Differential Response of Macrobenthic Abundance and Community Composition to Mangrove Vegetation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Macrobenthic Sampling
2.3. Sediment Features
2.4. Mangrove Forest Structure
2.5. Statistical Analyses
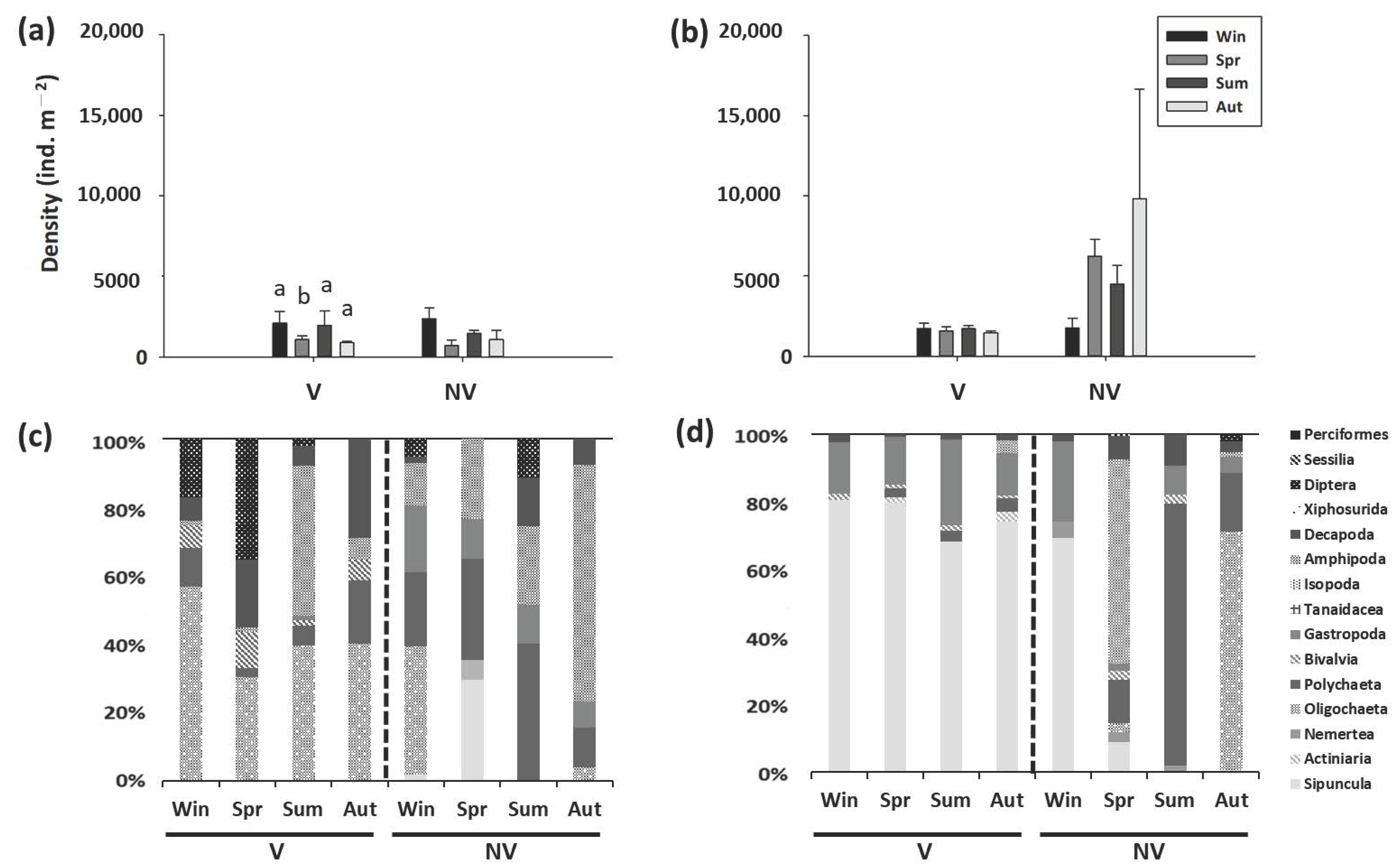
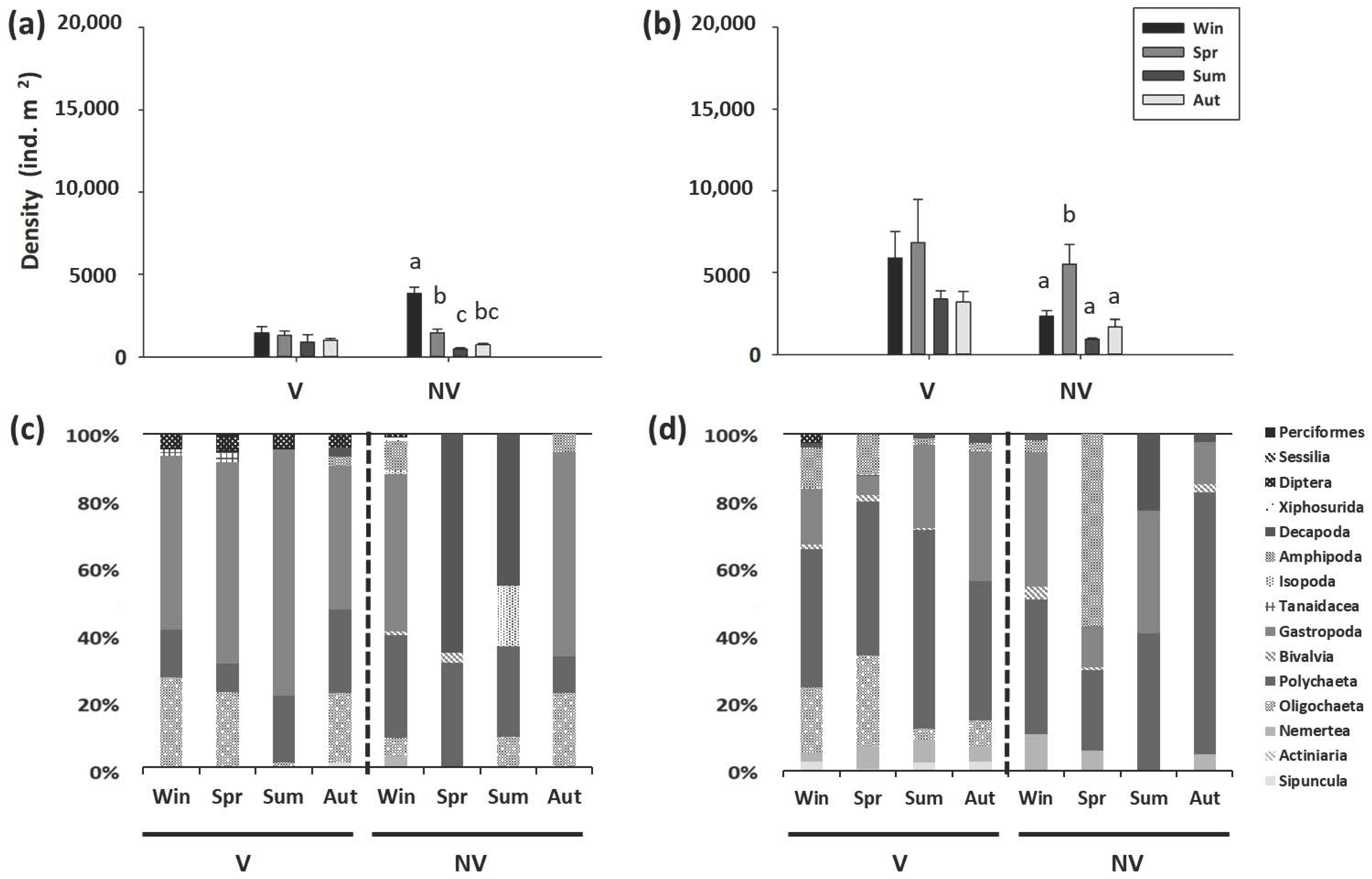
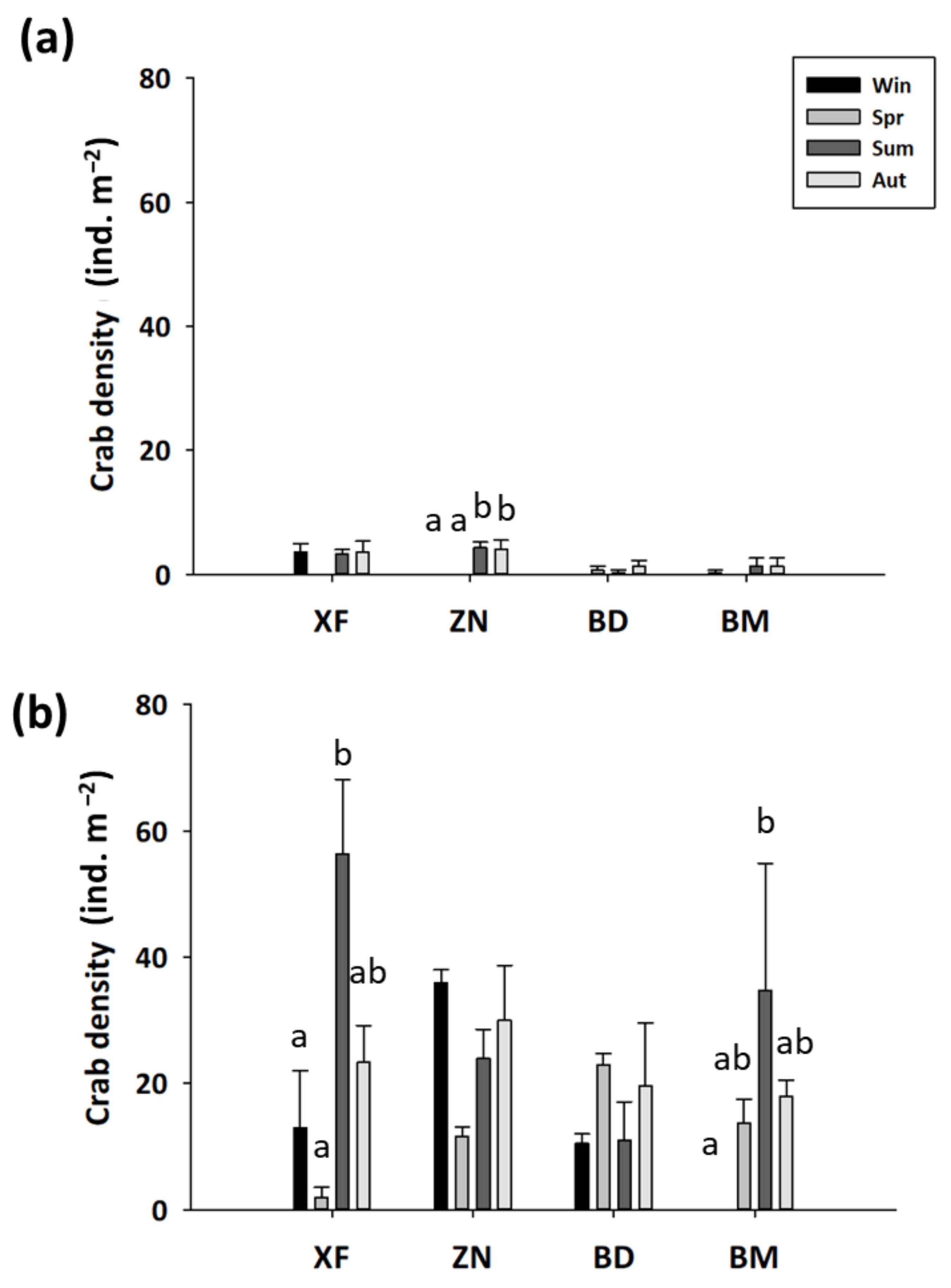
