Response of Soil CO2 Emission to Addition of Biochar and Dissolved Organic Carbon along a Vegetation Restoration Gradient of Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection and Preparation
2.2. Incubation Experiment
2.3. Gas Sample Collection and Measurement
2.4. Soil Properties and Enzyme Analyses
2.5. Statistical Analysis
3. Results
3.1. Soil CO2 Emission
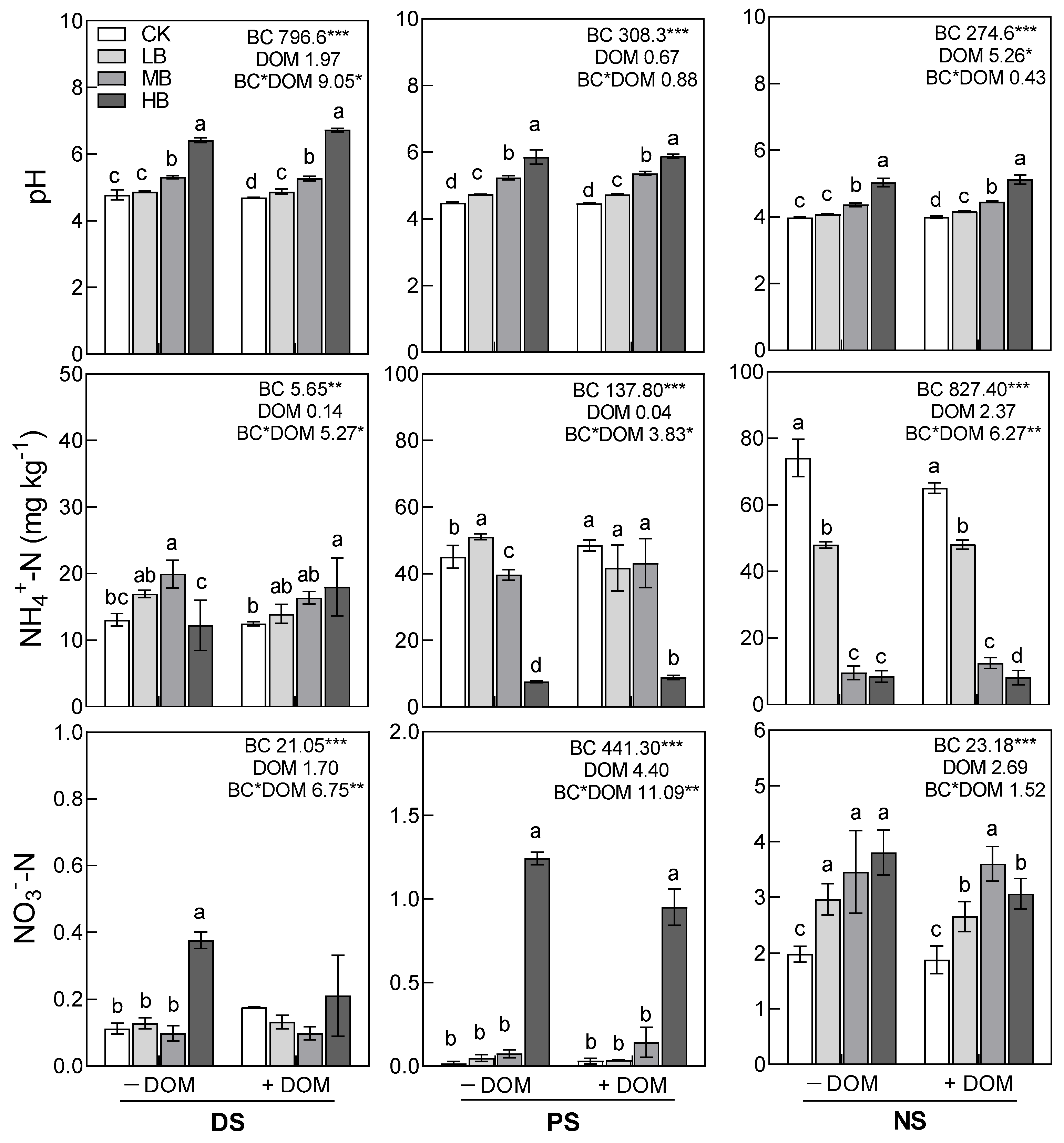
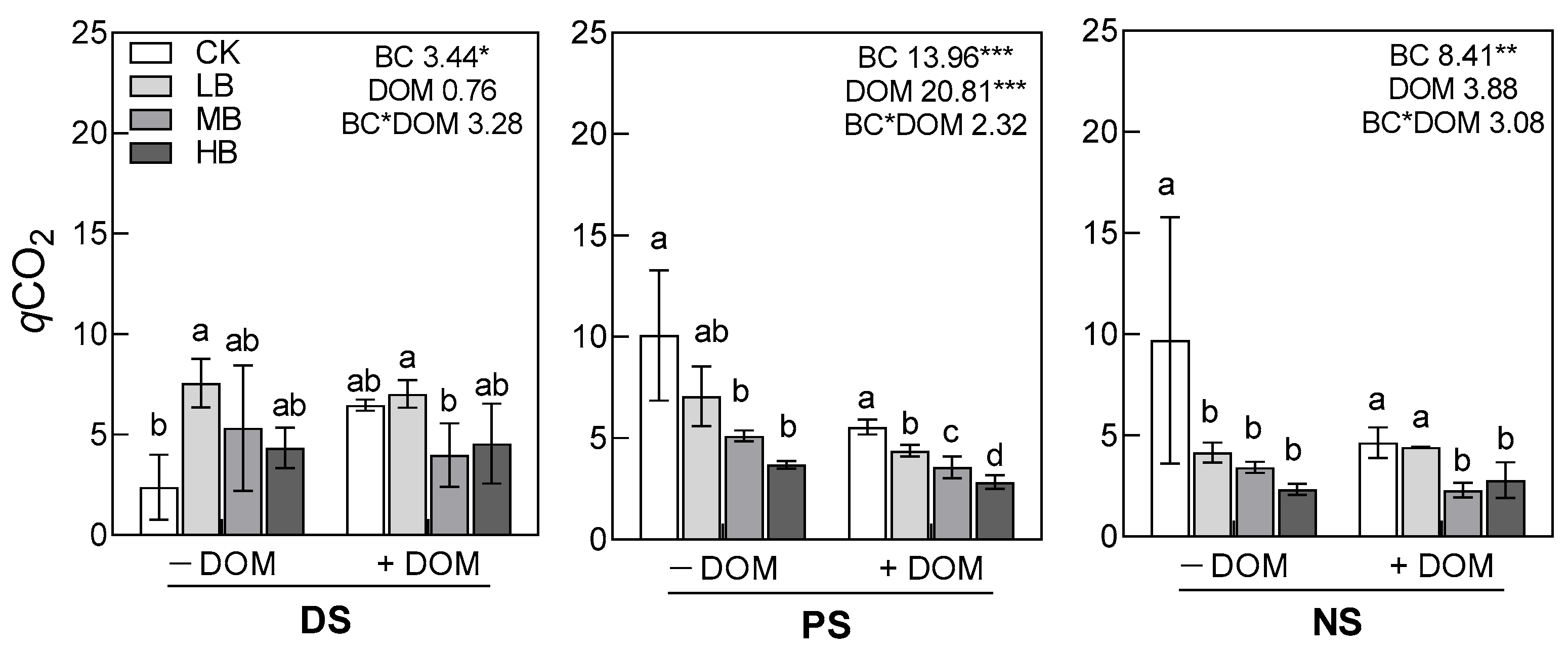
3.2. Soil pH, Available Nitrogen, MBC, DOC, and qCO2
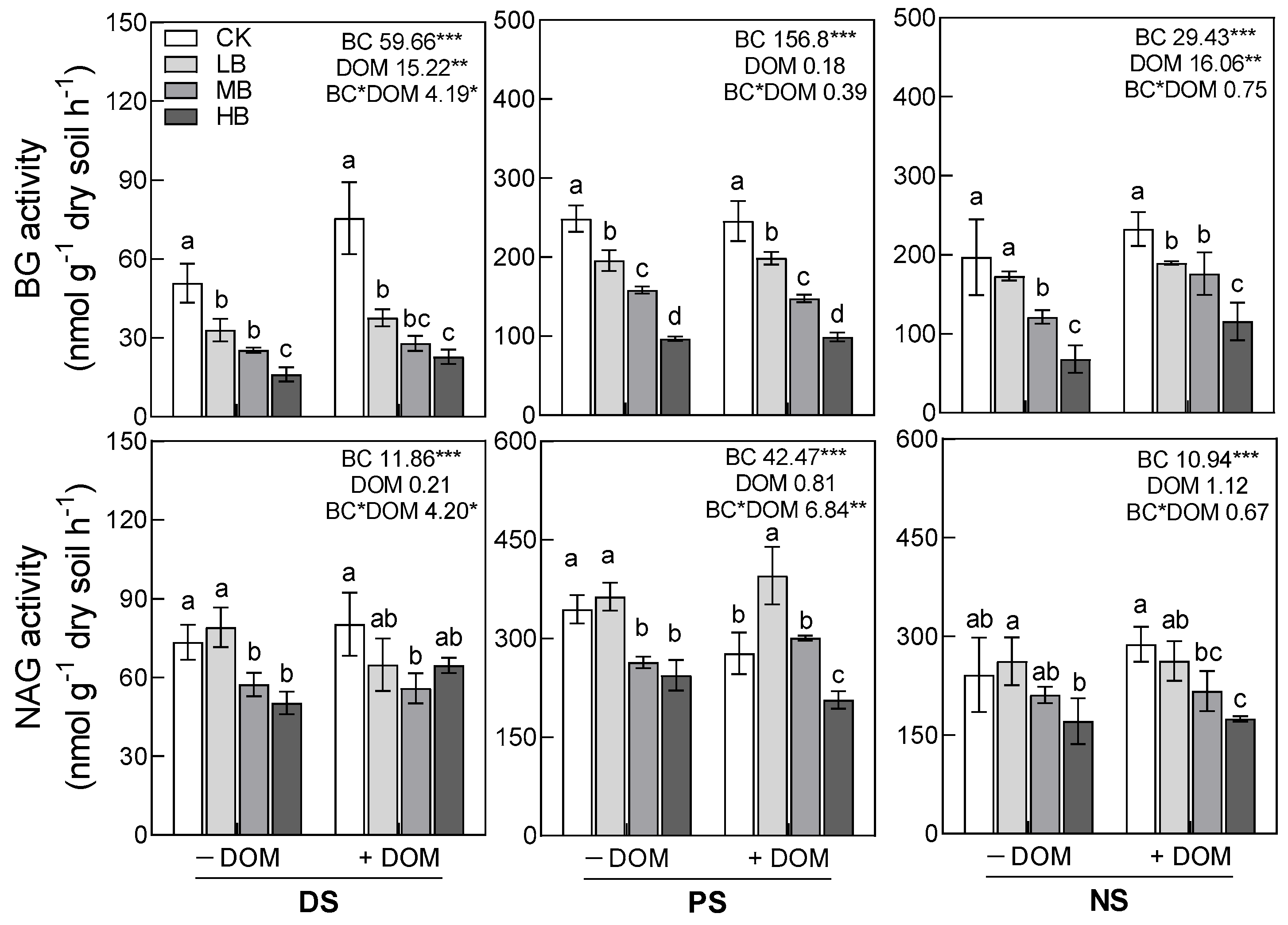
3.3. Soil Enzyme Activities
3.4. Main Factors Influencing Changes in Soil CO2-C Emission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. Forest Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.F.; Sun, W.J.; Zhu, J.J.; Liu, G.B.; Zhou, W.M.; Zhang, Q.F.; Shi, P.L.; Liu, X.P.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Prac. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef]
- Pang, D.B.; Cui, M.; Liu, Y.G.; Wang, G.Z.; Cao, J.H.; Wang, X.R.; Dan, X.Q.; Zhou, J.X. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Denef, K.; Stewart, C.E.; Cotrufo, M.F. Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol. Fert. Soils 2016, 52, 1–14. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Koide, R.T.; Nguyen, B.T.; Skinner, R.H.; Dell, C.J.; Peoples, M.S.; Adler, P.R.; Drohan, P.J. Biochar amendment of soil improves resilience to climate change. Glob. Change Biol. Bioenergy 2015, 7, 1084–1091. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.L.; Chen, Z.M.; Zhu, L.Z.; Schnoor, J.L. Insight into multiple and multi-level structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fert. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Zhang, K.L.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.M.; Luo, Y. The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol. Fert. Soils 2017, 53, 77–87. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.Q.; Zhan, L.P.; Xu, X.T.; Bi, R.Y.; Xiong, Z.Q. Biochar addition stabilized soil carbon sequestration by reducing temperature sensitivity of mineralization and altering the microbial community in a greenhouse vegetable field. J. Environ. Manag. 