Response of the Soil Fungal Community and Its Function during the Conversion of Forestland to Tea Plantations: A Case Study in Southeast China
Abstract
1. Introduction
2. Material and Methods
2.1. Site Selection and Soil Sampling
2.2. Soil Physical and Chemical Analyses
2.3. Soil DNA Extraction, MiSeq Sequencing, Data Processing and Analysis
2.4. Statistical Analysis
3. Results
3.1. Effects of Forestland Conversion to Tea Plantations on Soil Fungal Communities
3.2. Effects of Forestland Conversion to Tea Plantations on Soil Fungal Alpha and Beta Diversities
3.3. Fungal Co-Occurrence Networks and Their Topological Features
3.4. Effects of Forestland Conversion to Tea Plantations on Soil Functional Fungal Groups
3.5. Relationship between Fungal Community Structure and Soil Properties
4. Discussion
4.1. Forestland Conversion to Tea Plantations Decreases Soil Fungal Diversity
4.2. Forestland Conversion to Tea Plantations Shifts Soil Fungal Community Compositions
4.3. Effects of Forestland Conversion to Tea Plantations on Fungal Co-Occurrence Networks and Functional Fungal Groups
4.4. Effects of Soil Properties on Soil Fungal Community Compositions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Singh, D.; Tomlinson, K.W.; Yang, X.; Ogwu, M.C.; Slik, J.W.F.; Adams, J.M. Tropical forest conversion to rubber plantation in southwest China results in lower fungal beta diversity and reduced network complexity. FEMS Microbiol. Ecol. 2019, 95, fiz092. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, B. Changes in soil greenhouse gas fluxes by land use change from primary forest. Glob. Chang. Biol. 2020, 26, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, C.; Sarria-Guzman, Y.; Chavez-Romero, Y.A.; Luna-Guido, M.; Munoz-Arenas, L.C.; Dendooven, L.; Estrada-Torres, A.; Navarro-Noya, Y.E. Land use is the main driver of soil organic carbon spatial distribution in a high mountain ecosystem. Peer. J. 2019, 7, e7897. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Kim, K.H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A review on the role of organic inputs in maintaining the soil carbon pool of the terrestrial ecosystem. J. Environ. Manag. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Fan, L.; Shao, G.; Pang, Y.; Dai, H.; Zhang, L.; Yan, P.; Zou, Z.; Zhang, Z.; Xu, J.; Zamanian, K.; et al. Enhanced soil quality after forest conversion to vegetable cropland and tea plantations has contrasting effects on soil microbial structure and functions. Catena 2022, 211, 106029. [Google Scholar] [CrossRef]
