Response of C:N:P Stoichiometry to Phosphorus Addition and Homeostasis of Plant Tissues in a Subtropical Slash Pine Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
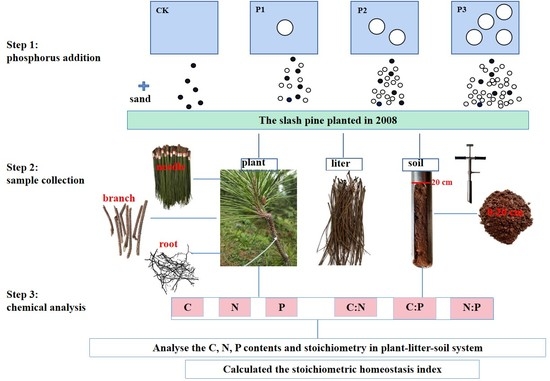
2.2. Experimental Design
2.3. Sample Collection
2.4. Chemical Analysis
2.5. Statistical Analyses
3. Results
3.1. The Contents of C, N, and P and the C:N:P Stoichiometry in Plant Tissues
3.2. The C, N, and P Contents and the C:N:P Stoichiometry of Soil
3.3. Relationships of C, N, and P Contents and Stoichiometry
3.4. Stoichiometric Homeostasis
4. Discussion
4.1. The C, N, and P Contents and Stoichiometry
4.2. Stoichiometric Homeostasis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wang, T.; Song, J.C. Effects of different application amont of phosphorus fertilizer on olive growth and rhizosphere soil micro environment. Soil Fert. Sci. Chin. 2021, 11, 1–13. [Google Scholar]
- Hou, E.Q.; Luo, Y.Q.; Kuang, Y.W.; Chen, C.R.; Wen, D.Z. Global meta-analysis shows pervasive phosphorus limitation of above ground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef]
- Mirriam, A.; Mugwe, J.; Raza, M.A.; Seleiman, M.F.; Maitra, S.; Gitari, H.H. Aggrandizing soybean yield, phosphorus use efficiency and economic returns under phosphatic fertilizer application and inoculation with Bradyrhizobium. J. Soil Sci. Plant Nutr. 2022, 22, 5086–5098. [Google Scholar] [CrossRef]
- Zhu, J.X.; Wang, Q.F.; He, N.P.; Smith, M.D.; Elser, J.J.; Du, J.Q.; Yuan, G.F.; Yu, G.R.; Yu, Q. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation. J. Geophys. Res. Biogeosci. 2016, 121, 1605–1616. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Li, P.; Xu, G.C.; Li, Z.B.; Gao, H.D.; Zhao, B.H.; Wang, T.; Wang, F.C.; Cheng, S.D. Effects of soil erosion and land use on spatial distribution of soil total phosphorus in a small watershed on the Loess Plateau, China. Soil Till. Res. 2018, 184, 142–152. [Google Scholar] [CrossRef]
- Chapin, F.S., III. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Wang, P.A.; Ren, X.M.; Bao, F.J. Analysis of soil stoichiometric characteristics and soil physical and chemical properties of different forests in Guandi Mountain. J. Shanxi Agric. Sci. 2020, 48, 956–963. [Google Scholar]
- Elser, J.J.; Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biol. 2007, 5, e181. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.H.; Zheng, W.; Zhong, X.P.; Bin, Y. Stoichiometric characteristics of carbon, nitrogen and phosphorus in Zanthoxylum planispinum var. Dintanensis plantation of different ages. Agron. J. 2020, 113, 685–695. [Google Scholar]
- Zhou, J.; Wang, Q.G. Research progress on the effect of nitrogen addition on main functional characters of early spring herbaceous plants. Int. J. Ecol. 2021, 10, 129–137. [Google Scholar] [CrossRef]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.C.; Xu, M.P.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Fang, J.Y. Review on characteristics and main hypotheses of plant ecological stoichiometry. Chin. J. Plant Ecol. 2021, 45, 682–713. [Google Scholar] [CrossRef]
- Sterner, R.; Elser, J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2003; Volume 25, p. 1183. [Google Scholar]
- Yu, Q.; Elser, J.J.; He, N.P.; Wu, H.H.; Chen, Q.S.; Zhang, G.M.; Han, X.G. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, J.N.; Guo, W.H.; Li, Y.G.; Geoff, G.; Wu, T.G. Stoichiometric homeostasis, physiology, and growth responses of three tree species to nitrogen and phosphorus addition. Trees 2018, 32, 1377–1386. [Google Scholar] [CrossRef]
- Peng, H.; Chen, Y.; Yan, Z.; Han, W. Stage-dependent stoichiometric homeostasis and responses of nutrient resorption in Amaranthus mangostanus to nitrogen and phosphorus addition. Sci. Rep. 2016, 6, 37219. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Shangguan, Z. Stoichiometric homeostasis in response to variable water and nutrient supply in a robinia pseudoacacia plant-soil system. J. Plant Ecol. 2022, 15, 991–1006. [Google Scholar] [CrossRef]
- Jia, T.; Fang, X.M.; Yuan, Y.; Fu, Y.X.; Yi, M.; Yuan, S.G.; Guo, S.M.; Lai, M.; Xie, J.W.; Zhang, L. Phosphorus addition alter the pine resin flow rate by regulating tree growth and non-structural carbohydrates in a subtropical slash pine plantation. Ind. Crops Prod. 2023, 199, 116782. [Google Scholar] [CrossRef]
