Impacts of Prescribed Fire Frequency on Coarse Woody Debris Volume, Decomposition and Termite Activity in the Longleaf Pine Flatwoods of Florida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Design
| Time | Treatment or Sampling Conducted |
|---|---|
| 1956 | Entire study area burned to initialize the study |
| 1958–1964 | Plots burned at 0, 2, 4 or 6 year intervals |
| 1964–2012 | Plots burned at 0, 1, 2 or 4 year intervals |
| November 1994 | Present study begun, logs placed on the plots to measure decomposition |
| Winter 1994–1995 | 1 year interval plots were burned |
| April 1995 | Termite blocks placed on plots and checked bimonthly |
| Winter 1995–1996 | Logs sampled, 1, 2 and 4 year interval plots burned |
| Winter 1996–1997 | Logs sampled, 1 year interval plots burned |
| September 1997 | Termite blocks replaced and checked bimonthly |
| Winter 1997–1998 | Logs sampled, 1 and 2 year interval plots burned |
| Winter 1998–1999 | Logs sampled, 1 year interval plots burned |
| Winter 1999–2000 | 1, 2 and 4 year interval plots burned |
| December 2003 | CWD surveyed on all plots |
2.2. Coarse Woody Debris
2.3. Wood Decomposition and Nitrogen (N) Content
2.4. Termites
2.5. Statistical Analyses

3. Results
3.1. Coarse Woody Debris
| Burn Frequency | N | Logs 1 | Snags 1 | Total 1 |
|---|---|---|---|---|
| Annual | 6 | 3.9 ± 1.38 a | 5.2 ± 1.44 a | 9.1 ± 1.73 a |
| Biennial | 6 | 1.9 ± 0.60 a | 6.5 ± 1.00 a | 8.5 ± 1.23 a |
| Quadrennial | 6 | 1.6 ± 0.70 a | 7.2 ± 1.48 a | 8.9 ± 1.87 a |
| Unburned | 6 | 0.8 ± 0.27 a | 7.2 ± 0.81 a | 8.0 ± 1.01 a |
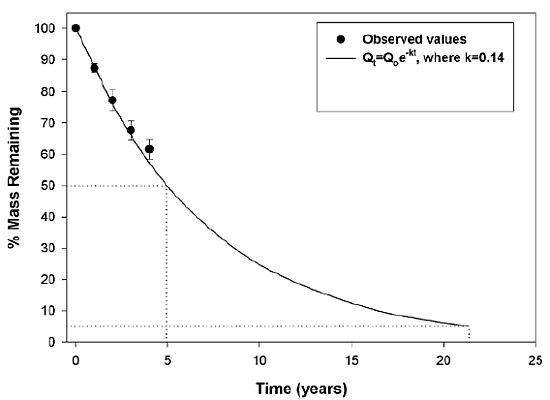
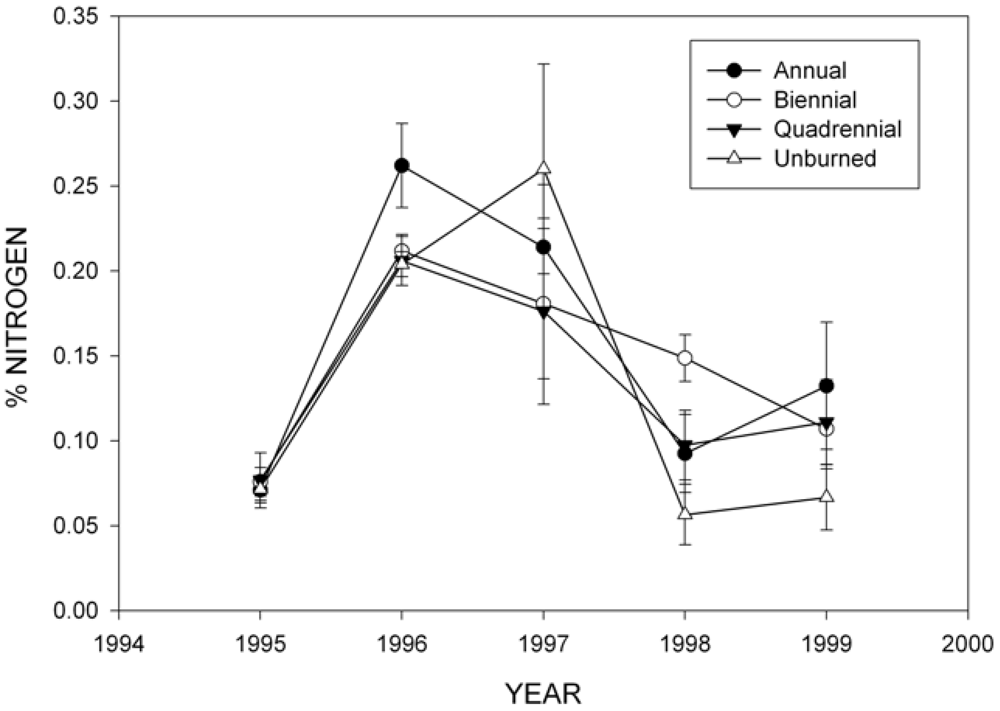
3.2. Wood Decomposition and Nitrogen (N) Content
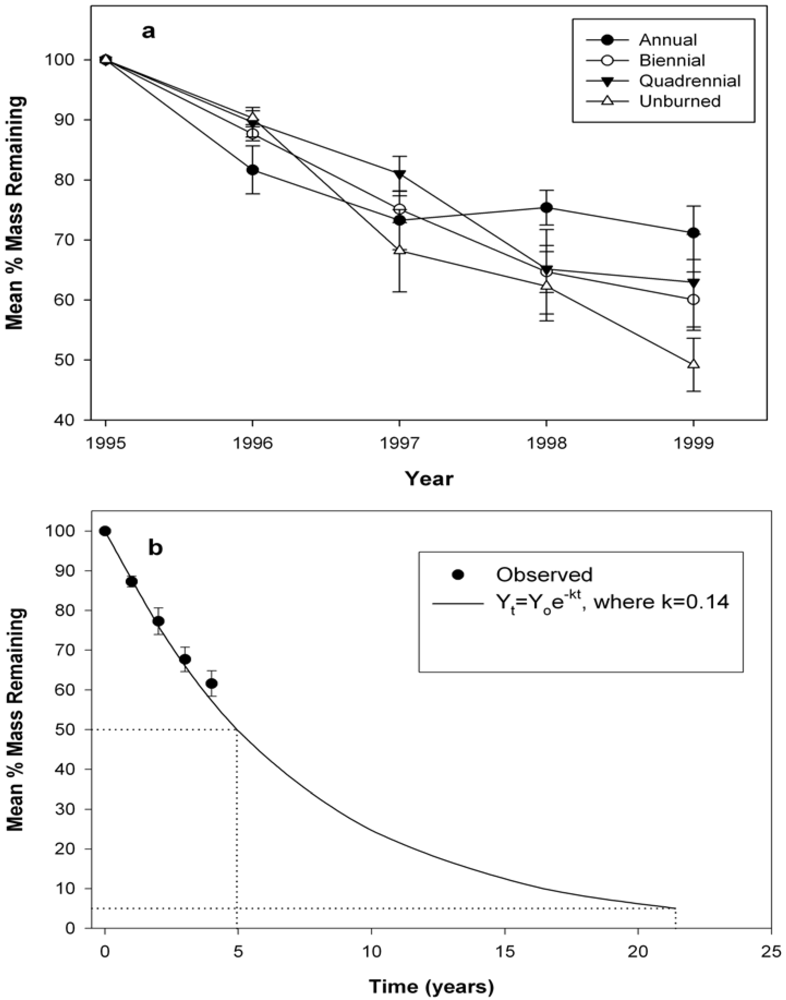
| Source | Mass remaining | Nitrogen | ||||||
|---|---|---|---|---|---|---|---|---|
| df | MS | F | P | df | MS | F | p | |
| Whole unit | ||||||||
| Treatment | 3 | 0.03 | F3,15 = 4.20 | 0.02 | 3 | 0.01 | F3,15 = 1.13 | 0.37 |
| Block | 5 | 0.03 | F5,15 = 4.10 | 0.02 | 5 | 0.00 | F5,15 = 0.98 | 0.46 |
| Treatment* Block | 15 | 0.01 | F15,55 = 0.37 | 0.98 | 15 | 0.00 | F15,58 = 0.74 | 0.74 |
| Sub unit | ||||||||
| Year | 3 | 0.41 | F3,55 = 24.74 | <0.01 | 3 | 0.10 | F3,58 = 15.98 | <0.01 |
