Forest Structure, Diversity and Dynamics in Terra Firme and Igapó Gallery Forests in the Colombian Orinoco Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.3.1. Diversity and Structure
2.3.2. Floristic and Soil Composition
2.3.3. Estimation of the Aboveground Biomass of Trees
2.3.4. Forest Dynamics
3. Results
3.1. Forest Structure and Diversity
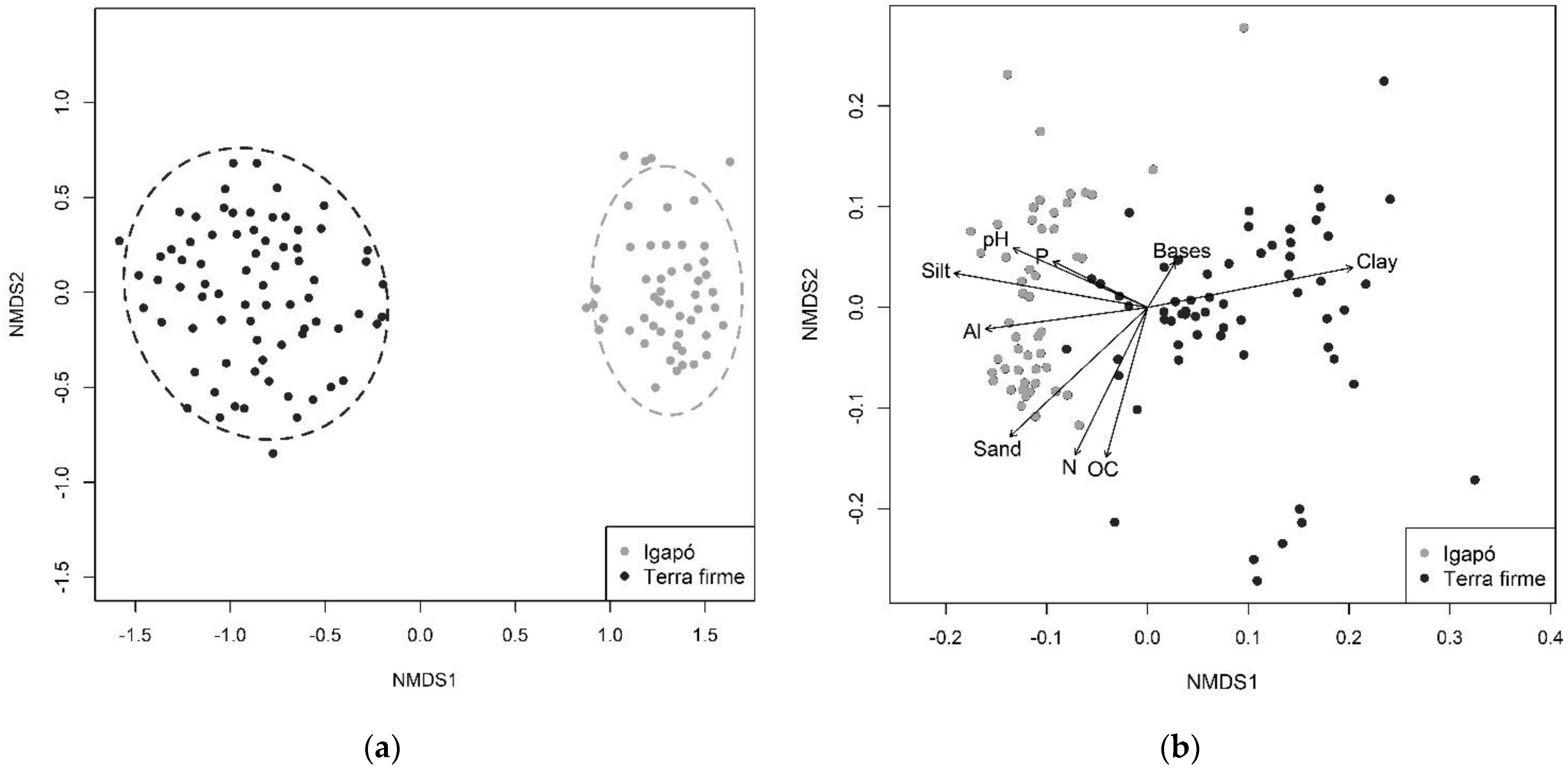
3.2. Floristic and Soil Composition
3.3. Forest Dynamics
3.4. Estimation of Biomass Stock and Carbon Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, D.M. Análisis Florístico, Estructural y Biotipológico Foliar de La Vegetación Leñosa En Bosques Ribereños de La Cuenca Baja Del Río Pauto (Casanare-Colombia). Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2014. [Google Scholar]
- Capon, S.J.; Dowe, J.L. Diversity and Dynamics of Riparian Vegetation. In Principles for Riparian Lands Management; Lovett, S., Price, P., Eds.; Land & Water Australia: Camberra, Australia, 2007; pp. 13–32. [Google Scholar]
- Veneklaas, E.J.; Fajardo, A.; Obregon, S.; Lozano, J. Gallery Forest Types and Their Environmental Correlates in a Colombian Savanna Landscape. Ecography 2005, 28, 236–252. [Google Scholar] [CrossRef]
- Casas, L.F.; Stevenson, P.R. Variación de Biomasa Aérea y Densidad de Madera En Bosques de Tierras Bajas Con Planos de Inundación de Aguas Negras y Aguas Blancas. Master’s Thesis, Universidad de los Andes, Bogotá, Colombia, 2013. [Google Scholar]
- Vásquez, L.C.; Matallana, C.L. Identificación de Los Servicios Ecosistémicos Que Proveen Las Diferentes Áreas Protegidas En La Orinoquia Colombiana. Biodivers. En La Práctica 2016, 1, 77–127. [Google Scholar]
- Redford, K.H.; Da Fonseca, G.A.B. The Role of Gallery Forests in The Zoogeography of the Cerrado’s Non-Volant Mammalian Fauna. Biotropica 1986, 18, 126–135. [Google Scholar] [CrossRef]
- Calderón García, A. Revisión Literaria de Los Bosques de Galería. 2010. Available online: http://repositorio.utn.edu.ec/bitstream/123456789/103/14/03%20REC%20119%20Cap2%20REVISION%20DE%20LITERATURA.pdf (accessed on 20 January 2021).
- Andrade, H.; Segura, M.; Somarriba, E.; Villalobos, M. Valoración Biofísica y Financiera de La Fijación de Carbono Por Uso Del Suelo En Fincas Cacaoteras Indígenas de Talamanca, Costa Rica. Agroforestería en las Américas 2008, 46, 45–50. [Google Scholar]
- Valera, V.; Carvajal, N. Valoración Económica Del Secuestro de Carbono En Un Sector Del Parque Nacional Waraira Repano (Caracas, Venezuela). An. de la Univ. Metrop. 2014, 14, 241–264. [Google Scholar]
- Kellman, M.; Tackaberry, R.; Rigg, L. Structure and Function in Two Tropical Gallery Forest Communities: Implications for Forest Conservation in Fragmented Systems. J. Appl. Ecol. 1998, 35, 195–206. [Google Scholar] [CrossRef]