- Marín, C.; Godoy, R.; Valenzuela, E.; Schloter, M.; Wubet, T.; Boy, J.; Gschwendtner, S. Functional land-use change effects on soil fungal communities in Chilean temperate rainforests. J. Soil Sci. Plant Nut. 2017, 17, 985–1002. [Google Scholar] [CrossRef]
- Luneberg, K.; Schneider, D.; Brinkmann, N.; Siebe, C.; Daniel, R. Land Use Change and Water Quality Use for Irrigation Alters Drylands Soil Fungal Community in the Mezquital Valley, Mexico. Front. Microbiol. 2019, 10, 1220. [Google Scholar] [CrossRef]
- Brinkmann, N.; Schneider, D.; Sahner, J.; Ballauff, J.; Edy, N.; Barus, H.; Irawan, B.; Budi, S.W.; Qaim, M.; Daniel, R.; et al. Intensive tropical land use massively shifts soil fungal communities. Sci. Rep. 2019, 9, 3403. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the loess plateau: A chronosequence approach. Plant Soil 2017, 423, 327–338. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Liu, J.; Dang, P.; Gao, Y.; Zhu, H.; Zhu, H.; Zhao, F.; Zhao, Z. Effects of tree species and soil properties on the composition and diversity of the soil bacterial community following afforestation. For. Ecol. Manag. 2018, 427, 342–349. [Google Scholar] [CrossRef]
- Mueller, R.C.; Rodrigues, J.L.M.; Nüsslein, K.; Bohannan, B.J.M.; Treseder, K. Land use change in the Amazon rain forest favours generalist fungi. Funct. Ecol. 2016, 30, 1845–1853. [Google Scholar] [CrossRef]
- Tian, Q.; Taniguchi, T.; Shi, W.-Y.; Li, G.; Yamanaka, N.; Du, S. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 2017, 7, 45289. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Le, T.H.; Zhu, H.; Yao, Y.; Zhu, H.; Cao, Y.; Zhao, Z. Afforestation of cropland fundamentally alters the soil fungal community. Plant Soil 2020, 457, 279–292. [Google Scholar] [CrossRef]
- Waymouth, V.; Miller, R.E.; Kasel, S.; Ede, F.; Bissett, A.; Aponte, C. Riparian fungal communities respond to land-use mediated changes in soil properties and vegetation structure. Plant Soil 2022, 475, 491–513. [Google Scholar] [CrossRef]
- Bachelot, B.; Uriarte, M.; Zimmerman, J.K.; Thompson, J.; Leff, J.W.; Asiaii, A.; Koshner, J.; McGuire, K. Long-lasting effects of land use history on soil fungal communities in second-growth tropical rain forests. Ecol. Appl. 2016, 26, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Feng, S.; Sun, H.; Wu, S.; Zhuang, G.; Deng, Y.; Bai, Z.; Jing, C.; Zhuang, X. Linking N2O Emissions from Biofertilizer-Amended Soil of Tea Plantations to the Abundance and Structure of N2O-Reducing Microbial Communities. Environ. Sci. Technol. 2018, 52, 11338–11345. [Google Scholar] [CrossRef]