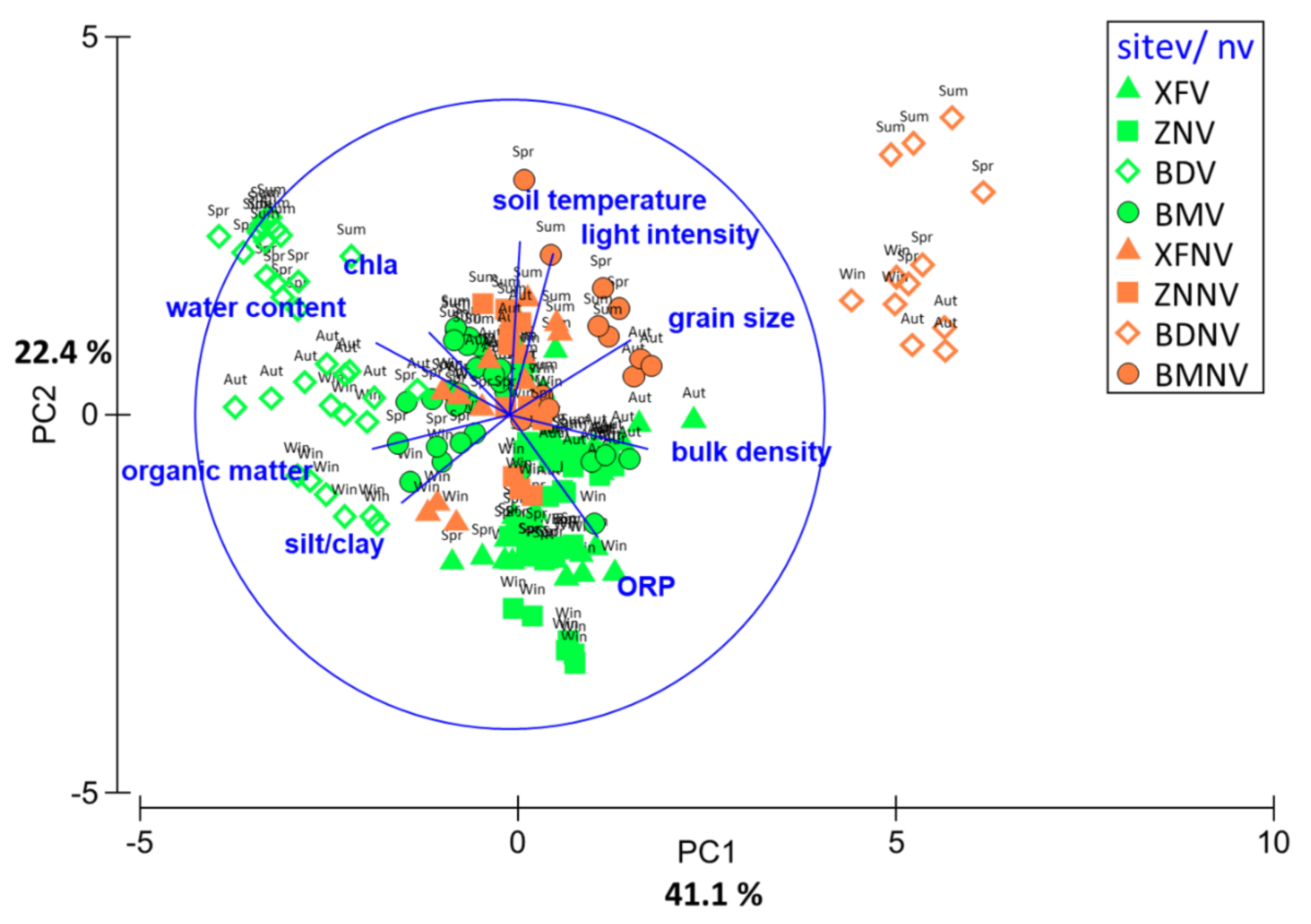
3. Results
