Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs
Abstract
:1. Introduction
1.1. Background
1.2. The Analogue Method
1.3. Research Objectives
2. Experimental Section
2.1. Camcore’s International Provenance Trial Data
2.2. Study Species’ Description
2.3. The Study Area

2.4. Climate Data
- a homogeneous world with rapid economic growth that relies on a balance of all energy sources (emission scenario A1b);
- a more divided world with focus on regionally oriented economic development and slower and more fragmented technological changes (emission scenario A2);
- an integrated world that is more ecologically friendly and emphasizes global solutions to achieve economic, social and environmental stability (emission scenario B1) [3].
| GCM | Developed by | Institute-/Model Name |
|---|---|---|
| BCCR-BCM 2.0 | Bjerknes Centre for Climate Research | University of Bergen, Norway |
| CNRM-CM 3 | Centre Européen de Recherche et de Formation Avancée en Calcul Scientifique | Centre National de la Recherche Scientifique |
| GFDL CM 2.0 and 2.1 | Geophysical Fluid Dynamics Laboratory | National Oceanic and Atmospheric Administration |
| GISS Model ER and AOM | Goddard Institute for Space Studies | National Aeronautics and Space Administration |
| IPSL CM 4 | Institute Pierre Simon Laplace | Centre National de la Recherche Scientifique |
| MIUB ECHO-G | Meteorologisches Institut der Universität Bonn | Friedrich-Wilhelms Universität Bonn |