- Bruening, E.F. Conservation and Management of Tropical Rainforests. In An Integrated Approach to Sustainability, 2nd ed.; CAB International: Wallingford, UK, 1996. [Google Scholar]
- Bawa, K.S.; Seidler, R. Natural Forest Management and Conservation of Biodiversity in Tropical Forests. Conserv. Biol. 1998, 12, 46–55. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar] [CrossRef]
- Londoño, A.C. Flora and Dynamics of an Upland and a Floodplain Forest in Peña Roja, Colombian Amazonia. Ph.D. Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2011. [Google Scholar]
- Silver, W.L.; Ostertag, R.; Lugo, A.E. The Potential for Carbon Sequestration through Reforestation of Abandoned Tropical Agricultural and Pasture Lands. Restor. Ecol. 2000, 8, 394–407. [Google Scholar] [CrossRef]
- Brown, S. Measuring Carbon in Forests: Current Status and Future Challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science. 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, J.; Baker, T.R.; Lewis, S.L.; Lopez-Gonzalez, G.; Phillips, O.L. The RAINFOR Database: Monitoring Forest Biomass and Dynamics. J. Veg. Sci. 2007, 18, 535–542. [Google Scholar] [CrossRef]
- Phillips, O.L.; Lewis, S.L. Recent Changes in Tropical Forest Biomass and Dynamics. In Forests and Global Change; Coomes, D., Burslem, D.F.R.P., Simonson, W.D., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 77–108. [Google Scholar] [CrossRef] [Green Version]
- Phillips, O.L.; Baker, T.R.; Arroyo, L.; Higuchi, N.; Killeen, T.J.; Laurance, W.F.; Lewis, S.L.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; et al. Pattern and Process in Amazon Tree Turnover, 1976–2001. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 381–407. [Google Scholar] [CrossRef]
- Veblen, T.T.; Kitzberger, T.; Villalba, R. Nuevos Conocimientos En Ecología y Su Influencia Sobre El Conocimiento de La Dinámica de Los Bosques Del Sur de Argentina y Chile. In Ecología y Manejo De Bosques en Argentina; Goya, J.F., Frangi, J.L., Arturi, M.F., Eds.; Universidad Nacional de la Plata: La Plata, Argentina, 2005; pp. 442–447. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-Wide Variations in Amazon Forest Structure and Function Are Mediated by Both Soils and Climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef] [Green Version]
- Martins, K.G.; Marques, M.C.M.; Dos Santos, E.; Marques, R. Effects of Soil Conditions on the Diversity of Tropical Forests across a Successional Gradient. For. Ecol. Manag. 2015, 349, 4–11. [Google Scholar] [CrossRef]