| Treatment* Year | 9 | 0.02 | F9,55 = 1.46 | 0.19 | 9 | 0.01 | F9,58 = 1.15 | 0.34 |
| Error | 55 | 0.02 | – | – | 58 | 0.01 | – | – |
| Total | 90 | – | – | – | 93 | – | – | – |
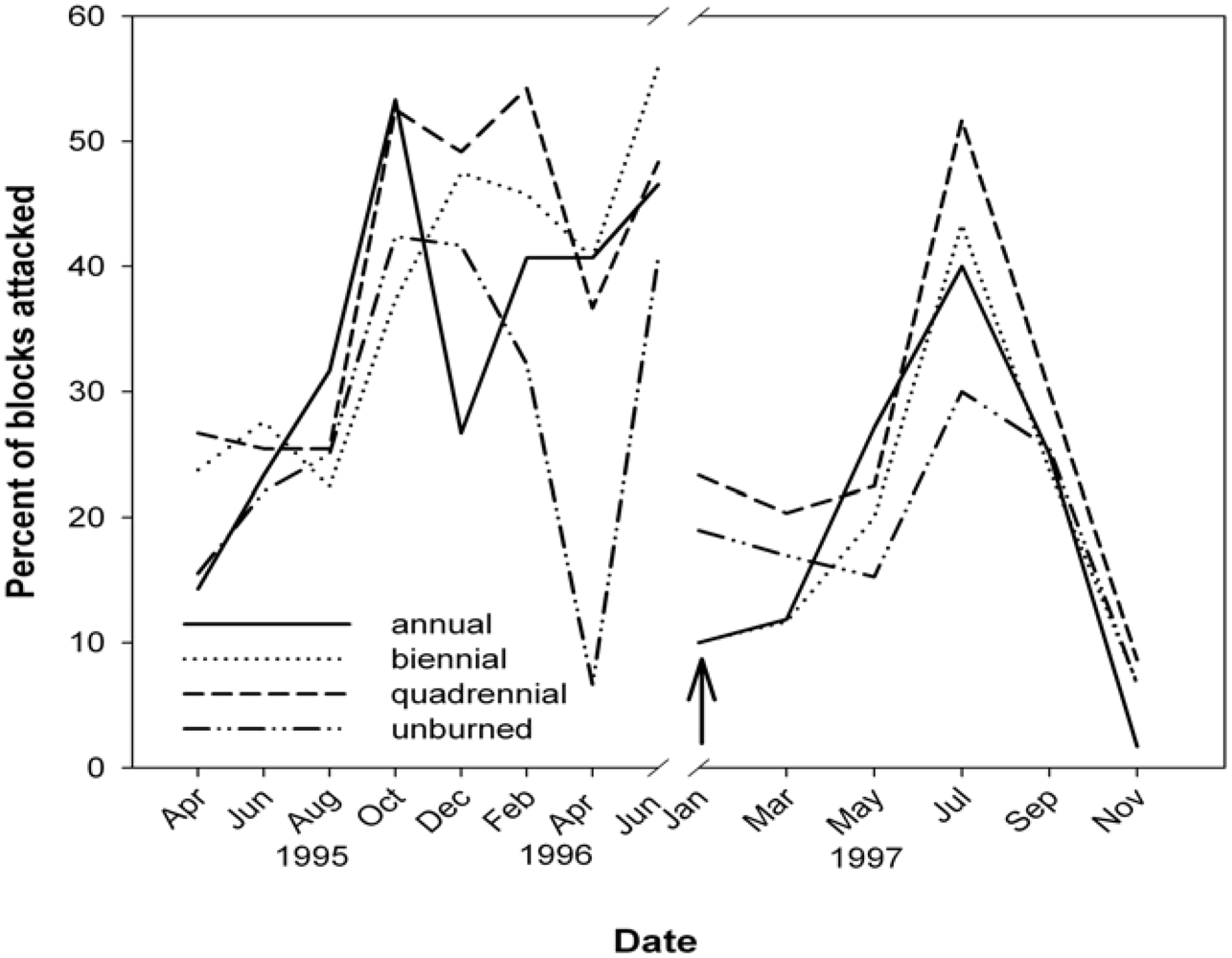
3.3. Termites
4. Discussion
Acknowledgments
Conflict of Interest
References and Notes
- Elton, C.S. The Pattern of Animal Communities; John Wiley & Sons, Inc.: New York, NY, USA, 1966. [Google Scholar]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; Lienkaemper, G.W., Jr.; Cromack, K.; Cummins, K.W. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar] [CrossRef]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Ann. Rev. Ecol. Sys. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Grove, S.J.; Hanula, J.L. Insect biodiversity and dead wood. In Proceedings of a Symposium for the 22nd International Congress of Entomology, Brisbane, Australia, 15–21 August 2004; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006. [Google Scholar]
- Dudley, N.; Vallauri, D. Deadwood: Living Forests: The Importance of Veteran Trees and Deadwood to Biodiversity; Technical Report for World Wildlife Fund: Washington, DC, USA, 2004. [Google Scholar]
- Woldendorp, G.; Keenan, R.J.; Ryan, M.F. Coarse Woody Debris in Australian Forest Ecosystems; Bureau of Rural Sciences: Canberra, Australia, 2002. [Google Scholar]
- McMinn, J.W.; Crossley, D.A., Jr. Biodiversity and Coarse Woody Debris in Southern Forests; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 1996. [Google Scholar]
- Outcalt, K.W.; Sheffield, R.M. The Longleaf Pine Forest: Trends and Current Conditions; U.S. Department Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 1996. [Google Scholar]
- Wahlenberg, W.G. Longleaf Pine, Its Use, Ecology, egeneration, Protection, Growth, and Management; U.S. Department of Agriculture: Washington, DC, USA, 1946. [Google Scholar]
- Landers, J.L. Disturbance influences on pine traits in southeastern United States. In Proceedings of Tall Timbers Fire Ecology Conference, Tallahassee, FL, USA, 30 May–June 2 1991; Tall Timbers Research Station: Tallahassee, FL, USA, 1993; pp. 61–98. [Google Scholar]