2022, 313, 114972. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhou, X.H.; Zhang, T.; Du, Z.G.; He, Y.H.; Wang, X.H.; Shao, J.J.; Cao, Y.; Xue, S.G.; Wang, H.L.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. Forest Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Xu, L.; Fang, H.J.; Ying, J.Y.; Lv, J.W.; Shi, Y.J.; Zhou, G.M.; Zhou, Y.F. Biochar application increased ecosystem carbon sequestration capacity in a Moso bamboo forest. Forest Ecol. Manag. 2020, 475, 118447. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X.; Yang, Y.F.; Fu, S.L.; Jiang, P.K.; Luo, Y.; Yang, M.; Chen, Z.H.; Hu, S.D.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhan, Y.; Zhu, L.Z. Reduced carbon sequestration potential of biochar in acidic soil. Sci. Total Environ. 2016, 572, 129–137. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xiong, Z.Q.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. Glob. Chang. Biol. Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J.; Kinyangi, J.; Cheng, C.; Thies, J.E.; Mugendi, D.N.; Pell, A.N. Soil organic C stabilization and thresholds in C saturation. Soil Biol. Biochem. 2009, 41, 2100–2104. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils: A Review. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conan, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Stewart, C.E.; Zheng, J.Y.; Botte, J.; Cotrufo, M.F. Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. Glob. Change Biol. Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Whitman, T.; Enders, A.; Lehmann, J. Pyrogenic carbon additions to soil counteract positive priming of soil carbon mineralization by plants. Soil Biol. Biochem. 2014, 73, 33–41. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xiong, Z.Q.; Yan, X.Y.; Kuzyakov, Y. Carbon budget by priming in a biochar-amended soil. Eur. J. Soil Biol. 2016, 76, 26–34. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Yan, T.T.; Zhu, Q.; Bu, X.L.; Chen, B.; Xue, J.H.; Wu, Y.B. Bacterial community structure shifts induced by biochar amendment to karst calcareous soil in southwestern areas of China. J. Soils Sediment 2019, 19, 356–365. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, D.; Xu, Q.F.; Fuhrmann, J.J.; Li, L.Q.; Pan, G.X.; Li, Y.F.; Qin, H. Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol. Fert. Soils 2019, 55, 185–197. [Google Scholar] [CrossRef]
