DAYCENT Simulations to Test the Influence of Fire Regime and Fire Suppression on Trace Gas Fluxes and Nitrogen Biogeochemistry of Colorado Forests
Abstract
:1. Introduction
- Evaluate the short-term impact of fire on trace gas fluxes and N biogeochemistry from burned landscapes.
- Examine trace gas fluxes and N biogeochemistry in response to hypothetical fire regimes among locations and pre-/post- fire suppression.
2. Experimental Section
2.1. Model Description
2.2. Study Sites
2.2.1. Fire History and Climate Data
| Location | |||||
|---|---|---|---|---|---|
| Boulder | Cheesman | Allenspark | Fort Collins | ||
| Station name | Boulder | Cheesman Reservoir | Allenspark | Fort Collins | |
| Station ID | 50,848 | 51,528 | 50,183 | 53,005 | 53,005 |
| County | Boulder | Jefferson | Boulder | Larimer | Larimer |
| Years of record | 1898–2006 | 1903–2006 | 1948–1993 | 1994–2006 | 1900–2006 |
| Data years | 108 | 103 | 45 | 13 | 106 |
| Latitude (°N) | 40° 0' | 39° 13' | 40° 12' | 40° 37' | |
| Longitude (°W) | 105° 16' | 105° 17' | 105° 32' | 105° 08' | |
| Elevation (m) | 1,671 | 2,097 | 2,606 | 1,525 | |
| MAP (cm) | 48.6 | 41.2 | 52.8 | 38.4 | |
| MAT (°C) | 11 | 7.2 | 4.7 | 8.9 | |
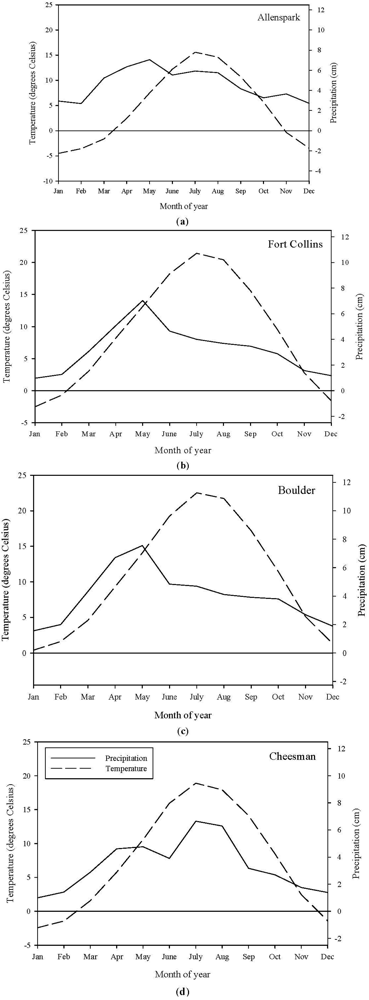
| Location | Modeled Period | Fire Return Interval (Years) or Fire Date (Year) | Source |
|---|---|---|---|
| Cheesman | 1–1285 | 9 | Brown et al. [3] |
| 1286–1500 | 12 | ||
| 1501–1600 | 6 | ||
| 1601–1715 | 10 | ||
| 1715–2000 | 1723, 1851 (no further fires) | ||
| Boulder | 1–1650 | 14 | Veblen et al. [18] |
| 1651–1920 | 1679,1691,1703,1708,1713,1716, 1722, 1725, 1732, 1737, 1747, 1786, 1789,1795, 1813, 1841, 1847, 1851, 1860, 1868, 1870, 1880, 1884, 1886, 1908, 1910 | Sites 15 (1853–1914 m, >10% of trees scarred) | |
| 1921–2000 | Fire suppression | ||
| Allenspark | 1–1500 | 40 | Veblen et al. [18] |
| 1501–1920 | 1541, 1602, 1654, 1745, 1768, 1814, 1859, 1880 | Sites 18, 19 (2414–2682 m, >10% of trees scarred) | |
| 1921–2000 | Fire suppression | ||
| Fort Collins | 1–19201921–2000 | 30Fire suppression | Sherrif and Veblen [20] Brown and Shepperd [19] |
2.2.2. Study Sites-Vegetation and Soils
| Layer thickness (cm) | Bulk density (mg cm3) | Sand and clay (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Allenspark | Boulder | Cheesman | Fort Collins | Allenspark | Boulder | Cheesman | Fort Collins | |
| 0 to 2 | 1.5 | 1.33 | 1.33 | 1.25 | 0.80, 0.13 | 0.74, 0.11 | 0.71, 0.21 | 0.70, 0.20 |
| 2 to 5 | 1.5 | 1.33 | 1.5 | 1.25 | 0.80, 0.13 | 0.74, 0.11 | 0.75, 0.15 | 0.70, 0.20 |
| 5 to 10 | 1.5 | 1.33 | 1.5 | 1.35 | 0.80, 0.13 | 0.74, 0.11 | 0.75, 0.15 | 0.70, 0.20 |
| 10 to 20 | 1.5 | 1.33 | 1.5 | 1.35 | 0.80, 0.13 | 0.74, 0.11 | 0.75, 0.15 | 0.70, 0.20 |
| 20 to 30 | 1.5 | 1.33 | 1.6 | 1.5 | 0.80, 0.13 | 0.74, 0.11 | 0.80, 0.10 | 0.70, 0.20 |