- Brose, P.; Wade, D. Potential fire behavior in pine flatwood forests following three different fuel reduction techniques. For. Ecol. Manag. 2002, 163, 71–84. [Google Scholar] [CrossRef]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood: Its Biology and Ecology; John Wiley & Sons, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Abe, T. Studies on the distribution and ecological role of termites in a lowland rain forest of West Malaysia. 4. The role of termites in the process of wood decomposition in Pasoh Forest Reserve. Rev. Ecol. Biol. Sol. 1980, 17, 23–40. [Google Scholar]
- Schuurman, G. Decomposition rates and termite assemblage composition in semiarid Africa. Ecology 2005, 86, 1236–1249. [Google Scholar] [CrossRef]
- Davies, R.G. Termite species richness in fire-prone and fire-protected dry deciduous dipterocarp forest in Doi Suthep-Pui National Park, Northern Thailand. J. Trop. Ecol. 1997, 13, 153–160. [Google Scholar] [CrossRef]
- Davies, A.B.; Eggleton, P.; van Rensburg, B.J.; Parr, C.L. The pyrodiversity-biodiversity hypothesis: A test with savanna termite assemblages. J. Appl. Ecol. 2012, 49, 422–430. [Google Scholar] [CrossRef]
- Glitzenstein, J.; Streng, D.R.; Wade, D.D. Fire frequency effects on longleaf pine (Pinus palustris P. Miller) vegetation in South Carolina and Northeast Florida, USA. Nat. Area. J. 2003, 23, 22–37. [Google Scholar]
- Avery, T.E. Natural Resources Measurements; McGraw-Hill Book Co.: New York, NY, USA, 1975. [Google Scholar]
- Clark, I.A.; Souter, R.A.; Schlaegel, B.E. Stem Profile Equations for Southern Trees Species; U.S. Department of Agriculture, Southeastern Forest Experiment Station: Asheville, NC, USA, 1991. [Google Scholar]
- Ausmus, B.S. Regulation of wood decomposition rates by arthropod and annelid populations. Ecol. Bull. 1977, 25, 180–192. [Google Scholar]
- Boddy, L.; Watkinson, S.C. Wood decomposition, higher fungi, and their role in nutrient redistribution. Can. J. Bot. 1995, 73, S1377–S1383. [Google Scholar] [CrossRef]
- Swift, M.J. The roles of fungi and animals in the immobilization and release of nutrient elements from decomposing branch-wood. Ecol. Bull. 1977, 25, 193–202. [Google Scholar]
- Takamura, K. Effects of termite exclusion on decay of heavy and light hardwood in a tropical rain forest of peninsular Malaysia. J. Trop. Ecol. 2001, 17, 541–548. [Google Scholar]