- Phillips, O.L.; Hall, P.; Gentry, A.H.; Sawyer, S.A.; Vásquez, R. Dynamics and Species Richness of Tropical Rain Forests. Proc. Natl. Acad. Sci. USA 1994, 91, 2805–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, R.E.; Fahey, T.J.; Patrick, M.H.; Battles, J.J. Patterns of Growth, Recruitment, Mortality and Biomass across an Altitudinal Gradient in a Neotropical Montane Forest, Dominican Republic. J. Trop. Ecol. 2012, 28, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Nebel, G.; Kvist, L.; Vanclay, J.; Vidaurre, H. Ecology and Management of Flood Plain Forests in the Peruvian Amazon. For. Ecol. Manag. 2001, 150, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.C.M.; Burslem, D.F.R.P.; Britez, R.M.; Silva, S.M. Dynamics and Diversity of Flooded and Unflooded Forests in a Brazilian Atlantic Rain Forest: A 16-Year Study. Plant Ecol. Divers. 2009, 2, 57–64. [Google Scholar] [CrossRef]
- Luize, B.G.; Magalhães, J.L.L.; Queiroz, H.; Lopes, M.A.; Venticinque, E.M.; Leão de Moraes Novo, E.M.; Silva, T.S.F. The Tree Species Pool of Amazonian Wetland Forests: Which Species Can Assemble in Periodically Waterlogged Habitats? PLoS ONE 2018, 13, e0198130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, P.; Wittmann, F. Struggle in the Flood: Tree Responses to Flooding Stress in Four Tropical Floodplain Systems. AoB Plants 2010, plq003. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, F.; Schöngart, J.; Junk, W.J. Phytogeography, Species Diversity, Community Structure and Dynamics of Central Amazonian Floodplain Forest. In Amazonian Floodplain Forest: Ecophysiology, Biodiversity and Sustainable Management; Junk, W.J., Piedade, M.T., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2010; pp. 61–102. [Google Scholar] [CrossRef]
- Junk, W.J. The Central Amazon Floodplain. Ecology of a Pulsing System; Springer-Verlag: Heidelberg, Berlin; New York, NY, USA, 1997. [Google Scholar] [CrossRef]
- Parolin, P.; De Simone, O.; Haase, K.; Waldhoff, D.; Rottenberger, S.; Kuhn, U.; Kesselmeier, J.; Kleiss, B.; Schmidt, W.; Piedade, M.T.; et al. Central Amazonian Floodplain Forest: Tree Adaptations in a Pulsing System. Bot. Rev. 2004, 70, 357–380. [Google Scholar] [CrossRef]
- Wittmann, F.; Schöngart, J.; Montero, J.C.; Motzer, T.; Junk, W.J.; Piedade, M.T.F.; Queiroz, H.L.; Worbes, M. Tree Species Composition and Diversity Gradients in White-Water Forests across the Amazon Basin. J. Biogeogr. 2006, 33, 1334–1347. [Google Scholar] [CrossRef]
- Silvertown, J.; Araya, Y.; Gowing, D. Hydrological Niches in Terrestrial Plant Communities: A Review. J. Ecol. 2015, 103, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, F.; Marques, M.C.M.; Júnior, G.D.; Budke, J.C.; Piedade, M.T.F.; De Wittmann, A.O.; Montero, J.C.; De Assis, R.L.; Targhetta, N.; Parolin, P.; et al. The Brazilian Freshwater Wetscape: Changes in Tree Community Diversity and Composition on Climatic and Geographic Gradients. PLoS ONE 2017, 12, e0175003. [Google Scholar] [CrossRef]