| NCAR CCM 3.0 | National Center for Atmospheric Research | Community Climate System Model |
| CCCMA CGCM 3.1 t46 and t63 | Canadian Centre for Climate Modelling and Analysis | Meteorological Service of Canada |
| CSIRO Mk 3.0 and 3.5 | Commonwealth Scientific and Industrial Research Organisation | Marine and Atmospheric Research Laboratories |
| IAP FGOALS g1.0 | Institute of Atmospheric Physics | Chinese Academy of Sciences |
| MIROC 3.2 Hires and Medres | Centre for Climate System Research, Tokyo | Model for Interdisciplinary Research On Climate |
| MPI ECHAM 5 | Max Planck Institute for Meteorology | European Centre Hamburg Model |
| UKMO HADCM 3 | United Kingdom Meteorological Office | Hadley Centre Climate Model |
| INM CM 3.0 | Institute of Numerical Mathematics | Russian Academy of Science |
| MRI CGCM 2.3 2a | Meteorological Research Institute, Japan | Coupled General Circulation Model |
2.5. Data Preparation
| Id Number | Provenance Name | Species | Subpopulation/Variety | Country, State of Origin | No of Trials |
|---|---|---|---|---|---|
| 1 | Jocón | P. tecunumanii | low elevation | Honduras, Yoro | 9 |
| 2 | Campamento | P. tecunumanii | low elevation | Honduras, Olancho | 7 |
| 3 | Chempil | P. tecunumanii | high elevation | Mexico, Chiapas | 6 |
| 4 | San Jerónimo | P. tecunumanii | high elevation | Guatemala, Baja Verapaz | 9 |
| 5 | Mountain Pine Ridge | P. tecunumanii | low elevation | Belize, Cayo | 6 |