- Fan, L.; Han, W. Soil respiration after forest conversion to tea gardens: A chronosequence study. Catena 2020, 190, 104532. [Google Scholar] [CrossRef]
- Arafat, Y.; Wei, X.; Jiang, Y.; Chen, T.; Saqib, H.; Lin, S.; Lin, W. Spatial Distribution Patterns of Root-Associated Bacterial Communities Mediated by Root Exudates in Different Aged Ratooning Tea Monoculture Systems. Int. J. Mol. Sci. 2017, 18, 1727. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Z.; Li, T.; Zhang, X.; Wang, Y.; Yu, H.; He, S.; Liu, T. Effect of tea plantation age on the distribution of soil organic carbon fractions within water-stable aggregates in the hilly region of Western Sichuan, China. Catena 2015, 133, 198–205. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, L.; Wang, Y.; Fan, K.; Xu, Q.; Li, Y.; Ma, Q.; Wang, J.; Ren, W.; Ding, Z. Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leng, Y.; Zhou, Z.; Shang, H.; Ni, K.; Ma, L.; Yi, X.; Cai, Y.; Ji, L.; Ruan, J.; et al. Ecological management model for the improvement of soil fertility through the regulation of rare microbial taxa in tea (Camellia sinensis L.) plantation soils. J. Environ. Manag. 2022, 308, 114595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, J.; Chung, J.-O.; Cho, D.; Roh, J.-H.; Hong, Y.-D.; Kim, W.-G.; Kang, H. Variations in the composition of tea leaves and soil microbial community. Biol. Fert. Soils 2022, 58, 167–179. [Google Scholar] [CrossRef]
- Gu, S.; Hu, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y.; et al. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil Till. Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Zi, H.; Jiang, Y.; Cheng, X.; Li, W.; Huang, X. Change of rhizospheric bacterial community of the ancient wild tea along elevational gradients in Ailao mountain, China. Sci. Rep. 2020, 10, 9203. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, F.; Wu, Z.D.; Jiang, F.Y.; Zhang, W.J.; Weng, B.Q.; You, Z.M. Effects of forestland to tea garden conversion on soil. bacterial community and diversity. J. Northwest AF Univ. (Nat. Sci. Ed.) 2020, 48, 97–106. [Google Scholar] [CrossRef]
- Pang, J.; Li, H.; Tang, X.; Geng, J. Carbon dynamics and environmental controls of a hilly tea plantation in Southeast China. Ecol. Evol. 2019, 9, 9723–9735. [Google Scholar] [CrossRef]