- Parolin, P.; Ferreira, L.V.; Junk, W.J. Central Amazonian Floodplains: Effect of Two Water Types on the Wood Density of Trees. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 1998, 26, 1106–1112. [Google Scholar] [CrossRef]
- Baker, T.R.; Burslem, D.F.R.P.; Swaine, M.D. Associations between Tree Growth, Soil Fertility and Water Availability at Local and Regional Scales in Ghanaian Tropical Rain Forest. J. Trop. Ecol. 2003, 19, 109–125. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Schöngart, J.; Wittmann, F. Biomass and Net Primary Production of Central Amazonian Floodplain Forests. In Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management; Junk, W.J., Piedade, M.T., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer Science+Business Media B.V: Berlin/Heidelberg, Germany, 2010; pp. 347–388. [Google Scholar] [CrossRef]
- Núñez-Avellaneda, L.A.; Castro, M.I.; Mestre, G.; Lozano, L. Los Bosques de Galería Conectores de Vida. Ámbito Investig. 2019, 4, 14–19. [Google Scholar]
- Garibello, J. Estructura de La Vegetación Leñosa Del Ecotono Bosque de Galería Sabana En La Altillanura de La Cuenca Alta Del Río Tomo (Estación Biológica Bachaqueros—Departamento Del Vichada). Bachelor´s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2000. [Google Scholar]
- Parrado-Rosselli, A.; Gonzales-M, R.; García, H. Estado Actual de La Investigación Científica Publicada Sobre Los Bosques de Colombia. Biodivers. En La Práctica 2016, 1, 177–197. [Google Scholar]
- Stevenson, P.R.; Aldana, A.M.; Cárdenas, S.; Negret, P.J. Flooding and Soil Composition Determine Beta Diversity of Lowland Forests in Northern South America. Biotropica 2018, 50, 568–577. [Google Scholar] [CrossRef]
- Correa-Gómez, D.F.; Stevenson, P.R. Estructura y Diversidad de Bosques de Los Llanos Orientales Colombianos (Reserva Tomo Grande, Vichada). Orinoquia 2010, 14, 31–48. [Google Scholar]
- Felfili, J.M. Diameter and Height Distributions in a Gallery Forest Tree Community and Some of Its Main Species in Central Brazil over a Six-Year Period (1985–1991). Braz. J. Bot. 1997, 20, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Parolin, P. Submerged in Darkness: Adaptations to Prolonged Submergence by Woody Species of the Amazonian Floodplains. Ann. Bot. 2009, 103, 359–376. [Google Scholar] [CrossRef] [Green Version]
- Duivenvoorden, J. Patterns of tree species richness in rain forest of the middle Caquetá area, Colombia, NW Amazonia. Biotropica 1996, 28, 142–158. [Google Scholar] [CrossRef]