- Kofoid, C.A. Termites and Termite Control; University of California Press: Berkeley, CA, USA, 1934. [Google Scholar]
- Forschler, B. Termite colony frequency in forests. Personal communication, Department of Entomology, University of Georgia: Athens, GA, USA, 1999. [Google Scholar]
- SAS Institute, SAS/STAT Guide for Personal Computers, Version 6 Edition, SAS Institute: Cary, NC, USA, 1987.
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Dowdy, S.; Wearden, S.; Chilko, D. Statistics for Research, 3rd ed; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2004. [Google Scholar]
- Stokes, M.E.; Davis, C.S.; Koch, G.G. Categorical Data Analysis Using the SAS System, 2nd ed; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Cornwell, W.K.; Cornelissen, J.H.C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.A.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant traits and wood fates across the globe: Rotted, burned, or consumed? Glo. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Conner, R.N.; Saenz, D. The longevity of large pine snags in eastern Texas. Wildl. Soc. Bull. 2005, 33, 700–705. [Google Scholar] [CrossRef]
- Ottmar, R.D.; Vihnanek, R.E. U.S. study of emissions of air pollutants from biomass fires in the United States: A progress report on fuel characterization—year 2. Department Agriculture, Forest Service, Pacific Northwest Research Station: Seattle, WA, USA, 1997; Unpublished work. [Google Scholar]
- Parresol, B.R.; Shea, D.; Ottmar, R. Creating a fuels baseline and establishing fire frequency relationships to develop a landscape management strategy at the Savannah River Site. In Proceeding Fuels Management—How to Measure Success, Portland, OR, USA, 28–30 March 2006; Andrews, P.L., Bret, B.W., Eds.; U.S. Department Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 351–366. [Google Scholar]
- Hanula, J.L.; Wade, D.D. Influence of long-term dormant-season burning and fire exclusion on ground-dwelling arthropod populations in longleaf pine flatwoods ecosystems. For. Ecol. Manag. 2003, 175, 163–184. [Google Scholar] [CrossRef]