| 30 to 45 | 1.5 | 1.33 | 1.6 | 1.5 | 0.80, 0.13 | 0.74, 0.11 | 0.80, 0.10 | 0.75, 0.15 |
| 45 to 60 | 1.5 | 1.33 | 1.6 | 1.7 | 0.80, 0.13 | 0.74, 0.11 | 0.80, 0.10 | 0.75, 0.15 |
| 60 to 75 | 1.5 | 1.33 | - | 1.7 | 0.80, 0.13 | 0.74, 0.11 | - | 0.75, 0.15 |
| 75 to 90 | 1.5 | 1.33 | - | 1.7 | 0.80, 0.13 | 0.74, 0.11 | - | 0.75, 0.15 |
| 90 to 105 | 1.5 | 1.33 | - | - | 0.80, 0.13 | 0.74, 0.11 | - | - |
| 105 to 120 | 1.5 | 1.33 | - | - | 0.80, 0.13 | 0.74, 0.11 | - | - |
| 120 to 150 | 1.5 | 1.33 | - | - | 0.80, 0.13 | 0.74, 0.11 | - | - |
2.3. Model Parameterization
2.3.1. Simulation Procedure
| Surface Fire | Canopy Fire | Parameter | Definition |
|---|---|---|---|
| 1 | 1 | EVNTYP | event type flag (=0 for cutting event, =1 for fire event) |
| 0.5 | 0.99 | REMF (1) | fraction of leaf component removed |
| 0.5 | 0.90 | REMF (2) | fraction of live branch component removed |
| 0.2 | 0.90 | REMF (3) | fraction of large wood live component removed |
| 0.8 | 0.99 | REMF (4) | fraction of fine branch dead component removed |
| 0.4 | 0.99 | REMF (5) | fraction of large wood dead component removed |
| 0.3 | 0.99 | FD (1) | fraction of fine root component that dies |
| 0.1 | 0.99 | FD (2) | fraction of coarse root component that dies |
| 0.5 | 0 | RETF (1,1) | fraction of C in killed live leaves that is returned to the system (ash or litter) |
| 0.5 | 0.3 | RETF (1,2) | fraction of N in killed live leaves that is returned to the system (ash or litter) |
| 1 | 1 | RETF (1,3) | fraction of P in killed live leaves that is returned to the system (ash or litter) |
| 0 | 0 | RETF(1,4) | fraction of S in killed live leaves that is returned to the system (ash or litter) |
| 0.5 | 0 | RETF(2,1) | fraction of C in killed fine branches that is returned to the system (ash or dead fine branches) |
| 0.5 | 0.3 | RETF (2,2) | fraction of N in killed fine branches that is returned to the system (ash or dead fine branches) |
| 1 | 1 | RETF (2,3) | fraction of P in killed fine branches that is returned to the system (ash or dead fine branches) |
| 0 | 0 | RETF (2,4) | fraction of S in killed fine branches that is returned to the system (ash or dead fine branches) |
| 0.3 | 0 | RETF (3,1) | fraction of C in killed large wood that is returned to the system (ash or dead large wood) |
| 0.3 | 0.3 | RETF (3,2) | fraction of N in killed large wood that is returned to the system (ash or dead large wood) |
| 1 | 1 | RETF (3,3) | fraction of P in killed large wood that is returned to the system (ash or dead large wood) |
| 0 | 0 | RETF (3,4) | fraction of S in killed large wood that is returned to the system (ash or dead large wood) |
| Fire Severity | Parameter | Definition | |
|---|---|---|---|
| Low | High | ||
| 0.6 | 0.8 | FLFREM | fraction of live shoots removed by a fire event |