- Ter Steege, H.; Sabatier, D.; Castellanos, H.; Van Andel, T.; Duivenvoorden, J.; De Oliveira, A.; Ek, R.; Lilwah, R.; Maas, P.; Mori, S. An Analysis of the Floristic Composition and Diversity of Amazonian Forests Including Those of the Guiana Shield. J. Trop. Ecol. 2000, 16, 801–828. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, A.O.; Lopes, A.; Dos Santos Conserva, A.; Wittmann, F.; Piedade, M.T. Seed Germination and Seedlong Establishment of Amazonian Floodplain Trees. In Amazonian Floodplain Forest: Ecophysiology, Biodiversity and Sustainable Management; Junk, W.J., Piedade, M.T., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2010; Volume 210, pp. 259–281. [Google Scholar]
- Furch, K. Chemistry of Várzea and Igapó Soils and Nutrient Inventory of Their Floodplain Forests. Ecol. Stud. 1997, 126, 47–67. [Google Scholar] [CrossRef]
- Hurtado, G.; González, O.C.; Montaña, J.A. Tercera Parte: Aspectos Departamentales; Henríquez, M., Ed.; Atlas climatológico de Colombia; Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia IDEAM: Bogotá, Colombia, 2005. [Google Scholar]
- Hernández, J.; Sánchez, H. Sabanas de Colombia. In Sabanas Naturales de Colombia; Banco de Occidente: Cali, Colombia, 1994; pp. 57–161. [Google Scholar]
- Romero, M. Estado de La Biodiversidad En El Piedemonte Orinoquense. In Sabanas y Bosques Asociados Al Norte Del Río Guaviare: Nivel de Ecosistemas; Romero, M., Maldonado-Ocampo, J., Eds.; Instituto de Investigaciones de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2009. [Google Scholar]
- Prance, G.T. Notes on the Vegetation of Amazonia III. The Terminology of Amazonian Forest Types Subject to Inundation. Brittonia 1979, 31, 26–38. [Google Scholar] [CrossRef]
- Quiñones-Fernández, M.; Flórez-Ayala, C.; Estupiñán-Suarez, L. Historias Contadas Por El Agua. Frecuencias de Inundación. In Colombia Anfibia. Un País De Humedales; Jaramillo, U., Cortés-Duque, J., Flórez-Ayala, C., Eds.; Instituto de Investigación de recursos biológicos Alexander von Humboldt: Bogotá, Colombia, 2015; pp. 78–81. [Google Scholar]
- Phillips, O.; Baker, T.; Feldpausch, T.; Brienen, R. RAINFOR Manual de Campo Para La Remedición y Establecimiento de Parcelas; Pan-Amazonia. 2009. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwir7Mb99pH0AhXlr1YBHZUZDcAQFnoECAMQAQ&url=http%3A%2F%2Fwww.rainfor.org%2Fupload%2FManualsSpanish%2FManual%2FRAINFOR_field_manual_version2016_ES.pdf&usg=AOvVaw3Cd2ugEh_fbtl2XKkH6eB4 (accessed on 11 April 2020).
- Ter Steege, H.; Pitman, N.; Sabatier, D.; Castellanos, H.; Van Der Hout, P.; Daly, D.C.; Silveira, M.; Phillips, O.; Vasquez, R.; Van Andel, T.; et al. A Spatial Model of Tree α-Diversity and Tree Density for the Amazon. Biodivers. Conserv. 2003, 12, 2255–2277. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 26 August 2021).