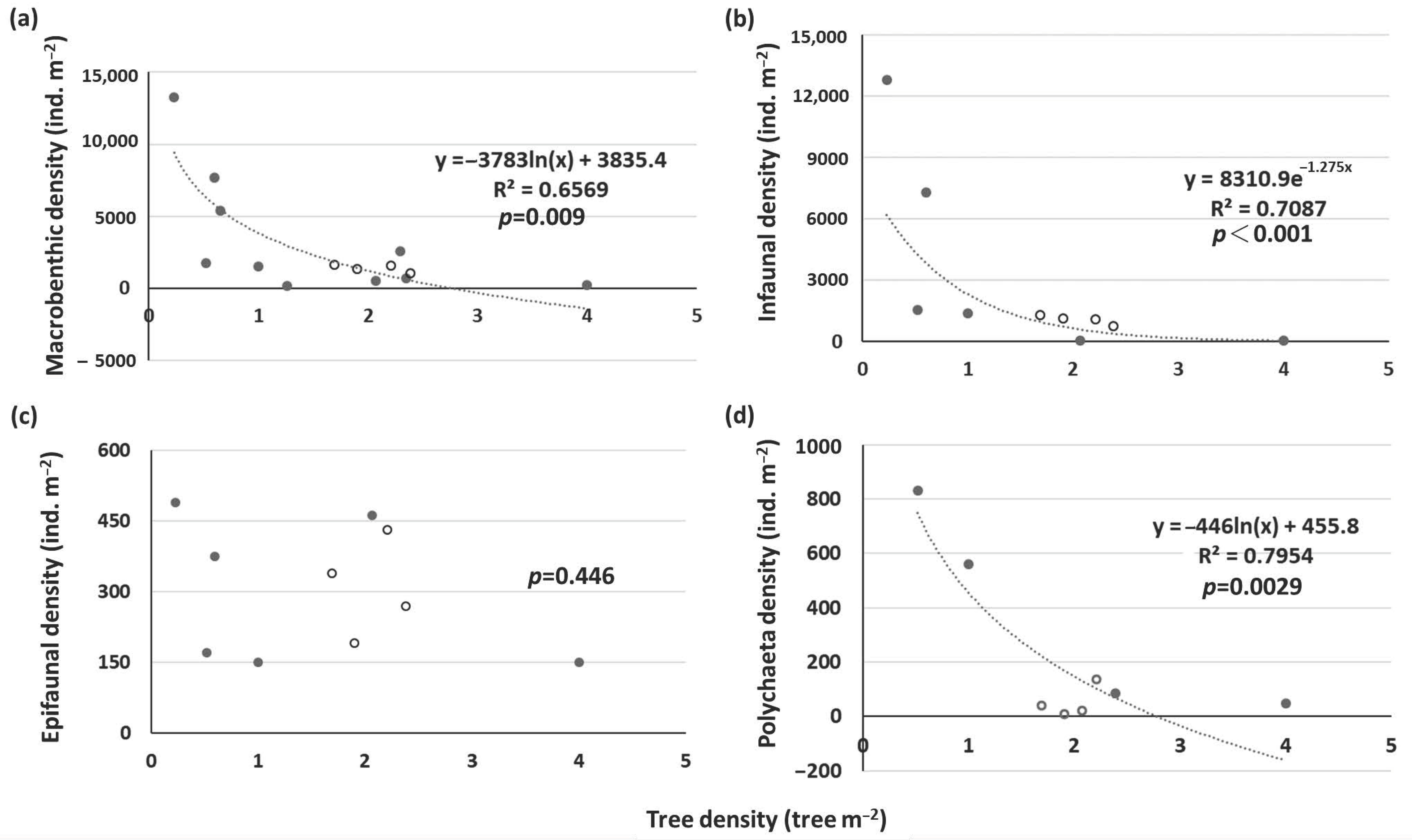
3.1. Mangrove Forest Structure
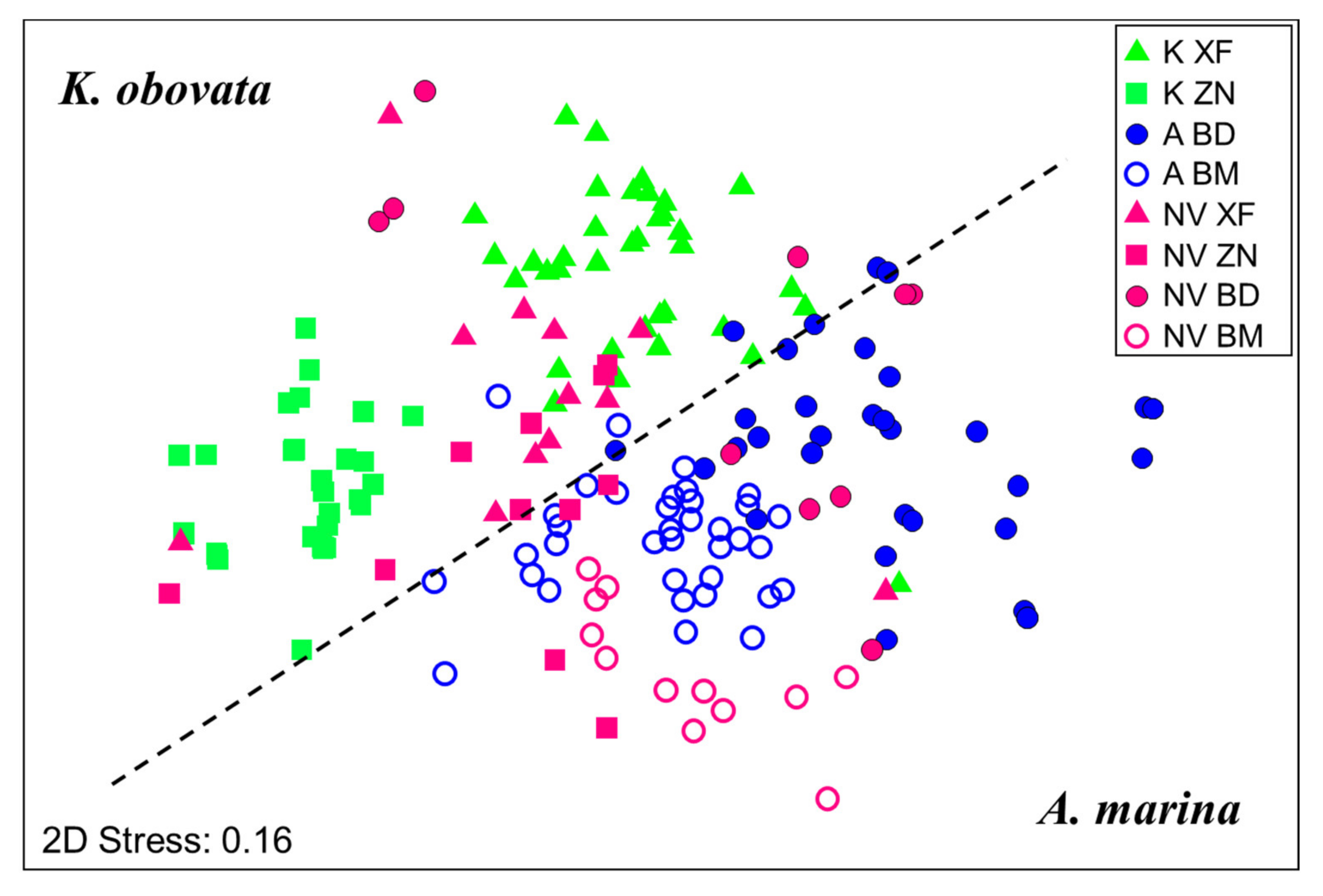