| 0.6 | 0.8 | FDFREM(1) | fraction of standing dead plant material removed by a fire event |
| 0.2 | 0.9 | FDFREM(2) | fraction of surface litter removed by a fire event |
| 0.6 | 0.9 | FDFREM(3) | fraction of dead fine branches removed by a fire event |
| 0.4 | 0.9 | FDFREM(4) | fraction of dead large wood removed by a fire event |
| 0.1 | 0.01 | FRET(1,1) | fraction of C in the burned aboveground material (live shoots, standing dead, and litter) returned to the system following a fire event |
| 0.4 | 0.4 | FRET(1,2) | fraction of N in the burned aboveground material (live shoots, standing dead, and litter) returned to the system following a fire event |
| 1 | 0.4 | FRET(1,3) | fraction of P in the burned aboveground material (live shoots, standing dead, and litter) returned to the system following a fire event |
| 1 | 0.4 | FRET(1,4) | fraction of S in the burned aboveground material (live shoots, standing dead, and litter) returned to the system following a fire event |
| 0.003 | 0.003 | FRET(2,1) | fraction of C in the burned dead fine branch material returned to the system following a fire event |
| 0.2 | 0.2 | FRET(2,2) | fraction of N in the burned dead fine branch material returned to the system following a fire event |
| 0 | 0.4 | FRET(2,3) | fraction of P in the burned dead fine branch material returned to the system following a fire event |
| 0 | 0.4 | FRET(2,4) | fraction of S in the burned dead fine branch material returned to the system following a fire event |
| 0.003 | 0.003 | FRET(3,1) | fraction of C in the burned dead dead large wood material returned to the system following a fire event |
| 0.2 | 0.2 | FRET(3,2) | fraction of N in the burned dead dead large wood material returned to the system following a fire event |
| 0 | 0.4 | FRET(3,3) | fraction of P in the burned dead dead large wood material returned to the system following a fire event |
| 0 | 0.4 | FRET(3,4) | fraction of S in the burned dead dead large wood material returned to the system following a fire event |
| 0.2 | 0.2 | FRTSH | additive effect of burning on root/shoot ratio |
| 10 | 10 | FNUE(1) | effect of fire on increase in maximum C/N ratio of shoots |
| 30 | 30 | FNUE(2) | effect of fire on increase in maximum C/N ratio of roots |
2.4. Comparison of Model Output and Statistical Analyses
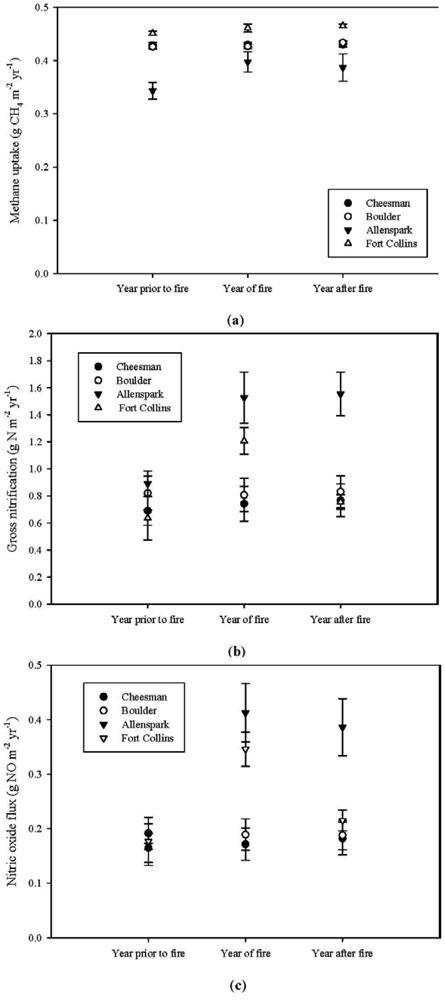
3. Results and Discussion
3.1. Simulated and Observed Biogeochemistry of Front Range Forests
| Variable | Site | Mean | Minimum | Maximum | CV (%) |
|---|---|---|---|---|---|
| CH4 uptake | Fort Collins | 0.448 | 0.378 | 0.501 | 4.2 |
| Allenspark | 0.377 | 0.248 | 0.511 | 14.8 | |
| Boulder | 0.427 | 0.356 | 0.468 | 5.2 | |
| Cheesman | 0.398 | 0.331 | 0.432 | 5.2 | |
| N2O flux | Fort Collins | 0.011 | 0.002 | 0.048 | 49.3 |