- Henderson, A. Evolution and Ecology of Palms; The New York Botanical Garden Press: Bronx, Nueva York, NY, USA, 2002. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Rangel-Ch, J.O.; Lowy-C, P.D.; Aguilar-P, M. Métodos de Estudio de la Vegetación. Colombia Diversidad Biótica II: Tipos de Vegetación en Colombia; Universidad Nacional de Colombia, Instituto de Ciencias Naturales: Bogotá, Colombia, 1997; pp. 59–87. [Google Scholar]
- Alvarez, E.; Duque, A.; Saldarriaga, J.; Cabrera, K.; de las Salas, G.; del Valle, I.; Lema, A.; Moreno, F.; Orrego, S.; Rodríguez, L. Tree Above-Ground Biomass Allometries for Carbon Stocks Estimation in the Natural Forests of Colombia. For. Ecol. Manag. 2012, 267, 297–308. [Google Scholar] [CrossRef]
- Holdridge, L.R.; Grenke, W.C.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environments in Tropical Life Zones; Pergamon Press: Oxford, UK, 1971. [Google Scholar]
- Casas, L.F.; Aldana, A.M.; Henao-Diaz, F.; Villanueva, B.; Stevenson, P.R. Specific Gravity of Woody Tissue from Lowland Neotropical Plants: Differences among Forest Types. Ecology 2017, 98, 1474. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.L.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Malhi, Y.; Almeida, S.; Higuchi, N.; Laurance, W.F.; Neill, D.A.; Silva, J.N.M.; et al. Concerted Changes in Tropical Forest Structure and Dynamics: Evidence from 50 South American Long-Term Plots. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Phillips, O.L.; Sheil, D.; Vinceti, B.; Baker, T.R.; Brown, G.; Andrew, W.H.; Niro, H.; David, W.; Laurance, W.F.; et al. Tropical forest tree mortality, recruitment and turnover rates: Calculation, interpretation and comparison when census intervals vary. J. Ecol. 2004, 92, 929–944. [Google Scholar] [CrossRef]
- Umaña, M.N.; Norden, N.; Cano, Á.; Stevenson, P.R. Determinants of Plant Community Assembly in a Mosaic of Landscape Units in Central Amazonia: Ecological and Phylogenetic Perspectives. PLoS ONE 2012, 7, e45199. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments a 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.V. Effects of the Duration of Flooding on Species Richness and Floristic Composition in Three Hectares in the Jau National Park in Floodplain Forests in Central Amazonia. Biodivers. Conserv. 1997, 6, 1353–1363. [Google Scholar] [CrossRef]
- De Jesus Veiga Carim, M.; Wittmann, F.K.; Piedade, M.T.F.; Da Silva Guimarães, J.R.; de Cássia Leôncio Tostes, L. Composition, Diversity, and Structure of Tidal “Várzea” and “Igapó” Floodplain Forests in Eastern Amazonia, Brazil. Rev. Bras. Bot. 2017, 40, 115–124. [Google Scholar] [CrossRef]
- Mori, G.B.; Schietti, J.; Poorter, L.; Piedade, M.T.F. Trait Divergence and Habitat Specialization in Tropical Floodplain Forests Trees. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Targhetta, N.; Kesselmeier, J.; Wittmann, F. Effects of the Hydroedaphic Gradient on Tree Species Composition and Aboveground Wood Biomass of Oligotrophic Forest Ecosystems in the Central Amazon Basin. Folia Geobot. 2015, 50, 185–205. [Google Scholar] [CrossRef]