- Chen, F.S.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H.M. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.M. Methods of Soil Analysis, Part 3: Chemical Methods. Soil Sci. Soc. Am. J. 1996, 5, 1085–1121. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual; Sacred African Publishers: Nairobi, Kenya, 2002. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Persson, J.; Fink, P.; Goto, A.; James, M.H.; Jayne, J.; Satoshi, K. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Zhen, Y.M. Study on the Stoichiometric Characteristics of Plant Carbon, Nitrogen and Phosphorus of Sandy Mountain Soil in Poyang Lake. Jiangxi Norm. Univ. 2014. [Google Scholar]
- Li, Y.H. Stoichiometric characteristics of different aged Pinus sylvestris Var. Mongolica plantations. Liaoning Univ. Eng. Tech. 2019. [CrossRef]
- Chapin, F.S., III; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Ann. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Piper, F.I. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Würth, M.K.; Pelaez-Riedl, S.; Wright, S.J.; Körner, C. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, M.; Huang, L.; Li, M.; Zhang, X.; Cao, Y. Response of plant, litter, and soil C:N:P stoichiometry to growth stages in Quercus secondary forests on the Loess Plateau, China. J. For. Res. 2023, 34, 595–607. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, R.L.; Yan, J.H.; Sha, L.Q.; Han, S.J. C:N:P stoichiometric characteristics of four forest types’ dominant tree species in China. Chin. J. Plant Ecol. 2011, 35, 587–595. [Google Scholar]
- He, J.S.; Wang, L.; Flynn, D.F.B.; Wang, X.P.; Ma, W.H.; Fang, J.Y. Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, H.; He, N.; Lü, X.T.; Wang, Z.P.; Elser, J.J.; Wu, J.G.; Han, X.G. Testing the growth rate hypothesis in vascular plants with above- and below-ground biomass. PLoS ONE 2012, 7, e32162. [Google Scholar] [CrossRef] [Green Version]
- Koerselman, W.; Meuleman, A.M.F. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. Chin. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Sabine, G. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Melillo, J.M.; Hall, C. Pattern and variation of C:N:P ratios in China’s soil: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Du, P.C.; Pan, Y.Z.; Hou, S.L.; Wang, Z.H.; Wang, H.Y. Effects of nitrogen and phosphorus addition on litter decomposition in Hulunber steppe. Acta Pratac. Sin. 2023, 32, 44–53. [Google Scholar]
- Fan, H.B.; Wu, J.P.; Liu, W.F.; Yuan, Y.H.; Hu, L.; Cai, Q.K. Linkages of plant and soil C: N: P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Bai, X.; Wang, B.; An, S.; Zeng, Q.; Zhang, H. Response of forest species to C:N:P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau, China. Catena 2019, 183, 104–186. [Google Scholar] [CrossRef]
- Jia, T.; Chen, M.Y.; Zhang, L.; Yi, M.; Guo, S.M.; Cheng, Z.S.; Li, X.; Zhong, Q.W. Ecological stoichiometry and homeostasis index of needles, branches, roots and soil in Pinus elliottii plantations of different ages. Acta Agric. Nucl. Sin. 2023, 37, 397–404. [Google Scholar]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Gu, Q.; Zamin, T.J.; Grogan, P. Stoichiometric homeostasis: A test to predict tundra vascular plant species and community-level response to climate change. Arct. Sci. 2017, 3, 320–333. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A reevaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Blouin, M.; Mathieu, J.; Leadley, P.W. Plant homeostasis, growth and development in natural and artificial soils. Ecol. Complex 2012, 9, 10–15. [Google Scholar] [CrossRef]





| Trait | CK | P1 | P2 | P3 |
|---|---|---|---|---|
| C (g kg−1) | 11.50 ± 0.44 c | 12.28 ± 0.56 bc | 13.41 ± 0.51 ab | 14.31 ± 0.56 a |
| N (g kg−1) | 0.94 ± 0.04 | 0.98 ± 0.05 | 1.00 ± 0.04 | 1.06 ±0.04 |
| P (g kg−1) | 0.69 ± 0.03 b | 0.70 ± 0.03 b | 0.77 ± 0.03 ab | 0.83 ± 0.02 a |
| C:N | 12.58 ± 0.73 | 12.94 ±0.74 | 13.84 ± 0.72 | 14.13 ± 1.00 |
| C:P | 17.46 ± 1.20 | 17.98 ±1.06 | 18.04 ± 1.10 | 17.47 ± 0.80 |
| N:P | 1.41 ± 0.09 | 1.43 ± 0.08 | 1.33 ± 0.08 | 1.29 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, T.; Yi, M.; Chen, F.; Lai, M.; Jin, C.; Nie, Z.; Zhou, L.; Xie, J.; Zhang, L. Response of C:N:P Stoichiometry to Phosphorus Addition and Homeostasis of Plant Tissues in a Subtropical Slash Pine Plantation. Forests 2023, 14, 1355. https://doi.org/10.3390/f14071355
Jia T, Yi M, Chen F, Lai M, Jin C, Nie Z, Zhou L, Xie J, Zhang L. Response of C:N:P Stoichiometry to Phosphorus Addition and Homeostasis of Plant Tissues in a Subtropical Slash Pine Plantation. Forests. 2023; 14(7):1355. https://doi.org/10.3390/f14071355
Chicago/Turabian StyleJia, Ting, Min Yi, Fusheng Chen, Meng Lai, Cangfu Jin, Zixuan Nie, Linjin Zhou, Jinwen Xie, and Lu Zhang. 2023. "Response of C:N:P Stoichiometry to Phosphorus Addition and Homeostasis of Plant Tissues in a Subtropical Slash Pine Plantation" Forests 14, no. 7: 1355. https://doi.org/10.3390/f14071355