| Allenspark | 0.029 | 0.004 | 0.099 | 52.4 | |
| Boulder | 0.011 | 0.003 | 0.066 | 82.7 | |
| Cheesman | 0.014 | 0.004 | 0.077 | 78.6 | |
| NO flux | Fort Collins | 0.152 | 0.030 | 0.674 | 52.1 |
| Allenspark | 0.198 | 0.058 | 0.694 | 43.8 | |
| Boulder | 0.129 | 0.033 | 0.676 | 85.9 | |
| Cheesman | 0.129 | 0.036 | 0.637 | 80.0 | |
| Nitrification | Fort Collins | 0.558 | 0.106 | 2.422 | 49.2 |
| Allenspark | 0.861 | 0.205 | 2.348 | 37.5 | |
| Boulder | 0.564 | 0.148 | 3.304 | 83.1 | |
| Cheesman | 0.678 | 0.188 | 3.866 | 78.2 | |
| Precipitation | Fort Collins | 38.9 | 19.4 | 72.6 | 27.2 |
| Allenspark | 49.1 | 23.2 | 87.3 | 32.5 | |
| Boulder | 47.1 | 22.0 | 75.5 | 23.7 | |
| Cheesman | 35.4 | 33.8 | 49.1 | 20.0 |
3.2. Effects of Fire on Trace Gas Fluxes
3.2.1. Year Before, of and After Fire
3.2.2. Pre- versus Post-Fire Suppression
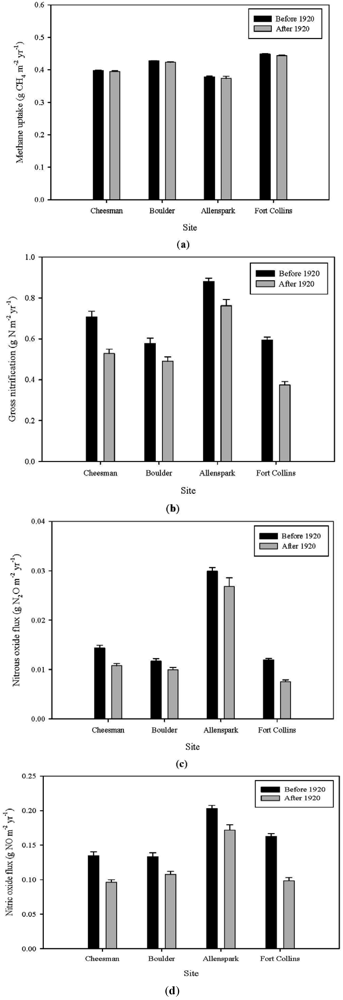
4. Conclusions
Acknowledgments
References
- Moritz, M.A.; Morais, M.E.; Summerell, L.A.; Carlson, J.M.; Doyle, J. Wildfires, complexity, and highly optimized tolerance. Proc. Natl. Acad. Sci. USA 2005, 102, 17912–17917. [Google Scholar]
- Donnegan, J.A.; Veblen, T.T.; Sibold, J.S. Climatic and human influences on fire history in pike national forest, central Colorado. Can. J. For. Res. 2001, 31, 1526–1539. [Google Scholar] [CrossRef]
- Brown, P.M.; Kaufmann, M.R.; Shepperd, W.D. Long-term, landscape patterns of past fire events in a montane ponderosa pine forest of central Colorado. Landsc. Ecol. 1999, 14, 513–532. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Allen, C.D.; Betancourt, J.L. Applied historical ecology: Using the past to manage for the future. Ecol. Appl. 1999, 9, 1189–1206. [Google Scholar] [CrossRef]
- Fule, P.Z.; Covington, W.W.; Moore, M.M. Determining reference conditions for ecosystem management of southwestern ponderosa pine forests. Ecol. Appl. 1997, 7, 895–908. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.-P.; Harnik, N.; Leetmaa, A.; Lau, N.-C.; et al. Model projections of an imminent transition to a more arid climate in southwestern north america. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western U.S. Forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar]
- Randerson, J.T.; Liu, H.; Flanner, M.G.; Chambers, S.D.; Jin, Y.; Hess, P.G.; Pfister, G.; Mack, M.C.; Treseder, K.K.; Welp, L.R.; et al. The impact of boreal forest fire on climate warming. Science 2006, 314, 1130–1132. [Google Scholar]
- Del Grosso, S.J.; Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Potter, C.S.; Borken, W.; Brumme, R.; Butterbach-Bahl, K.; Crill, P.M.; Dobbie, K.; et al. General CH4 oxidation model and comparisons of CH4 oxidation in natural and managed systems. Glob. Biogeochem. Cycles 2000, 14, 999–1019. [Google Scholar] [CrossRef]