| 6 | Sierra Huayacocotla | P. patula | Patula | Mexico, Veracruz | 9 |
| 7 | Potrero de Monroy | P. patula | Patula | Mexico, Veracruz | 22 |
| 8 | Chanal | P. tecunumanii | high elevation | Mexico, Chiapas | 11 |
| 9 | El Cielo | P. patula | Patula | Mexico, Tamaulipas | 7 |
| 10 | Conrado Castillo | P. patula | Patula | Mexico, Tamaulipas | 14 |
2.6. The Analog Methods
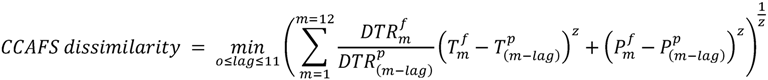
2.6.1. CCAFS Dissimilarity, Hereafter Method A
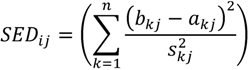
2.6.2. CCAFS with Stepwise Selected BIOCLIM Variables, Hereafter Method B
2.7. Evaluating Dissimilarities in Climate as a Provenance Performance Indicator

2.8. Spatialization of Results and Growth Predictions
3. Results
3.1. Definition of Study Area
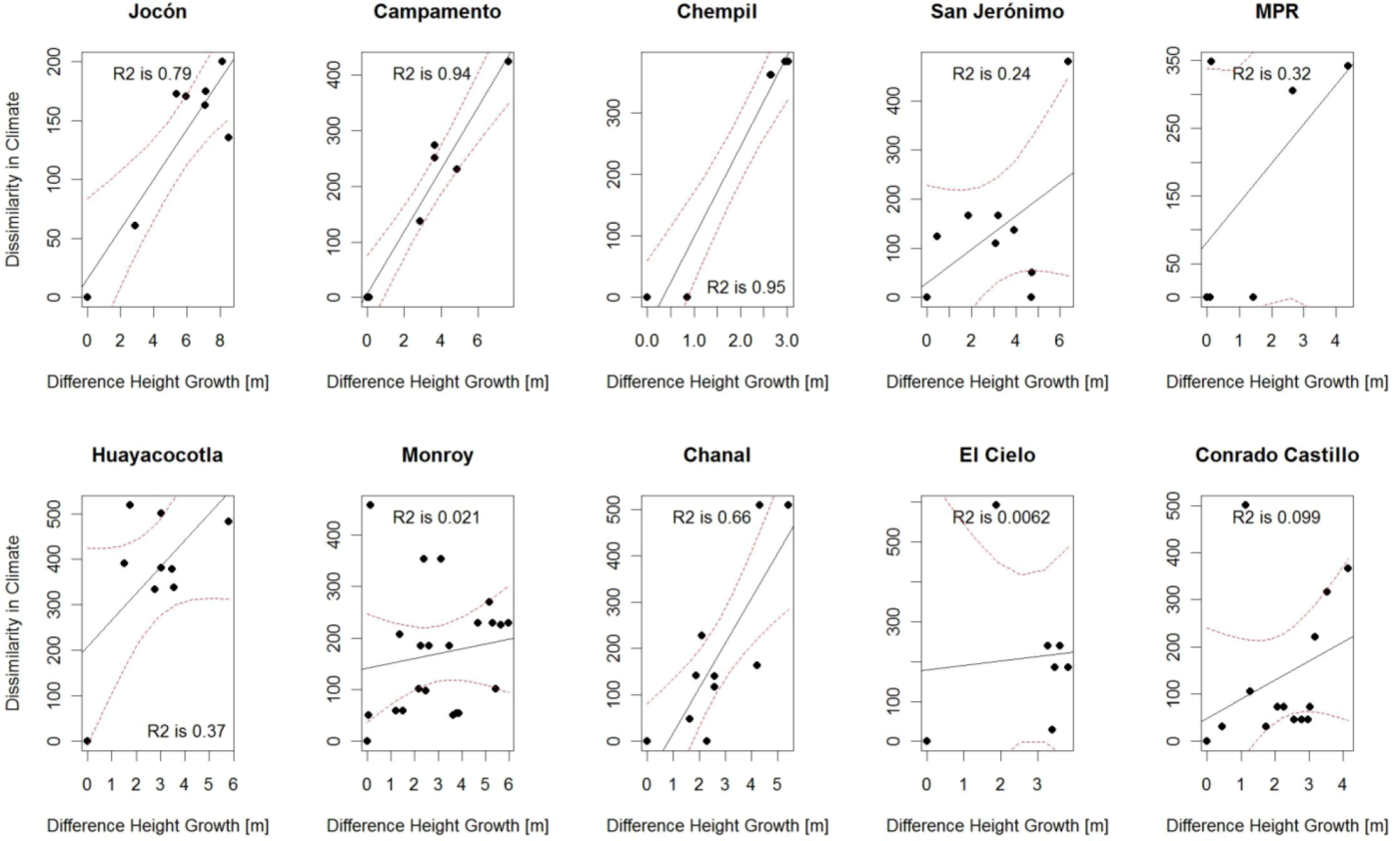
3.2. Comparison of Analog Methods in Their Ability to Predict Provenance Performance
| Pinus tecunumanii provenances | Pinus patula provenances | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 8 | Average1R2 | 6 | 7 | 9 | 10 | Average2R2 | |
| CCAFS | 0.79 ** | 0.94 *** | 0.95 *** | 0.44 . | 0.32 | 0.66 ** | 0.68 | 0.37 . | 0.02 | 0.01 | 0.10 | 0.12 |
| BIOCLIM | 0.53* | 0.36 | 0.95 ** | 0.25 | 0.77 * | 0.70** | 0.59 | 0.02 | 0.03 | 0.19 | 0.37 * | 0.15 |

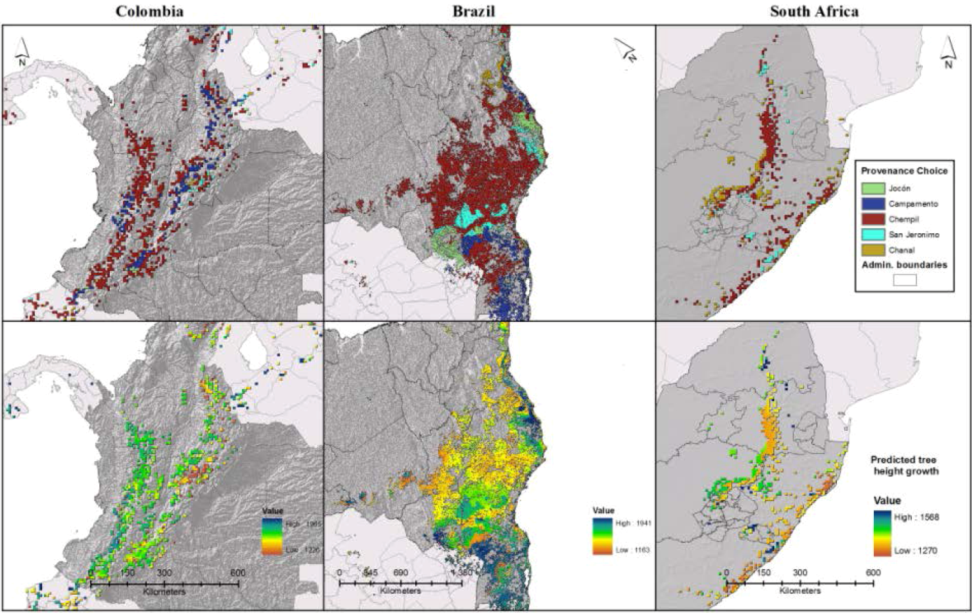
3.3. Expected Impact of Climate Change on Wood Productivity and Choice of Seed Material