- Ferreira, L.V. Species Richness and Floristic Composition in Four Hectares in the Jaú National Park in Upland Forest in Central Amazonia. Biodivers. Conserv. 1998, 7, 1349–1364. [Google Scholar] [CrossRef]
- Parolin, J.A.; Rodrigues, W.A.; Amaral, I.; Piedade, M.T.F. Floristic Study of Igapó Floodplain in Central Amazonia, Brazil (Tarumã-Mirim, Rio Negro). Amazoniana 2004, 18, 29–47. [Google Scholar]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Short-Term dynamics of a neotropical forest. Bioscience 1992, 42, 822–828. [Google Scholar] [CrossRef]
- Parolin, P. Radial Gradients in Wood Specific Gravity in Trees of Central Amazonian Floodplains. IAWA J. 2002, 23, 449–457. [Google Scholar] [CrossRef]
- Saatchi, S.; Houghton, R.A.; Dos Santos Alvalá, R.C.; Soares, J.V.; Yu, Y. Distribution of Aboveground Live Biomass in the Amazon Basin. Glob. Chang. Biol. 2007, 13, 816–837. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J.; Holland, E.A. Net Primary Production in Tropical Forests: An Evaluation and Synthesis of Existing Field Data. Ecol. Appl. 2001, 11, 371–384. [Google Scholar] [CrossRef]
- Yepes, A.; Herrera, J.; Phillips, J.; Cabrera, E.; Galindo, G.; Granados, E.; Duque, Á.; Barbosa, A.; Olarte, C.; Cardona, M. Contribución de Los Bosques Tropicales de Montaña En El Almacenamiento de Carbono En Colombia. Rev. Biol. Trop. 2014, 63, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, I.C.; Aldana, A.M.; Stevenson, P. Dinámica de Bosques En Diferentes Escenarios de Tala Selectiva En El Magdalena Medio (Colombia). Colomb. For. 2016, 19, 195–208. [Google Scholar] [CrossRef]
- Landínez, A.; Linares, E. Plantas Dendroenergéticas Utilizadas Por Una Comunidad Indígena Piapoco En Guainía, Colombia. Cult. Científica 2006, 4, 29–35. [Google Scholar]



| Forest Type | Mean No. of Individuals/ha (Min.–Max.) | Mean No. of Species /ha (Min.–Max.) | Fisher’s α | eH | Tilted Trees (%) (Min.–Max.) |
|---|---|---|---|---|---|
| Terra firme | 450.3 (440–478) | 77 (65–85) | 30.39 | 49.89 | 2.07 (3.2–1.1) |
| Igapó | 691.5 (607–776) | 41 (36–46) | 12.52 | 22.59 | 8.53 (6.1–10.4) |
| Forest Type | Mean AGB (t/ha) | Change in AGB (t/ha/Year) | Carbon Stock (t/ha) | Carbon Accumulation (t/ha/Year) |
|---|---|---|---|---|
| Terra firme (n = 3) | 128.7 ± 22.14 | 0.73 ± 1.15 | 57.92 ± 9.96 | 0.33 ± 0.51 |
| Igapó (n = 2) | 169.9 ± 0.83 | 1.47 ± 0.06 | 76.46 ± 0.37 | 0.66 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Abella, J.S.; Aldana, A.M.; Correa, D.F.; Casas, L.F.; Stevenson, P.R. Forest Structure, Diversity and Dynamics in Terra Firme and Igapó Gallery Forests in the Colombian Orinoco Basin. Forests 2021, 12, 1568. https://doi.org/10.3390/f12111568
González-Abella JS, Aldana AM, Correa DF, Casas LF, Stevenson PR. Forest Structure, Diversity and Dynamics in Terra Firme and Igapó Gallery Forests in the Colombian Orinoco Basin. Forests. 2021; 12(11):1568. https://doi.org/10.3390/f12111568
Chicago/Turabian StyleGonzález-Abella, Juan S., Ana M. Aldana, Diego F. Correa, Luisa Fernanda Casas, and Pablo R. Stevenson. 2021. "Forest Structure, Diversity and Dynamics in Terra Firme and Igapó Gallery Forests in the Colombian Orinoco Basin" Forests 12, no. 11: 1568. https://doi.org/10.3390/f12111568