- Penttilä, R.; Kotiranta, H. Short-term effects of prescribed burning on wood-rotting fungi. Silva Fenn. 1996, 30, 399–419. [Google Scholar]
- Mayfield, J.E.; Wade, D.D. Occurrence of macrofungi in burned and unburned hurricane-impacted loblolly pine forest. In Proceedings of the 12th International Conference on Fire and Forest Meteorology, Jekyll Island, GA, USA, 26–28 October 1993; Society of American Foresters: Bethesda, MD, USA, 1994; pp. 642–654. [Google Scholar]
- Peterson, C.J. Review of termite forest ecology and opportunities to investigate the relationship of termites to fire. Sociobiology 1996, 56, 313–352. [Google Scholar]
- Hu, X.P.; Appel, A.G. Seasonal variation of critical thermal limits and temperature tolerance in Formosan and eastern subterranean termites (Isoptera: Rhinotermitidae). Environ. Entomol. 2004, 33, 197–205. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Massman, W.J.; Frank, J.M.; Shepperd, W.D.; Platten, M.J. In situ soil temperature and heat flux measurements during controlled surface burns at a southern Colorado forest site. In Fire, Fuel Treatments, and Ecological Restoration; Rocky Mountain Research Station: Fort Collins, CO, USA, 2003; pp. 69–87. [Google Scholar]
- Ulyshen, M.D.; Horn, S.; Barnes, B.; Gandhi, K.J.K. Impacts of prescribed fire on saproxylic beetles in loblolly pine logs. Insect Conserv. Divers. 2010, 3, 247–251. [Google Scholar] [CrossRef]
- Peterson, C.J.; Gerard, P.D.; Wagner, T.L. Charring does not affect wood infestation by subterranean termites. Entomol. Exp. Appl. 2008, 126, 78–84. [Google Scholar]
- Horn, S.; Hanula, J.L. Relationship of coarse woody debris to arthropod availability for red-cockaded woodpeckers and other bark foraging birds on loblolly pine boles. J. Entomol. Sci. 2008, 43, 153–168. [Google Scholar]
- Abensperg-Traun, M.; Milewski, A.V. Abundance and diversity of termites (Isoptera) in unburnt versus burnt vegetation at the Barrens in Mediterranean Western Australia. Aust. J. Ecol. 1995, 20, 413–417. [Google Scholar] [CrossRef]
- Dawes-Gromadzki, T.Z. Short-term effects of low intensity fire on soil macroinvertebrate assemblages in different vegetation patch types in an Australian tropical savanna. Aust. Ecol. 2007, 32, 663–668. [Google Scholar] [CrossRef]