| Current Climate | Change by 2030 | Change by 2050 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Provenance | Colombia | Brazil | South Africa | Colombia | Brazil | South Africa | Colombia | Brazil | South Africa |
| Jocon ** | 1.94 | 6.96 | 0.07 | −1.19 | −0.89 | - | −1.38 | −0.67 | - |
| Campamento *** | 21.07 | 14.02 | 0.04 | 1.00 | 2.31 | - | 2.19 | 4.02 | - |
| Chempil *** | 74.61 | 69.08 | 58.23 | 0.16 | −0.74 | −2.23 | −1.50 | −2.16 | −9.63 |
| San Jeronimo . | 1.57 | 7.41 | 10.50 | −0.66 | −0.01 | 3.08 | −0.60 | −0.62 | 4.45 |
| Huay . | - | - | - | - | - | - | - | - | - |
| Chanal ** | 0.82 | 2.54 | 31.16 | 0.69 | −0.67 | −0.74 | 1.29 | −0.58 | 5.29 |
| Colombia | Brazil | South Africa | |
|---|---|---|---|
| current climate [m] | 15.27 | 13.62 | 13.44 |
| change by 2030 [Δm] | −0.19 | −0.07 | −0.12 |
| change by 2050 [Δm] | −0.35 | −0.04 | 0.02 |
3.4. Influence of Socioeconomic Scenarios on the Prediction’s Outcome
3.5. Available Viable Seed Choices

3.6. Uncertainty in Climate Change Projections
4. Discussion
4.1. Climate Dissimilarity as an Estimator for Provenance Performance
4.2. “Analogues’” Performance in Relation to Species’ Stability across Environments
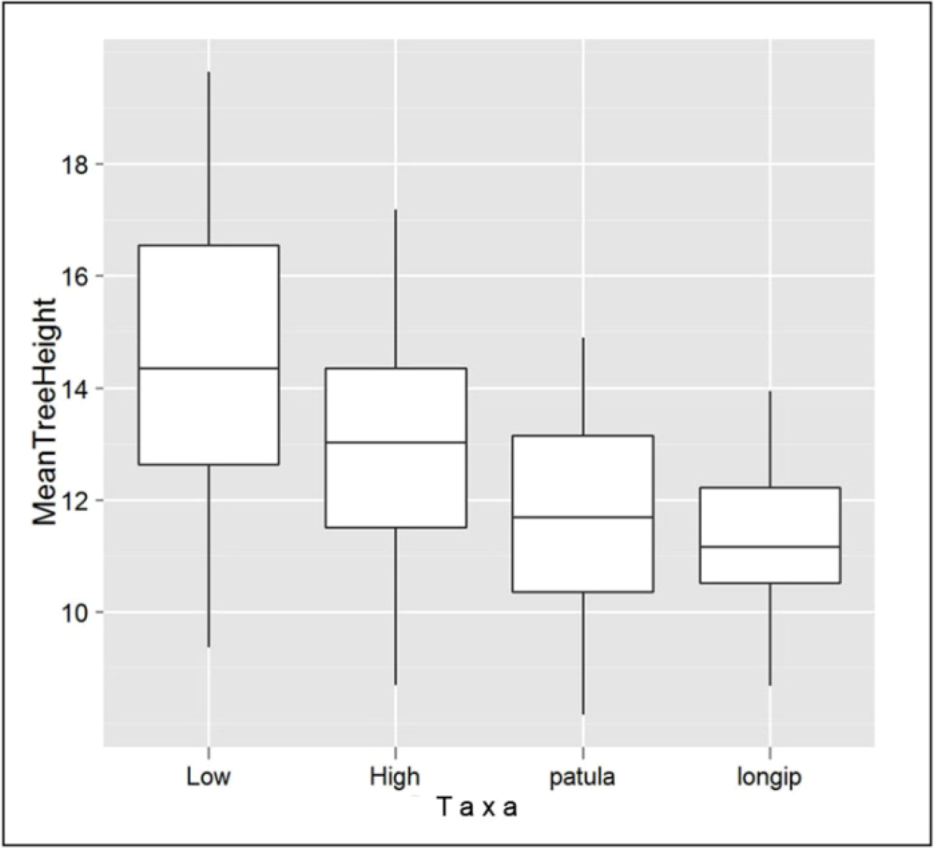
4.3. Implications for Plantation Forestry
Projection’s Uncertainties
5. Conclusions
Competing Interests
Acknowledgements
References
- IPCC, Climate Change 2007: Synthesis Report; Cambridge University Press: New York, NY, USA, 2007.
