75c |
| PR2 | 85BCa | 91ABa | 89ABa | 87ABa | 85BCa | 89ABa | 96Aa | 91ABa | 89a |
| PR3 | 54Cd c,d | 63Bc | 71ABb | 64Bc | 68Bb | 75ABb | 80Ab | 78ABb | 69d |
| R4 | 73BCb | 78BCb | 71Cb | 80ABCab | 77BCab | 89Aa | 82ABb | 85ABab | 80b |
| Ave. (R & PR) | 67D | 75B | 74BC | 76B | 69CD | 74BC | 82A | 78AB | 74 |
| S1 | 87 | 96 | 96 | 97 | 98 | 97 | 98 | 97 | 96 |
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Griggs, M.M.; Schmidt, R.A. Increase and Spread of Fusiform Rust. In Proceedings of Management of Fusiform Rust in Southern Pines, Gainesville, FL, USA, 7–8 December 1977; pp. 32–38.
- Dinus, R.J. Knowledge about Natural Ecosystems as a Guide to Disease Control in Managed Forests. In Proceedings of American Phytopathology Society; American Phytopathological Society: Vancouver, Canada, 1974; pp. 184–190. [Google Scholar]
- Schmidt, R.A. Diseases in Forest Ecosystems: The Importance of Functional Diversity. In Plant Disease: An Advanced Treatise. Vol. II. How Disease Develops in Populations; Horsfall, J.G., Cowling, E.B., Eds.; Academic Press: New York, NY, USA, 1978; pp. 187–315. [Google Scholar]
- Pye, J.M.; Wagner, J.E.; Holmes, T.P.; Cubbage, F.W. Positive Returns from Investment in Fusiform Rust Research; U. S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 1997; p. 55.
- Schmidt, R.A. Fusiform rust of southern pines: A major success story for forest disease management. Phytopathology 2003, 93, 1048–1051. [Google Scholar] [CrossRef]
- Kuhlman, E.G. Frequency of single-gall isolates of Cronartium quercuum F. sp. fusiforme with virulence toward three resistant loblolly pine families. Phytopathology 1990, 80, 614–617. [Google Scholar] [CrossRef]
- Powers, H.R., Jr. The use of survivors of artificial inoculation tests in developing fusiform rust resistant seed orchards. Phytopathol. Medit. 1980, 19, 17–20. [Google Scholar]
- Snow, G.A.; Dinus, R.J.; Kais, A.G. Variation in pathogenicity of diverse sources of Cronartium fusiforme on selected slash pine families. Phytopathology 1975, 65, 170–175. [Google Scholar] [CrossRef]
- Snow, G.A.; Kais, A.G. Pathogenic variability in isolates of Cronartium fusiforme from five southern states. Phytopathology 1970, 60, 1730–1731. [Google Scholar] [CrossRef]
- Wilcox, P.L.; Amerson, H.V.; Kuhlman, E.G.; Liu, B.H.; O’Malley, D.M.; Sederoff, R.R. Detection of a Major Gene for Resistance to Fusiform Rust Disease in Loblolly Pine by Genomic Mapping. Proc. Natl. Acad. Sci. USA 1996, 93, 3859–3864. [Google Scholar]
- Nelson, C.D.; Doudrick, R.L.; Nance, W.L.; Hamaker, J.M.; Capo, B. Specificity of Host: Pathogen Genetic Interaction for Fusiform Rust Disease on Slash Pine. In Proceedings of the 22nd Southern Forest Tree Improvement Conference, Atlanta, GA, USA, 14–17 June 1993; pp. 403–410.
- Kong, X. RAPD Mapping and Its Application to Slash Pine Breeding. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 1996. [Google Scholar]
- Amerson, H.V. Personal communication, Department of Forestry, North Carolina State University: Raleigh, NC, USA, 2002.
- Knighten, J.L.; Young, C.H.; McCartney, T.C.; Anderson, R.L. Resistance Screening Center Procedures Manual: A Step-by-Step Guide Used in the Operational Screening of Southern Pines for Resistance to Fusiform Rust; U. S. Department of Agriculture Forest Service: Asheville, NC, USA, 1988; p. 62.
- Gramacho, K.P. Disease Resistance and Pathogenic Variability in the Fusiform Rust Slash Pine Pathosystem. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1999. [Google Scholar]
- Walkinshaw, C.H.; Dell, T.R.; Hubbard, S.D. Predicting Field Performance of Slash Pine Families from Inoculated Greenhouse Seedlings; Forest Experiment Station Research Paper SO-160U; U. S. Department of Agriculture Forest Service South: Asheville, NC, USA, 1980.
- Schmidt, R.A.; Goddard, R.E. Preliminary Results of Fusiform Rust Resistance from Field Progeny Tests of Selected Slash Pines. In Proceedings of the Eleventh Conference of Southern Forest Tree Improvement, Atlanta, GA, USA, 11–12 June 1971; pp. 37–44.
- Sohn, S.I.; Goddard, R.E.; Schmidt, R.A. Comparative Performances of Slash Pine for Fusiform Rust Resistance in High Rust Hazard Locations. In Proceedings of the Thirteenth Forest Tree Improvement Conference, Raleigh, NC, USA, 10–11 June 1975; pp. 204–211.
- Goddard, R.E.; Schmidt, R.A. Early Identification of Rust-Resistant Slash Pine through Controlled Inoculations. In Proceedings of the Eleventh Conference of Southern Forest Tree Improvement, Atlanta, GA, USA, 11–12 June 1971; pp. 31–36.
- Schmidt, R.A.; Gramacho, K.P.; Miller, T.; Young, C.H. Components of partial resistance in the slash pine—Fusiform rust pathosystem. Phytopathology 2000, 90, 1005–1010. [Google Scholar] [CrossRef]
- Isik, F.; Amerson, H.V.; Whetten, R.W.; Garcia, S.A.; McKeand, S.E. Interaction of Fr genes and mixed-pathogen inocula in the loblolly pine-fusiform rust pathosystem. Tree Genet. Genomes 2012, 8, 15–25. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gramacho, K.P.; Schmidt, R.A.; Miller, T. Stability of Slash Pine Families with Major Gene and Partial Resistance to Single-Gall and Mixed-Gall Inocula of Cronartium quercuum fusiforme in Greenhouse Studies. Forests 2013, 4, 488-499. https://doi.org/10.3390/f4020488
Gramacho KP, Schmidt RA, Miller T. Stability of Slash Pine Families with Major Gene and Partial Resistance to Single-Gall and Mixed-Gall Inocula of Cronartium quercuum fusiforme in Greenhouse Studies. Forests. 2013; 4(2):488-499. https://doi.org/10.3390/f4020488
Chicago/Turabian StyleGramacho, Karina P., Robert A. Schmidt, and Thomas Miller. 2013. "Stability of Slash Pine Families with Major Gene and Partial Resistance to Single-Gall and Mixed-Gall Inocula of Cronartium quercuum fusiforme in Greenhouse Studies" Forests 4, no. 2: 488-499. https://doi.org/10.3390/f4020488