- Parton, W.J.; Hartman, M.; Ojima, D.; Schimel, D. Daycent and its land surface submodel: Description and testing. Glob. Planet. Change 1998, 19, 35–48. [Google Scholar] [CrossRef]
- Parton, W.J.; Holland, E.A.; Del Grosso, S.J.; Hartman, M.D.; Martin, R.E.; Mosier, A.R.; Ojima, D.S.; Schimel, D.S. Generalized model for nox and N2O emissions from soils. J. Geophys. Res.-Atmos. 2001, 106, 17403–17419. [Google Scholar]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar]
- Parton, W.J.; Stewart, J.W.B.; Cole, C.V. Dynamics of C, N, P, and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V. Testing the hole-in-the-pipe model of nitric and nitrous oxide emissions from soils using the tragnet database. Glob. Biogeochem. Cycles 2000, 14, 1035–1043. [Google Scholar] [CrossRef]
- Conrad, R. Soil microbial processes and the cycling of atmospheric trace gases. Philos. Trans. Phys. Sci. Eng. 1995, 351, 219–230. [Google Scholar] [CrossRef]
- Keane, R.E.; Ryan, K.C.; Veblen, T.T.; Allen, C.D.; Logan, J.; Hawkes, B. Cascading Effects of Fire Exclusion in Rocky Mountian Ecosystems: A Literature Review; RMRS-GTR-91; Rocky Mountain Research Station, Forest Service, United States Department of Agriculture: Washington, DC, USA, 2002; p. 31. [Google Scholar]
- NOAA. Western regional climate center. National Climatic Data Center. 2012. Available online: http://www.wrcc.dri.edu/Climsum.html (accessed on July 2007).
- Veblen, T.T.; Kitzberger, T.; Donnegan, J. Climatic and human influences on fire regimes in ponderosa pine forests in the Colorado Front Range. Ecol. Appl. 2000, 10, 1178–1195. [Google Scholar] [CrossRef]
- Brown, P.M.; Shepperd, W.D. Fire history and fire climatology along a 5-degree gradient in latitude in Colorado and Wyoming, USA. Paleobotanist 2001, 50, 133–140. [Google Scholar]
- Sherriff, R.L.; Veblen, T.T. Ecological effects of changes in fire regimes in pinus ponderosa ecosystems in the Colorado Front Range. J. Veg. Sci. 2006, 17, 705–718. [Google Scholar]
- Mast, J.N.; Veblen, T.T. Tree spatial patterns and stand development along the pine-grassland ecotone in the Colorado Front Range. Can. J. For. Res. 1999, 29, 575–584. [Google Scholar] [CrossRef]
- Mast, J.N.; Veblen, T.T.; Linhart, Y.B. Disturbance and climatic influences on age structure of ponderosa pine at the pine/grassland ecotone, Colorado Front Range. J. Biogeogr. 1998, 25, 743–755. [Google Scholar]
- Peet, R.K. Forest vegetation of the Colorado Front Range—Composition and dynamics. Vegetatio 1981, 45, 3–75. [Google Scholar]
- Peet, R.K. Forest vegetation of Colorado Front Range—patterns of species-diversity. Vegetatio 1978, 37, 65–78. [Google Scholar] [CrossRef]
- USDA. Web soil survey. USDA Natural Resource Conservation Service. 2012. Available online: http://websoilsurvey.nrcs.usda.gov/app/HomePage.htm (accessed on July 2007).