- McKenney, D.; Pedlar, J.; O’Neill, G. Climate change and forest seed zones: Past trends, future prospects and challenges to ponder. For. Chron. 2009, 85, 258–266. [Google Scholar]
- Fairbanks, D. South African Country Study on Climate Change: Vulnerability and Adaptation Assessment for Plantation Forestry; National Research Foundation: Pretoria, South Africa, 1999. [Google Scholar]
- Spittlehouse, D.L.; Stewart, R.G. Adaptation to climate change in forest management. BC J. Ecosyst. Manag. 2003, 4, 1–11. [Google Scholar]
- Maginnis, S.; Jackson, W. The Role of Planted Forests in Forest Landscape Restoration. In Proceedings of the UNFF Intersessional Experts Meeting on the Role of Planted Forests in Sustainable Forest Management, Christchurch, New Zealand, 25–27 March 2003; pp. 87–99.
- Carle, J.; Holmgren, P. Wood from planted forests: A global outlook 2005–2030. For. Prod. J. 2008, 58, 6–18. [Google Scholar]
- FRA, Global Forest Resources Assessment 2010; Forestry Paper for FAO: Rome, Italy, 2010; p. 136.
- IPCC, Land Use, Land Use Change and Forestry; Cambridge University Press: Cambridge, UK, 2000.
- Guariguata, M.R.; Cornelius, J.P.; Locatelli, B.; Forner, C.; Sánchez-Azofeifa, G.A. Mitigation needs adaptation: Tropical forestry and climate change. Mitig. Adapt. Strategy Glob. Chang. 2008, 13, 793–808. [Google Scholar] [CrossRef]
- Dvorak, W.S.; Hodge, G.R.; Romero, J.L. Results of twenty years of research on Pinus tecunumanii by the CAMCORE Cooperative. For. Genet. Resour. 2001, 29, 2–6. [Google Scholar]
- Glantz, M.H. Societal Responses to Climate Change: Forecasting by Analogy; Westview Press: Boulder, CO, USA, 1988. [Google Scholar]
- Glantz, M.H. Does history have a future—Forecasting climate change effects on fisheries by analogy. Fisheries 1990, 15, 39–44. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef]
- Hallegatte, S.; Hourcade, J.C.; Ambrosi, P. Using climate analogues for assessing climate change economic impacts in urban areas. Clim. Chang. 2007, 82, 47–60. [Google Scholar] [CrossRef]
- Ford, J.D.; Keskitalo, E.C.H.; Smith, T.; Pearce, T.; Berrang-Ford, L.; Duerden, F.; Smit, B. Case study and analogue methodologies in climate change vulnerability research. Wiley Interdiscip. Rev. 2010, 3, 374–392. [Google Scholar]
- Robertson, C.C. A Reconnaissance of The Forest Trees of Australia from the Point of View of Their Cultivation in South Africa; South African Government Printer: Capetown, South Africa, 1926. [Google Scholar]
- Golfari, L.; Caser, R.L.; Moura, V.P.G. Zoneamento ecologico esquematico para reflorestamento no Brasil. Série Técnica 1978, 11, 1–66. [Google Scholar]
- Booth, T.H. A new method for assisting species selection. Commonw. For. Rev. 1985, 64, 241–250. [Google Scholar]
- Dvorak, W.S.; Donahue, J.H.; Vásquez, J.A. Early performance of CAMCORE introductions of Pinus patula in Brazil, Colombia and South Africa. S. Afr. For. J. 1995, 174, 23–33. [Google Scholar]
- Dvorak, W.S.; Jordan, A.P.; Romero, J.L.; Hodge, G.R.; Furman, B.J. Quantifying the geographic range of Pinus patula var longipedunculata in southern Mexico using morphologic and RAPD marker data. S. Afr. For. J. 2001, 192, 19–30. [Google Scholar]