- Hu, L.; Xiang, Z.; Wang, G.; Rafique, R.; Liu, W.; Wang, C. Changes in soil physicochemical and microbial properties along elevation gradients in two forest soils. Scand. J. For. Res. 2016, 31, 242–253. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 2013, 79, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.D.; Brown, G.R.; Hiatt, S.M.; Thibaud-Nissen, F.; Astashyn, A.; Ermolaeva, O.; Farrell, C.M.; Hart, J.; Landrum, M.J.; McGarvey, K.M.; et al. RefSeq: An update on mammalian reference sequences. Nucleic. Acids. Res. 2014, 42, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shi, P.; Hu, Q.; Li, B.; Zhang, T.; Zhang, X.X. Bacterial Community Shift Drives Antibiotic Resistance Promotion during Drinking Water Chlorination. Environ. Sci. Technol. 2015, 49, 12271–12279. [Google Scholar] [CrossRef]
- Watson, M.; McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome. Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms–A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Schops, R.; Goldmann, K.; Herz, K.; Lentendu, G.; Schoning, I.; Bruelheide, H.; Wubet, T.; Buscot, F. Land-Use Intensity Rather Than Plant Functional Identity Shapes Bacterial and Fungal Rhizosphere Communities. Front. Microbiol. 2018, 9, 2711. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Yu, Y.; Wang, J.; Chapman, S.J.; Yao, H.; Zhang, Y. The conversion of subtropical forest to tea plantation changes the fungal community and the contribution of fungi to N(2)O production. Environ. Pollut. 2020, 265, 115106. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xun, W.; Yan, H.; Ma, A.; Xiong, W.; Shen, Q.; Zhang, R. Functional compensation dominates the assembly of plant rhizospheric bacterial community. Soil Biol. Biochem. 2020, 150, 107968. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Montoya-Ciriaco, N.; Munoz-Arenas, L.C.; Hereira-Pacheco, S.; Estrada-Torres, A.; Dendooven, L. Conversion of a High-Altitude Temperate Forest for Agriculture Reduced Alpha and Beta Diversity of the Soil Fungal Communities as Revealed by a Metabarcoding Analysis. Front. Microbiol. 2021, 12, 667566. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.-H.; Guo, H.-J.; Sheng, C.-Y. Assessment of plant species diversity of ancient tea garden communities in Yunnan, Southwest of China. Agrofor. Syst. 2012, 87, 465–474. [Google Scholar] [CrossRef]