- Randall-Parker, T.; Miller, R. Effects of prescribed fire in ponderosa pine on key wildlife habitat components: preliminary results and a method for monitoring. In Proceedings of the Symposium on the Ecology and Management of Dead Wood in Western Forests, Reno, NV, USA, 2–4 November 1999; Laudenslayer, W.F., Jr., Shea, P.J., Valentine, B.E., Weatherspoon, C.P., Lisle, T.E., Eds.; U.S. Department Agriculture, Forest Service, Pacific Southwest Experiment Station: Albany, CA, USA, 2002; pp. 823–834. [Google Scholar]
- Barber, B.L.; van Lear, D.H. Weight loss and nutrient dynamics in decomposing woody loblolly pine logging slash. Soil Sci. Soc. Am. J. 1984, 48, 906–910. [Google Scholar]
- Harris, W.F. Nutrient release from decaying wood. In Environmental Science Division Annual Report; Auerbach, S.I., Reichle, D.E., Struxness, E.G., Eds.; Oak Ridge National Laboratory: Oakridge, TN, USA, 1976; pp. 169–170. [Google Scholar]
- Eaton, R.J.; Sanchez, F.G. Quantitative and qualitative measures of decomposition: Is there a link? S. J. Appl. For. 2009, 33, 137–141. [Google Scholar]
- Jonsell, M.; Weslien, J.; Ehnström, B. Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodiv. Conserv. 1998, 7, 749–764. [Google Scholar] [CrossRef]
- Wikars, L.O. Dependence on fire in wood-living insects: An experiment with burned and unburned spruce and birch logs. J. Insect Conserv. 2002, 6, 1–12. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hanula, J.L.; Ulyshen, M.D.; Wade, D.D. Impacts of Prescribed Fire Frequency on Coarse Woody Debris Volume, Decomposition and Termite Activity in the Longleaf Pine Flatwoods of Florida. Forests 2012, 3, 317-331. https://doi.org/10.3390/f3020317
Hanula JL, Ulyshen MD, Wade DD. Impacts of Prescribed Fire Frequency on Coarse Woody Debris Volume, Decomposition and Termite Activity in the Longleaf Pine Flatwoods of Florida. Forests. 2012; 3(2):317-331. https://doi.org/10.3390/f3020317
Chicago/Turabian StyleHanula, James L., Michael D. Ulyshen, and Dale D. Wade. 2012. "Impacts of Prescribed Fire Frequency on Coarse Woody Debris Volume, Decomposition and Termite Activity in the Longleaf Pine Flatwoods of Florida" Forests 3, no. 2: 317-331. https://doi.org/10.3390/f3020317