- Hijmans, R.J.; Schreuder, M.; de la Cruz, J.; Guarino, L. Using GIS to check coordinates of genebank accessions. Genet. Resour. Crop Evol. 1999, 46, 291–296. [Google Scholar] [CrossRef]
- Birks, J.S.; Barnes, R.D. The Genetic Control of Wood Quality in Pinus patula; ODA Research Scheme R4616; Oxford Forestry Institute, University of Oxford: Oxford, UK, 1991. [Google Scholar]
- Hodge, G.R.; Dvorak, W.S. Growth potential and genetic parameters of four mesoamerican pine planted in the southern hemisphere. South. For. 2012, 74. in press. [Google Scholar]
- Dvorak, W.S.; Hodge, G.R.; Gutiérrez, E.A.; Osorio, L.F.; Malan, F.S.; Stanger, T.K. Pinus tecunumanii. In Conservation and Testing of Tropical and Subtropical Forest Tree Species; CAMCORE Cooperative: Raleigh, NC, USA, 2000; pp. 188–209. [Google Scholar]
- Dvorak, W.S.; Balocchi, C.E.; Raymond, R.H. Performance and Stability of Provenances and Families of Pinus tecunumanii in the Tropics and Subtropics. In Breeding Tropical Trees: Population Structure And Genetic Improvement Strategies In Clonal And Seedling Forestry; Gibson, G.L., Griffin, A.R., Matheson, A.C., Eds.; Oxford Forestry Institute: Oxford, UK, 1989; pp. 187–196. [Google Scholar]
- Dvorak, W.S.; Potter, K.M.; Hipkins, V.D.; Hodge, G.R. Genetic diversity and gene exchange in Pinus oocarpa, a Mesoamerican pine with resistance to the pitch canker fungus (Fusarium circinatum). Int. J. Plant Sci. 2009, 170, 609–626. [Google Scholar] [CrossRef]
- Kanzler, A. Genotype × environment interaction in Pinus patula and its implications in South Africa. PhD thesis, Department of Forestry, North Carolina State University, Raleigh, NC, USA, 2010. [Google Scholar]
- Louw, J.H. Site Classification and Evaluation for Commercial Forestry in the Crocodile River Catchment; University of Stellenbosch: Stellenbosch, South Africa, 1995. [Google Scholar]
- IUCN and UNEP, The World Database on Protected Areas (WDPA); UNEP-WCMC: Cambridge, UK, 2009.
- Eva, H.D.; de Miranda, E.E.; di Bella, C.M.; Gond, V.; Sgrenzaroli, O.M.; Jones, S.; Coutinho, A.; Dorado, A.; Guimarães, M.; Elvidge, C.; et al. A vegetation map of South America, European Commission; EUR 20159 EN; Joint Research Centre: Brussels, Belgium, 2002. [Google Scholar]
- Mayaux, P.; Bartholomé, E.; Massart, M.; van Cutsem, C.; Cabral, A.; Nonguierma, A. A Land-Cover Map of Africa; EUR 20665 EN; Office for Official Publications of the European Communities: Luxembourg, Luxembourg, 2003. [Google Scholar]
- Bartholomé, E.; Belward, A.S.; Achard, F.; Bartalev, S.; Carmona Moreno, C.; Eva, H.; Fritz, S.; Grégoire, J.M.; Mayaux, P.; Stibig, H.J. Global Land Cover Mapping for the Year 2000—Project Status November 2002; ref EUR 20524; JRC: Ispra, Italy, 2003. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM-A Bioclimatic Analysis and Prediction System. In Nature Conservation: Cost Effective Biological Surveys and Data Analysis; Margules, C.R., Austin, M.P., Eds.; CSIRO: Canberra, Australia, 1991; pp. 64–68. [Google Scholar]
- R Development Core Team: R. A Language and Environment for Statistical Computing, Version 2.13.0; R Foundation for Statistical Computing: Vienna, Austria, 2011. Available online: http://www.R-project.org/ (accessed on 6 May 2011).