- Grosso, F.; Bååth, E.; De Nicola, F. Bacterial and fungal growth on different plant litter in Mediterranean soils: Effects of C/N ratio and soil pH. Appl. Soil Ecol. 2016, 108, 1–7. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Effects of forest degradation on microbial communities and soil carbon cycling: A global meta-analysis. Glob. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z.; Chen, H.Y.H. Soil aggregate-associated bacterial metabolic activity and community structure in different aged tea plantations. Sci. Total Environ. 2019, 654, 1023–1032. [Google Scholar] [CrossRef]
- Zaller, J.G.; Cantelmo, C.; Santos, G.D.; Muther, S.; Gruber, E.; Pallua, P.; Mandl, K.; Friedrich, B.; Hofstetter, I.; Schmuckenschlager, B.; et al. Herbicides in vineyards reduce grapevine root mycorrhization and alter soil microorganisms and the nutrient composition in grapevine roots, leaves, xylem sap and grape juice. Environ. Sci. Pollut. Res. Int. 2018, 25, 23215–23226. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, X.; Nie, C.; Wu, T.; Dai, W.; Liu, H.; Yang, R. Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J. Microb. Biot. 2012, 28, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, L.; Ji, L.; Shi, Y.; Yi, X.; Yang, Q.; Ni, K.; Ruan, J. Long-term nitrogen fertilization indirectly affects soil fungi community structure by changing soil and pruned litter in a subtropical tea (Camellia sinensis L.) plantation in China. Plant Soil 2019, 444, 409–426. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol. Fert. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Harikamal, B.; Aniruddha, R.; Shaon, K.D. Evaluation of plant products and antagonistic microbes against grey blight (Pestalotiopsis theae), a devastating pathogen of tea. Afr. J. Microbiol. Res. 2015, 9, 1263–1267. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, L.; Shu, N.; Jiang, M.; Wang, H.; Huang, Y.; Tong, H. Pestalotiopsis-Like Species Causing Gray Blight Disease on Camellia sinensis in China. Plant Dis. 2018, 102, 98–106. [Google Scholar] [CrossRef]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities Under Different Monoculture Spans. Microb. Ecol. 2017, 75, 739–750. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-Term Coffee Monoculture Alters Soil Chemical Properties and Microbial Communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Liu, L.; Dou, Y.; An, S. Comparison of soil microbial community between planted woodland and natural grass vegetation on the Loess Plateau. For. Ecol. Manag. 2020, 460, 117817. [Google Scholar] [CrossRef]
- Ko, D.; Yoo, G.; Yun, S.-T.; Jun, S.-C.; Chung, H. Bacterial and fungal community composition across the soil depth profiles in a fallow field. J. Ecol. Environ. 2017, 41, 34. [Google Scholar] [CrossRef]