- Liu, S.W.; Zhang, Y.J.; Zong, Y.J.; Hu, Z.Q.; Wu, S.; Zhou, J.; Jin, Y.G.; Zou, J.W. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. Glob. Change Biol. Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Bu, X.L.; Su, J.; Xue, J.H.; Wu, Y.B.; Zhao, C.X.; Wang, L.M. Effect of rice husk biochar addition on nutrient leaching and microbial properties of Calcaric Cambisols. J. Soil Water Conserv. 2019, 74, 172–179. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, X.Y.; Zheng, J.Y.; Zhang, B.; Lu, H.F.; Chi, Z.Z.; Pan, G.X.; Li, L.Q.; Zheng, J.F.; Zhang, X.H.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Li, Y.F.; Hu, S.D.; Chen, J.H.; Müller, K.; Li, Y.C.; Fu, W.J.; Lin, Z.W.; Wang, H.L. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediment 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Wu, F.P.; Jia, Z.K.; Wang, S.G.; Chang, S.X.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fert. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Kumar, S.; Masto, R.E.; Ram, L.C.; Sarkar, P.; George, J.; Selvi, V.A. Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 2013, 55, 67–72. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Global Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Fery, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhu, M.T.; Wang, J.M.; Liu, X.X.; Guo, W.J.; Zheng, J.F.; Bian, R.J.; Wang, G.M.; Zhang, X.H.; Cheng, K.; et al. The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. Glob. Chang. Biol. Bioenergy 2019, 11, 1408–1420. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.Q.; Zhang, R.D. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.K.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer, and biochar on decomposition of soil organic matter and plant residues as determined by 14C and enzyme activities. Eur. J. Soil Biol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L.; Gallo, M.; Lauber, C. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol. Appl. 2004, 14, 1172–1177. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic ‘latch’ on a global carbon store—A shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 2001, 409, 149. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. Data from: World Reference Base for Soil Resources 2006—A Framework for International Classification, Correlation and Communication; World Soil Resources Reports; FAO: Rome, Italy, 2006; 103p. [Google Scholar]
- Fang, X.; Zhu, Y.L.; Liu, J.D.; Lin, X.P.; Sun, H.Z.; Tang, X.H.; Hu, Y.L.; Huang, Y.P.; Yi, Z.G. Effects of moisture and temperature on soil organic carbon decomposition along a vegetation restoration gradient of subtropical China. Forests 2022, 13, 578. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; McNamara, N.P.; Nottingham, A.T.; Stott, A.W.; Bardgett, R.D.; Salinas, N.; Ccahuana, A.J.Q.; Meir, P. Microbial carbon mineralization intropical lowland and montane forest soils of Peru. Front. Microbiol. 2014, 5, 720. [Google Scholar] [CrossRef]
- Liu, J.X.; Liu, S.G.; Li, Y.Y.; Liu, S.Z.; Yin, G.C.; Huang, J.; Xu, Y.; Zhou, G.Y. Warming effects on the decomposition of two litter species in model subtropical forests. Plant Soil 2017, 409, 277–287. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA Book Ser; SSSA: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Fang, X.; Zhou, G.Y.; Qu, C.; Huang, W.J.; Zhang, D.Q.; Li, Y.L.; Yi, Z.G.; Liu, J.X. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Liu, J.X.; Fang, X.; Tang, X.L.; Wang, W.T.; Zhou, G.Y.; Huang, W.J.; Wang, G.X.; Yan, J.H.; Ma, K.P.; Du, S.; et al. Patterns and controlling factors of plant nitrogen and phosphorus stoichiometry across China’s forests. Biogeochemistry 2019, 143, 191–205. [Google Scholar] [CrossRef]
- Zhang, K.R.; Li, X.S.; Cheng, X.L.; Zhang, Z.H.; Zhang, Q.F. Changes in soil properties rather than functional gene abundance control carbon and nitrogen mineralization rates during long-term natural revegetation. Plant Soil 2019, 443, 293–306. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Tuo, D.F.; Wang, C.; Fu, B.J.; Lv, Y.H.; Liu, G.H. Land use change and stand age regulate soil respiration by influencing soil substrate supply and microbial community. Geoderma 2020, 359, 113991. [Google Scholar] [CrossRef]