- Arc 9.3 Arc/Info; Environmental Systems Research Institute: Redlands, CA, USA, 2009.
- Arnell, N.A.; Osborne, T.; Hiiker, J. Using Agriculturally Meaningful Measures of Climatic Similarity to Identify Present and Future Analogue Climates; Report to CCAFS: Development of Prototype Climate Analogue Tool 2011; Walker Institute for Climate System Research, University of Reading: West Berkshire, UK, 2011. [Google Scholar]
- Huston, M. Soil nutrients and tree species richness in Costa Rican forests. J. Biogeogr. 1980, 7, 147–157. [Google Scholar] [CrossRef]
- Dise, N.B.; Wright, R.F. Nitrogen leaching from European forests in relation to nitrogen deposition. For. Ecol. Manag. 2000, 71, 153–161. [Google Scholar] [CrossRef]
- Yang, Y.; Watanabe, M.; Li, F.; Zhang, J.; Zhang, W.; Zhai, J. Factors affecting forest growth and possible effects of climate change in the Taihang Mountains, northern China. Forestry 2006, 79, 135–147. [Google Scholar]
- Bedrick, E.J. Checking for lack of fit in linear models with parametric variance functions. Technometrics 2000, 42, 227–236. [Google Scholar] [CrossRef]
- Mac Nally, R. Regression and model-building in conservation biology, biogeography and ecology: The distinction between—and reconciliation of—‘predictive’ and ‘explanatory’ models. Biodivers. Conserv. 2000, 9, 665–671. [Google Scholar] [CrossRef]
- O’Brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- PCMDI, IPCC Model Output 2007. Available online: http://www-pcmdi.llnl.gov/ipcc/about_ipcc.php (accessed on 20 June 2011).
- IPCC Special Report on Emissions Scenarios (SRES); Nakicenovic, N.; Swart, R. (Eds.) Cambridge University Press: Cambridge, UK, 2000.
- Lugo, A.E.; Brown, S.; Chapman, J. An analytical review of production rates and stemwood biomass of tropical forest plantations. For. Ecol. Manag. 1988, 23, 189–200. [Google Scholar]
- Mearns, L.O.; Rosenzweig, C.; Goldberg, R. Mean and variance change in climate scenarios: Methods, agricultural applications, and measures of uncertainty. Clim. Chang. 1997, 34, 367–396. [Google Scholar]
- Whittingham, M.J.; Stephens, P.A.; Bradbury, R.B.; Freckleton, R.P. Why do we still use stepwise modeling in ecology and behaviour? J. Anim. Ecol. 2006, 75, 1182–1189. [Google Scholar] [CrossRef]
- Rencher, A.C.; Pun, F.C. Inflation of R2 in best subset regression. Technometrics 1980, 22, 49–53. [Google Scholar] [CrossRef]
- Gonzalez, R.W.; Salón, D.; Dasmohaptra, S.; Cubbage, F. South America industrial roundwood supply potential. BioResources 2008, 3, 255–269. [Google Scholar]
- Matalaa, J.; Ojansuu, R.; Peltola, H.; Sievänen, R.; Kellomäki, S. Introducing effects of temperature and CO2 elevation on tree growth into a statistical growth and yield model. Ecol. Model. 2005, 181, 173–190. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Tchebakova, N.M.; Parfenova, Y.I.; Wykoff, W.R.; Kuzmina, N.A.; Milyutin, L.I. Intraspecific responses to climate in Pinus sylvestris. Glob. Chang. Biol. 2002, 8, 912–929. [Google Scholar] [CrossRef]
- Fairbanks, D. South African Country Study on Climate Change: Vulnerability and Adaptation Assessment for Plantation Forestry; National Research Foundation: Pretoria, South Africa, 1999. [Google Scholar]
- Rowhani, P.; Lobell, D.B.; Linderman, M.; Ramankutty, N. Climate variability and crop production in Tanzania. Agric. For. Meteorol. 2001, 151, 449–460. [Google Scholar]