- Morrien, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buee, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Tian, J.; He, N.; Kong, W.; Deng, Y.; Feng, K.; Green, S.M.; Wang, X.; Zhou, J.; Kuzyakov, Y.; Yu, G. Deforestation decreases spatial turnover and alters the network interactions in soil bacterial communities. Soil Biol. Biochem. 2018, 123, 80–86. [Google Scholar] [CrossRef]
- Nakayama, M.; Imamura, S.; Taniguchi, T.; Tateno, R. Does conversion from natural forest to plantation affect fungal and bacterial biodiversity, community structure, and co-occurrence networks in the organic horizon and mineral soil? For. Ecol. Manag. 2019, 446, 238–250. [Google Scholar] [CrossRef]
- Lan, G.; Wu, Z.; Yang, C.; Sun, R.; Chen, B.; Zhang, X. Tropical rainforest conversion into rubber plantations results in changes in soil fungal composition, but underling mechanisms of community assembly remain unchanged. Geoderma 2020, 375, 114505. [Google Scholar] [CrossRef]
- Du, L.; Zheng, Z.; Li, T.; Wang, Y.; Huang, H.; Yu, H.; Ye, D.; Liu, T.; Yao, T.; Zhang, X. Variations of fungal communities within the soils of different tea varieties (Camellia sinensis L.) following long-term plantation. Plant Soil 2022, 477, 665–677. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Brodribb, T.J.; Wang, J.; Yao, X.; Li, S. Long term effects of management practice intensification on soil microbial community structure and co-occurrence network in a non-timber plantation. For. Ecol. Manag. 2020, 459, 117805. [Google Scholar] [CrossRef]
- Ahmed, W.; Jing, H.; Kaillou, L.; Qaswar, M.; Khan, M.N.; Jin, C.; Geng, S.; Qinghai, H.; Yiren, L.; Guangrong, L.; et al. Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China. PLoS ONE 2019, 14, e0216881. [Google Scholar] [CrossRef]
- Yan, P.; Shen, C.; Zou, Z.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Dong, C.; Fu, J.; Han, W.; et al. Increased Soil Fertility in Tea Gardens Leads to Declines in Fungal Diversity and Complexity in Subsoils. Agronomy 2022, 12, 1751. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biot. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Guo, X.P.; Yang, Y.; Niu, Z.S.; Lu, D.P.; Zhu, C.H.; Feng, J.N.; Wu, J.Y.; Chen, Y.R.; Tou, F.Y.; Liu, M.; et al. Characteristics of microbial community indicate anthropogenic impact on the sediments along the Yangtze Estuary and its coastal area, China. Sci. Total Environ. 2019, 648, 306–314. [Google Scholar] [CrossRef]