- Keogh, C. Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO, USA, Personal communication. 2007. [Google Scholar]
- Shinneman, D.J.; Baker, W.L. Nonequilibrium dynamics between catastrophic disturbances and old-growth forests in ponderosa pine landscapes of the Black Hills. Conserv. Biol. 1997, 11, 1276–1288. [Google Scholar] [CrossRef]
- Li, X.; Meixner, T.; Sickman, J.O.; Miller, A.E.; Schimel, J.P.; Melack, J.M. Decadal-scale dynamics of water, carbon, and nitrogen in a California chaparral ecosystem: Daycent modeling results. Biogeochemistry 2006, 77, 217–245. [Google Scholar] [CrossRef]
- Omi, P.N.; Kalabokidis, K.D. Fire damage on extensively versus intensively managed forest stands within the north fork fire, 1988. Northwest Sci. 1991, 65, 49–157. [Google Scholar]
- Omi, P.N.; Martinson, E.J.; Chong, G.W. Effectiveness of Pre-Fire Fuel Treatments; Report; United States Forest Service’s Joint Fire Science Program: Fort Collins, CO, USA, 2006. [Google Scholar]
- Law, B.E.; Thornton, P.E.; Irvine, J.; Anthoni, P.M.; van Tuyl, S. Carbon storage and fluxes in ponderosa pine forests at different developmental stages. Glob. Change Biol. 2001, 7, 755–777. [Google Scholar] [CrossRef]
- Hicke, J.A.; Sherriff, R.L.; Veblen, T.T.; Asner, G.P. Carbon accumulation in Colorado ponderosa pine stands. Can. J. For. Res.-Rev. 2004, 34, 1283–1295. [Google Scholar] [CrossRef]
- Hall, S.A.; Burke, I.C.; Hobbs, N.T. Litter and dead wood dynamics in ponderosa pine forests along a 160-year chronosequence. Ecol. Appl. 2006, 16, 2344–2355. [Google Scholar] [CrossRef]
- Irvine, J.; Law, B.E. Contrasting soil respiration in young and old-growth ponderosa pine forests. Glob. Change Biol. 2002, 8, 1183–1194. [Google Scholar] [CrossRef]
- Law, B.E.; Turner, D.; Campbell, J.; Sun, O.J.; van Tuyl, S.; Ritts, W.D.; Cohen, W.B. Disturbance and climate effects on carbon stocks and fluxes across western Oregon USA. Glob. Change Biol. 2004, 10, 1429–1444. [Google Scholar] [CrossRef]
- Hall, S.A.; Burke, I.C. Considerations for characterizing fuels as inputs for fire behavior models. For. Ecol. Manag. 2006, 227, 102–114. [Google Scholar] [CrossRef]
- Gathany, M.A.; Burke, I.C. Post-fire soil fluxes of CO2, CH4 and N2O along the Colorado Front Range. Int. J. Wildland Fire 2011, 20, 838–846. [Google Scholar] [CrossRef]
- Hart, S.C. Potential impacts of climate change on nitrogen transformations and greenhouse gas fluxes in forests: A soil transfer study. Glob. Change Biol. 2006, 12, 1032–1046. [Google Scholar] [CrossRef]
- Kaye, J.P.; Burke, I.C.; Mosier, A.R.; Guerschman, J.P. Methane and nitrous oxide fluxes from urban soils to the atmosphere. Ecol. Appl. 2004, 14, 975–981. [Google Scholar] [CrossRef]
- Sommerfeld, R.A.; Mosier, A.R.; Musselman, R.C. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 1993, 361, 140–142. [Google Scholar] [CrossRef]
- Epstein, H.E.; Burke, I.C.; Mosier, A.R.; Hutchinson, G.L. Plant functional type effects on trace gas fluxes in the shortgrass steppe. Biogeochemistry 1998, 42, 145–168. [Google Scholar] [CrossRef]
- Mosier, A.R.; Parton, W.J.; Valentine, D.W.; Ojima, D.S.; Schimel, D.S.; Delgado, J.A. CH4 and N2O fluxes in the Colorado shortgrass steppe. 1. Impact of landscape and nitrogen addition. Glob. Biogeochem. Cycles 1996, 10, 387–399. [Google Scholar] [CrossRef]