- Brook, J.R.; Flanagan, L.B.; Ehleringer, J.R. Responses of boreal conifers to climate fluctuations: Indications from tree-ring widths and carbon isotope analyses. Can. J. For. Res. 1998, 29, 524–533. [Google Scholar]
- Clark, D.A.; Clark, D.B. Climate induces annual variation in canopy tree growth in a Costa Rican tropical rain forest. J. Ecol. 1994, 82, 865–872. [Google Scholar] [CrossRef]
- Mátyás, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- William, R.L.; Barner, H. Matching Seed Source to Planting Site; Lecture Note B.3; Danida Forest Seed Centre: Humlebaek, Denmark, 1993. [Google Scholar]
- Finley, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding program. Aust. J. Agric. Res. 1963, 14, 342–354. [Google Scholar]
- Lortie, C.J.; Aarssen, L.W. The specialization hypothesis for phenotypic plasticity in plants. Int. J. Plant Sci. 1996, 15, 484–487. [Google Scholar]
- Via, S.; Gomulkiewicz, R.; de Jong, G.; Scheiner, S.M.; Schlichting, C.D. Adaptive phenotypic plasticity: Consensus and controversy. Trends Ecol. Evol. 1995, 10, 212–217. [Google Scholar] [CrossRef]
- Emery, R.J.N.; Chinnappa, C.C.; Chmielewski, J.G. Specialization, plant strategies, and phenotypic plasticity in populations of Stellaria longipes along an elevational gradient. Int. J. Plant Sci. 1994, 155, 203–219. [Google Scholar]
- Hodge, G.R.; Dvorak, W.S. Genetic parameters and provenance variation of Pinus tecunumanii in 78 international trials. For. Genet. 1999, 6, 157–180. [Google Scholar]
- Rosvall, O.; Ericsson, T. Förtlyttningseffekter I norrländska gran-proveniensförsörk. Föreningen Skogrsträds-förädling; Institutet for Skogsförbättring: Årsbok, Sweden, 1981. [Google Scholar]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- FAO, Adaptation to Climate Change in Agriculture, Forestry and Fisheries: Perspective, Framework and Priorities; Food Agricultural Organization of the United Nations: Rome, Italy, 2007; p. 2.
- Mitchell, R.G.; Steenkamp, E.T.; Coutinho, T.A.; Wingfield, M.J. The pitch canker fungus, Fusarium circinatum: Implications for South African forestry. South. For. 2011, 73, 1–13. [Google Scholar]
- Wilby, R.; Dawson, C. SDSM 4.1–A Decision Support Tool for the Assessment of Regional Climate Change Impacts; User Manual: London, UK, 2007; p. 93. [Google Scholar]
- Stott, P.A.; Kettleborough, J.A. Origins and estimates of uncertainty in predictions of twenty-first century temperature rise. Nature 2002, 416, 723–726. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leibing, C.; Signer, J.; Van Zonneveld, M.; Jarvis, A.; Dvorak, W. Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs. Forests 2013, 4, 155-178. https://doi.org/10.3390/f4010155
Leibing C, Signer J, Van Zonneveld M, Jarvis A, Dvorak W. Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs. Forests. 2013; 4(1):155-178. https://doi.org/10.3390/f4010155
Chicago/Turabian StyleLeibing, Christoph, Johannes Signer, Maarten Van Zonneveld, Andrew Jarvis, and William Dvorak. 2013. "Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs" Forests 4, no. 1: 155-178. https://doi.org/10.3390/f4010155
APA StyleLeibing, C., Signer, J., Van Zonneveld, M., Jarvis, A., & Dvorak, W. (2013). Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs. Forests, 4(1), 155-178. https://doi.org/10.3390/f4010155