- Munoz-Arenas, L.C.; Fusaro, C.; Hernandez-Guzman, M.; Dendooven, L.; Estrada-Torres, A.; Navarro-Noya, Y.E. Soil microbial diversity drops with land-use change in a high mountain temperate forest: A metagenomics survey. Environ. Microbiol. Rep. 2020, 12, 185–194. [Google Scholar] [CrossRef]
- Van Geel, M.; Verbruggen, E.; De Beenhouwer, M.; van Rennes, G.; Lievens, B.; Honnay, O. High soil phosphorus levels overrule the potential benefits of organic farming on arbuscular mycorrhizal diversity in northern vineyards. Agr. Ecosyst. Environ. 2017, 248, 144–152. [Google Scholar] [CrossRef]
- Li, P.; Liu, M.; Li, G.; Liu, K.; Liu, T.; Wu, M.; Saleem, M.; Li, Z. Phosphorus availability increases pathobiome abundance and invasion of rhizosphere microbial networks by Ralstonia. Environ. Microbiol. 2021, 23, 5992–6003. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; He, Y.; Lu, J.; Chen, H.; Feng, J. Seasonal variations in soil physicochemical properties and microbial community structure influenced by Spartina alterniflora invasion and Kandelia obovata restoration. Sci. Total Environ. 2021, 797, 149213. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef]
- Shen, C.; Ni, Y.; Liang, W.; Wang, J.; Chu, H. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 2015, 6, 582. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, J.; Yang, L.; He, Q.; Su, Y.; Li, J.; Wang, G.; Qiu, Q. Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China. Forests 2021, 13, 37. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R.; Wang, Z.T. Trends in soil microbial communities in afforestation ecosystem modulated by aggradation phase. For. Ecol. Manag. 2019, 441, 167–175. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Kim, J.M.; Roh, A.S.; Choi, S.C.; Kim, E.J.; Choi, M.T.; Ahn, B.K.; Kim, S.K.; Lee, Y.H.; Joa, J.H.; Kang, S.S.; et al. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Penuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Chang. Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
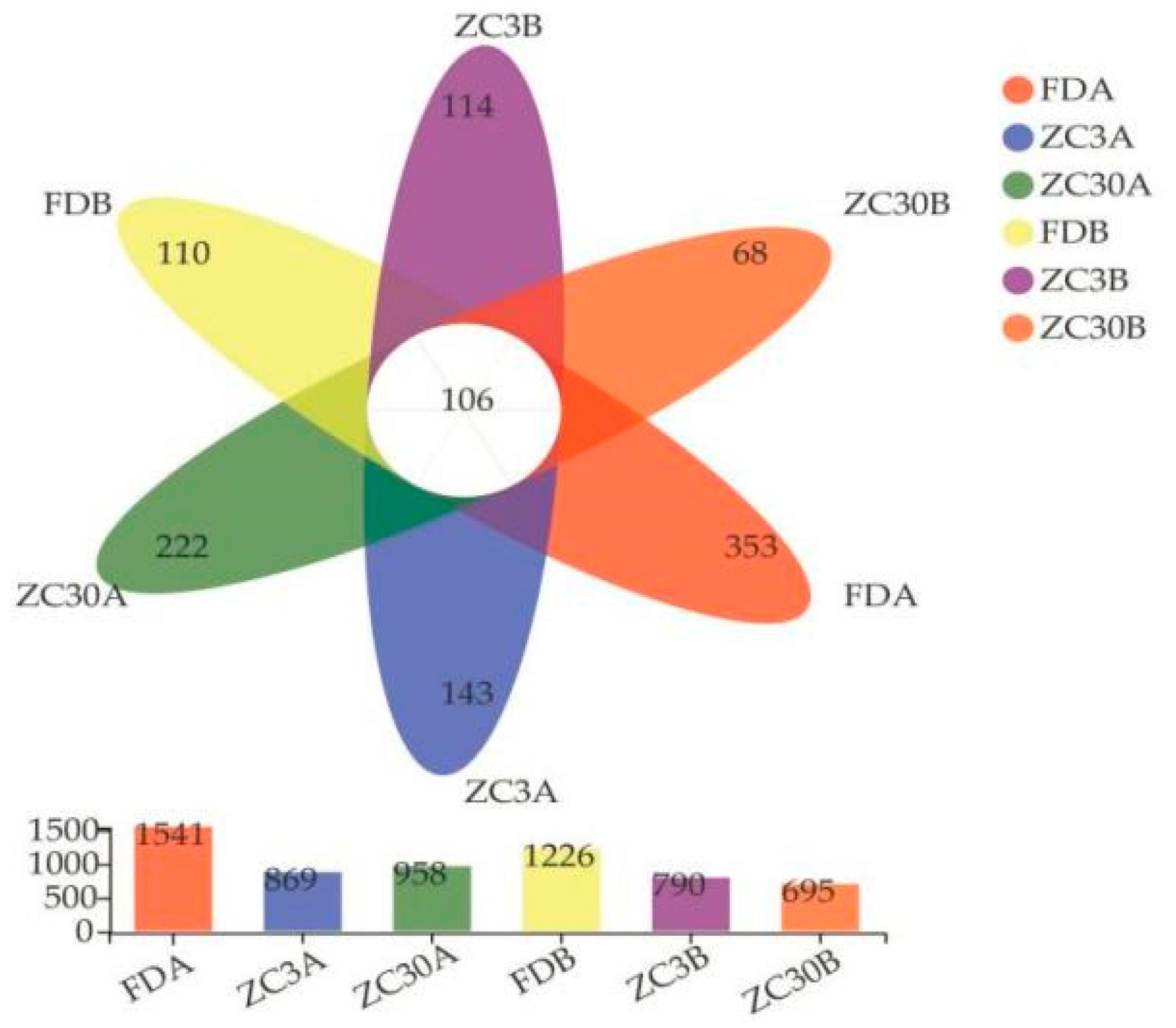

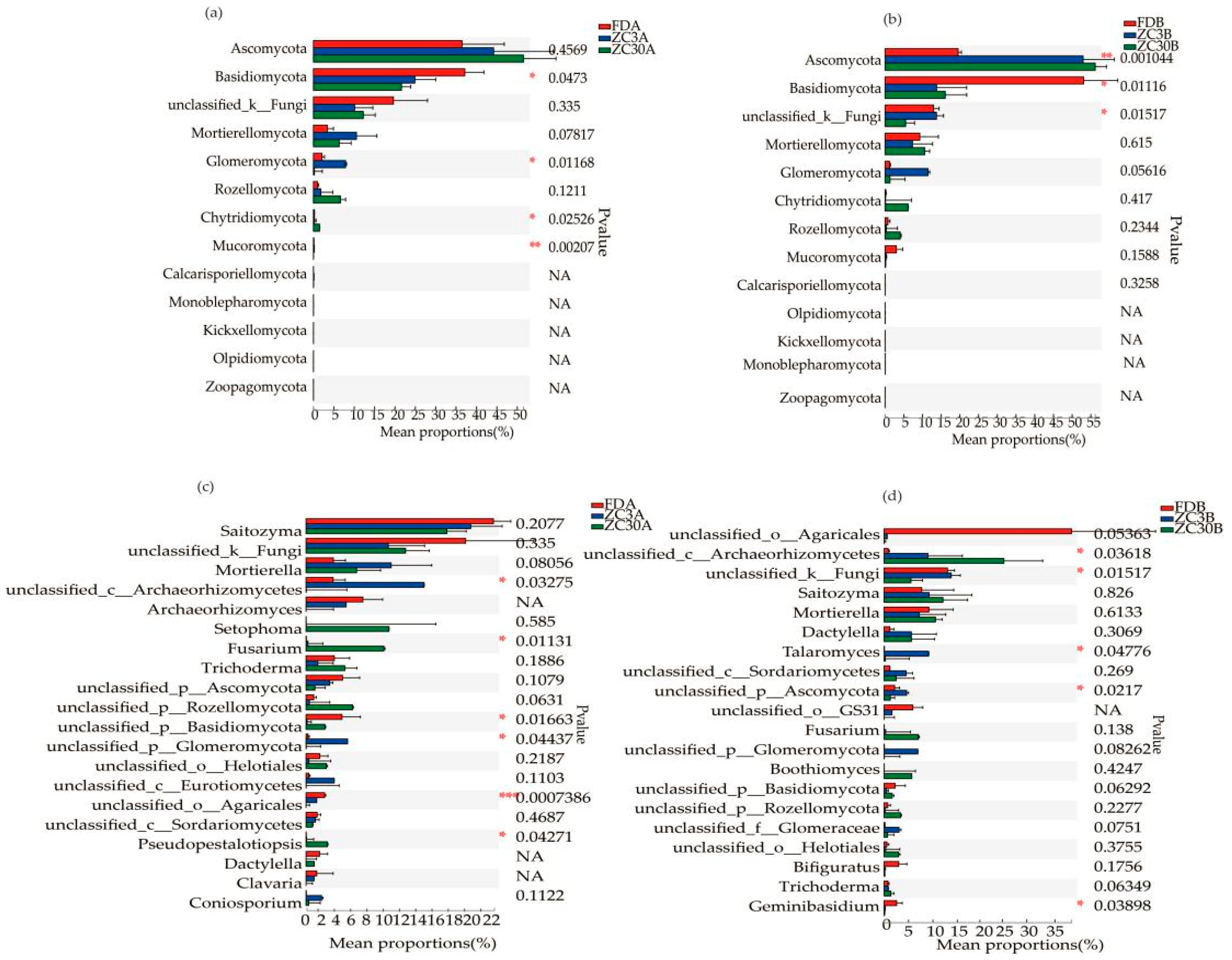


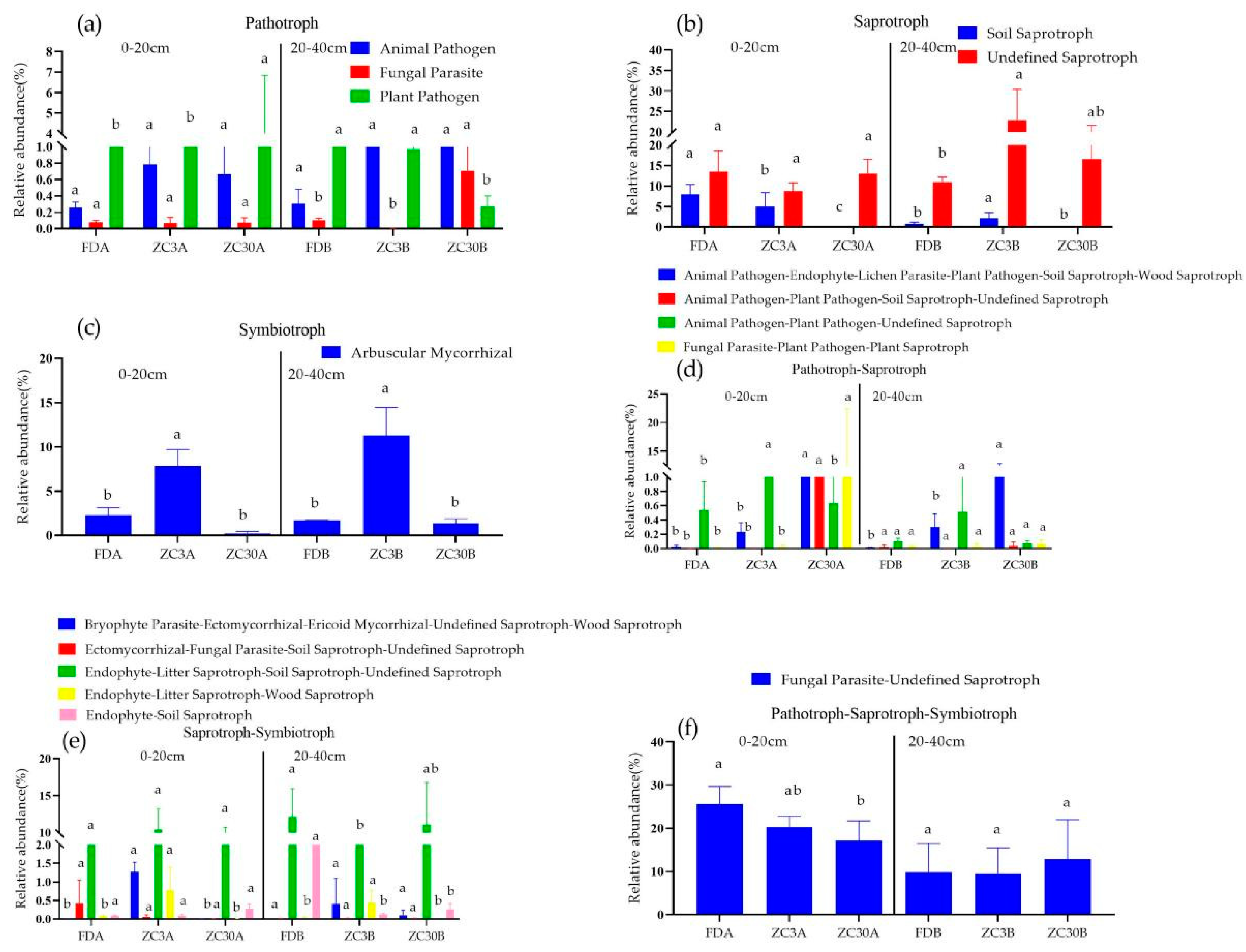

| Soil Depth/cm | Land-Use Types | Sobs | Shannon | Simpson | ACE | Chao1 |
|---|---|---|---|---|---|---|
| 0–20 cm | FDA | 1024.33 ± 62.17 Aa | 3.98 ± 0.21 Aa | 0.07 ± 0.01 Aa | 1164.86 ± 81.69 Aa | 1144.13 ± 62.95 Aa |
| ZC3A | 545.33 ± 29.5 Ac | 3.81 ± 0.36 Aa | 0.07 ± 0.02 Aa | 560.28 ± 36.22 Ac | 558.99 ± 34.59 Ac | |