- Zhang, S.B.; Fang, Y.Y.; Luo, Y.; Li, Y.C.; Ge, T.D.; Wang, Y.X.; Wang, H.L.; Yu, B.; Song, X.Z.; Chen, J.H.; et al. Linking soil carbon availability, microbial community composition and enzyme activities to organic carbon mineralization of a bamboo forest soil amended with pyrogenic and fresh organic matter. Sci. Total Environ. 2021, 801, 149717. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 2000, 31, 711–725. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Bio. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhu, T.T.; Pumpanen, J.; Palviainen, M.; Zhou, X.; Kulmala, L.; Bruckman, V.J.; Köster, E.; Köster, K.; Aaltonen, H.; et al. Short-term effects of biochar on soil CO2 efflux in boreal Scots pine forests. Ann. Forest Sci. 2020, 77, 59. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, G.S.; Robinson, D.; Yang, Z.J.; Guo, J.F.; Xie, J.S.; Fu, S.L.; Zhou, L.X.; Yang, Y.S. Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soilapplied black carbon: Downward migration, leaching and soil respiration. Global Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Singh, B.P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef]




| Parameters | Vegetation Restoration Stages | ||
|---|---|---|---|
| DS | PS | NS | |
| pH | 4.56 ± 0.05 a | 4.21 ± 0.15 b | 4.00 ± 0.12 b |
| SOC (g kg−1) | 3.53 ± 0.25 c | 16.03 ± 3.38 b | 34.13 ± 4.56 a |
| NH4+-N (mg kg−1) | 1.71 ± 0.24 b | 2.06 ± 0.40 b | 13.02 ± 3.40 a |
| NO3−-N (mg kg−1) | 0.04 ± 0.020 c | 0.44 ± 0.11 b | 0.69 ± 0.04 a |
| AP (mg kg−1) | 0.20 ± 0.08 c | 0.63 ± 0.20 b | 2.84 ± 0.33 a |
| TN (g kg−1) | 1.23 ± 0.11 b | 1.78 ± 0.30 b | 2.94 ± 0.37 a |
| TP (g kg−1) | 0.04 ± 0.00 c | 0.08 ± 0.01 b | 0.13 ± 0.01 a |
| MBC (mg kg−1) | 206.6 ± 52.4 b | 931.9 ± 190.2 a | 963.9 ± 45.4 a |
| DOC (mg kg−1) | 154.9 ± 24.5 c | 483.9 ± 160.0 b | 709.3 ± 40.8 a |
| Main Effect or Interaction | Dependent Variable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs | pH | NH4+-N | NO3−-N | MBC | DOC | BG | NAG | PhOx | Perox | qCO2 | |