- Mosier, A.R.; Parton, W.J.; Valentine, D.W.; Ojima, D.S.; Schimel, D.S.; Heinemeyer, O. CH4 and N2O fluxes in the Colorado shortgrass steppe. 2. Long-term impact of land use change. Glob. Biogeochem. Cycles 1997, 11, 29–42. [Google Scholar] [CrossRef]
- Smith, K.A.; Dobbie, K.E.; Ball, B.C.; Bakken, L.R.; Sitaula, B.K.; Hansen, S.; Brumme, R.; Borken, W.; Christensen, S.; Prieme, A.; et al. Oxidation of atmospheric methane in northern european soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob. Change Biol. 2000, 6, 791–803. [Google Scholar] [CrossRef]
- Matson, P.A.; Gower, S.T.; Volkmann, C.; Billow, C.; Grier, C.C. Soil-nitrogen cycling and nitrous-oxide flux in a rocky-mountain douglas-fir forest: Effects of fertilization, irrigation and carbon addition. Biogeochemistry 1992, 18, 101–117. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Martens, D.A. Moisture controls on trace gas fluxes in semiarid riparian soils. Soil Sci. Soc. Am. J. 2006, 70, 367–377. [Google Scholar] [CrossRef]
- Stark, J.M.; Smart, D.R.; Hart, S.C.; Haubensak, K.A. Regulation of nitric oxide emissions from forest and rangeland soils of western north america. Ecology 2002, 83, 2278–2292. [Google Scholar] [CrossRef]
- Levine, J.S.; Winstead, E.L.; Boston, P.J. The effects of fire on biogenic soil emissions of nitric oxide and nitrous oxide. Glob. Biogeochem. Cycles 1988, 2, 445–449. [Google Scholar] [CrossRef]
- Stark, J.M.; Hart, S.C. High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 1997, 385, 61–64. [Google Scholar] [CrossRef]
- Hamman, S.T. Altered fire regime impacts on the soil biogeochemistry and microbial community structure of mixed conifer and ponderosa pine forests. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2006. [Google Scholar]
- Hamman, S.T.; Burke, I.C.; Stromberger, M.E. Relationships between microbial community structure and soil environmental conditions in a recently burned system. Soil Biol. Biochem. 2007, 39, 1703–1711. [Google Scholar] [CrossRef]
- Carreira, J.A.; Niell, F.X.; Lajtha, K. Soil-nitrogen availability and nitrification in mediterranean shrublands of varying fire history and successional stage. Biogeochemistry 1994, 26, 189–209. [Google Scholar]
- Covington, W.W.; Sackett, S.S. Effect of periodic burning on soil nitrogen concentrations in ponderosa pine. Soil Sci. Soc. Am. J. 1986, 50, 452–457. [Google Scholar] [CrossRef]
- Covington, W.W.; Sackett, S.S. Soil mineral nitrogen changes following prescribed burning in ponderosa pine. For. Ecol. Manag. 1992, 54, 175–191. [Google Scholar] [CrossRef]
- Wan, S.Q.; Hui, D.F.; Luo, Y.Q. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Schimel, J.P.; Gulledge, J. Microbial community structure and global trace gases. Glob. Change Biol. 1998, 4, 745–758. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gathany, M.A.; Burke, I.C. DAYCENT Simulations to Test the Influence of Fire Regime and Fire Suppression on Trace Gas Fluxes and Nitrogen Biogeochemistry of Colorado Forests. Forests 2012, 3, 506-527. https://doi.org/10.3390/f3030506
Gathany MA, Burke IC. DAYCENT Simulations to Test the Influence of Fire Regime and Fire Suppression on Trace Gas Fluxes and Nitrogen Biogeochemistry of Colorado Forests. Forests. 2012; 3(3):506-527. https://doi.org/10.3390/f3030506
Chicago/Turabian StyleGathany, Mark A., and Ingrid C. Burke. 2012. "DAYCENT Simulations to Test the Influence of Fire Regime and Fire Suppression on Trace Gas Fluxes and Nitrogen Biogeochemistry of Colorado Forests" Forests 3, no. 3: 506-527. https://doi.org/10.3390/f3030506