| ZC30A | 665 ± 46.03 Ab | 3.82 ± 0.36 Aa | 0.07 ± 0.02 Aa | 729.15 ± 44.3 Ab | 732.4 ± 42.39 Ab | |
| 20–40 cm | FDB | 763.66 ± 71.84 Ba | 3.29 ± 0.49 Bb | 0.13 ± 0.08 Aa | 906.68 ± 82.26 Ba | 902.3 ± 74.68 Ba |
| ZC3B | 442.33 ± 125.11 Ab | 3.99 ± 0.30 Aa | 0.04 ± 0.01 Bb | 452.99 ± 127.94 Ab | 462.29 ± 127.85 Ab | |
| ZC30B | 406.66 ± 83.24 Bb | 3.26 ± 0.21 Bb | 0.10 ± 0.02 Aa | 429.81 ± 98.21 Bb | 434.66 ± 87.66 Bb | |
| Land-use types(L) | 50.32 ** | 1.78 NS | 1.94 NS | 69.29 ** | 75.71 ** | |
| Depth(D) L × D | 33.54 ** | 4.90 * | 1.18 NS | 31.03 ** | 33.22 ** | |
| 2.12 NS | 2.89 NS | 2.21 NS | 2.15 NS | 2.65 NS | ||
| R2 | p Value | R2 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Fungi | Topsoil (0–20 cm) | 0.038 | 0.538 | Fungi | FD vs. ZC3 | 0.60 | 0.003 |
| FD vs. ZC30 | 0.59 | 0.004 | |||||
| Subsoil (20–40 cm) | ZC3 vs. ZC30 | 0.28 | 0.016 | ||||
| Network Features | FD | ZC3 | ZC30 |
|---|---|---|---|
| Edges | 695 | 845 | 873 |
| Positive edges | 392 | 509 | 548 |
| Negative edges | 303 | 336 | 325 |
| Nodes | 98 | 99 | 98 |
| Network diameter | 8 | 8 | 7 |
| Modularity | 0.293 | 0.329 | 0.344 |
| Average degree | 14.18 | 17.071 | 17.816 |
| Average path length | 2.825 | 2.61 | 2.522 |
| Average clustering coefficient | 0.545 | 0.603 | 0.611 |
| Network density | 0.146 | 0.174 | 0.184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chen, Y.; Yu, X.; Yu, W.; You, Z.; Yang, Z. Response of the Soil Fungal Community and Its Function during the Conversion of Forestland to Tea Plantations: A Case Study in Southeast China. Forests 2023, 14, 209. https://doi.org/10.3390/f14020209
Wang F, Chen Y, Yu X, Yu W, You Z, Yang Z. Response of the Soil Fungal Community and Its Function during the Conversion of Forestland to Tea Plantations: A Case Study in Southeast China. Forests. 2023; 14(2):209. https://doi.org/10.3390/f14020209
Chicago/Turabian StyleWang, Feng, Yuzhen Chen, Xiaomin Yu, Wenquan Yu, Zhiming You, and Zhenbiao Yang. 2023. "Response of the Soil Fungal Community and Its Function during the Conversion of Forestland to Tea Plantations: A Case Study in Southeast China" Forests 14, no. 2: 209. https://doi.org/10.3390/f14020209
APA StyleWang, F., Chen, Y., Yu, X., Yu, W., You, Z., & Yang, Z. (2023). Response of the Soil Fungal Community and Its Function during the Conversion of Forestland to Tea Plantations: A Case Study in Southeast China. Forests, 14(2), 209. https://doi.org/10.3390/f14020209