| Vegetation restoration | 1190.1 *** | 985.94 *** | 350.49 *** | 1084.9 *** | 288.72 *** | 810.63 *** | 548.50 *** | 611.48 *** | 9.39 *** | 77.70 *** | 4.78 * |
| Biochar | 43.30 *** | 1240.6 *** | 423.87 *** | 50.33 *** | 18.54 *** | 139.96 *** | 142.75 *** | 45.76 *** | 1.21 | 0.98 | 13.82 *** |
| R×BC | 22.12 *** | 42.16 *** | 206.03 *** | 21.60 *** | 4.87 *** | 19.26 *** | 14.36 *** | 9.48 *** | 5.21 ** | 10.33 *** | 4.28 ** |
| DOM | 0.87 | 6.36 ** | 1.14 | 4.46 * | 29.76 *** | 56.08 *** | 17.07 *** | 0.15 | 3.83 | 4.49 * | 7.37 ** |
| R×DOM | 0.55 | 0.38 | 0.36 | 1.67 | 4.94 ** | 0.62 | 10.59 *** | 1.40 | 0.05 | 2.15 | 6.50 ** |
| BC×DOM | 1.15 | 4.03 ** | 4.21 ** | 3.71 ** | 3.92 ** | 2.63 | 0.48 | 0.72 | 8.58 *** | 2.43 | 0.84 |
| R×BC×DOM | 3.00 ** | 2.69 ** | 4.94 *** | 0.72 | 1.70 | 2.50 * | 1.02 | 3.99 ** | 2.85 * | 0.38 | 4.07 ** |
| Accumulative Release of CO2-C | |||
|---|---|---|---|
| DS | PS | NS | |
| pH | 0.622 ** | −0.845 *** | −0.626 ** |
| NH4+-N | 0.305 | 0.649 ** | 0.871 *** |
| NO3−-N | 0.281 | −0.551 * | −0.778 *** |
| MBC | 0.491 * | −0.277 | −0.617 ** |
| DOC | −0.204 | 0.809 *** | 0.811 *** |
| BG | −0.61 ** | 0.867 *** | 0.654 ** |
| NAG | −0.577 | 0.448 * | 0.466 * |
| PhOx | 0.24 | 0.083 | 0.025 |
| Perox | 0.602 ** | 0.427 * | 0.387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Tang, X.; Huang, Y.; Jiang, J.; Fang, X. Response of Soil CO2 Emission to Addition of Biochar and Dissolved Organic Carbon along a Vegetation Restoration Gradient of Subtropical China. Forests 2024, 15, 753. https://doi.org/10.3390/f15050753
Zhu Y, Tang X, Huang Y, Jiang J, Fang X. Response of Soil CO2 Emission to Addition of Biochar and Dissolved Organic Carbon along a Vegetation Restoration Gradient of Subtropical China. Forests. 2024; 15(5):753. https://doi.org/10.3390/f15050753
Chicago/Turabian StyleZhu, Yulin, Xinghao Tang, Yunpeng Huang, Jing Jiang, and Xiong Fang. 2024. "Response of Soil CO2 Emission to Addition of Biochar and Dissolved Organic Carbon along a Vegetation Restoration Gradient of Subtropical China" Forests 15, no. 5: 753. https://doi.org/10.3390/f15050753