3.2. Sites Response
3.3. Seasonal Response
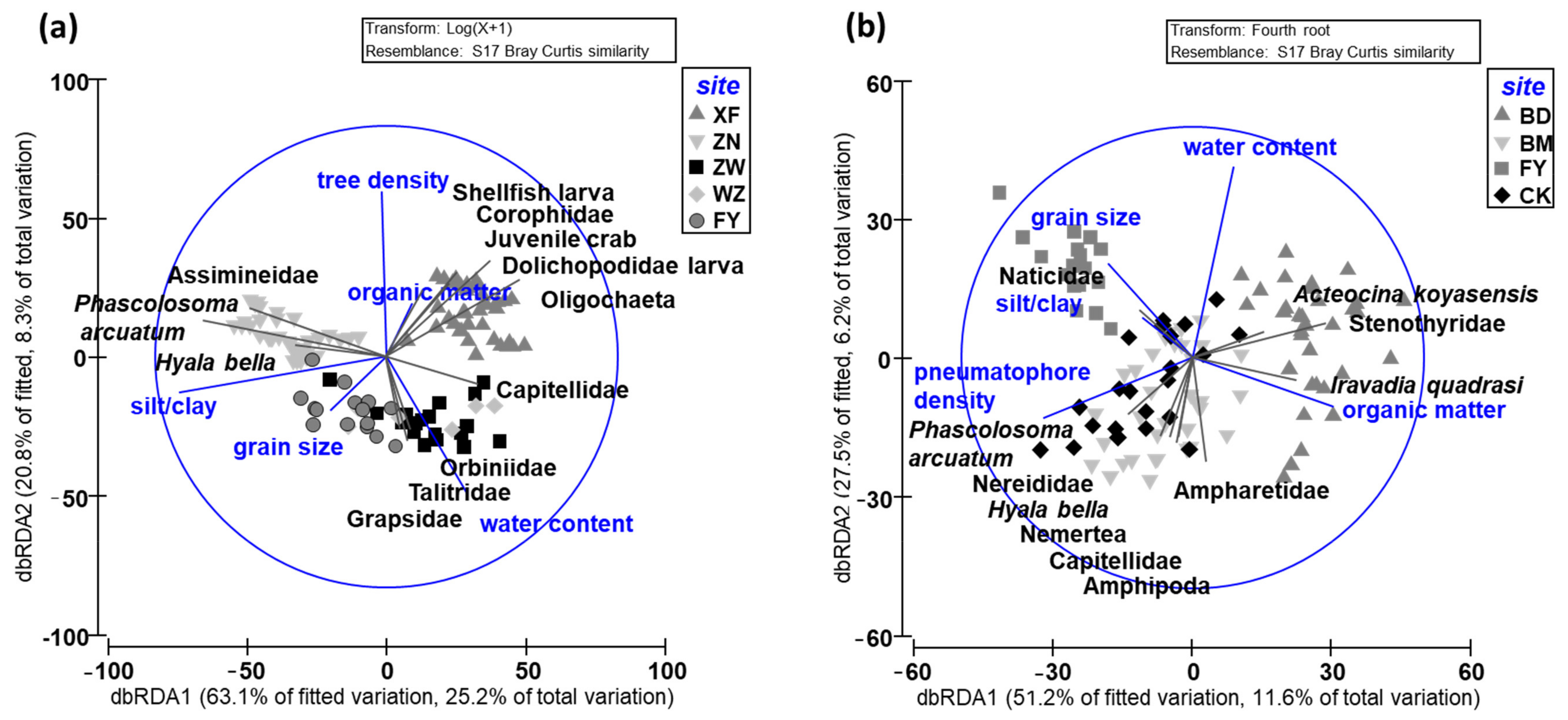
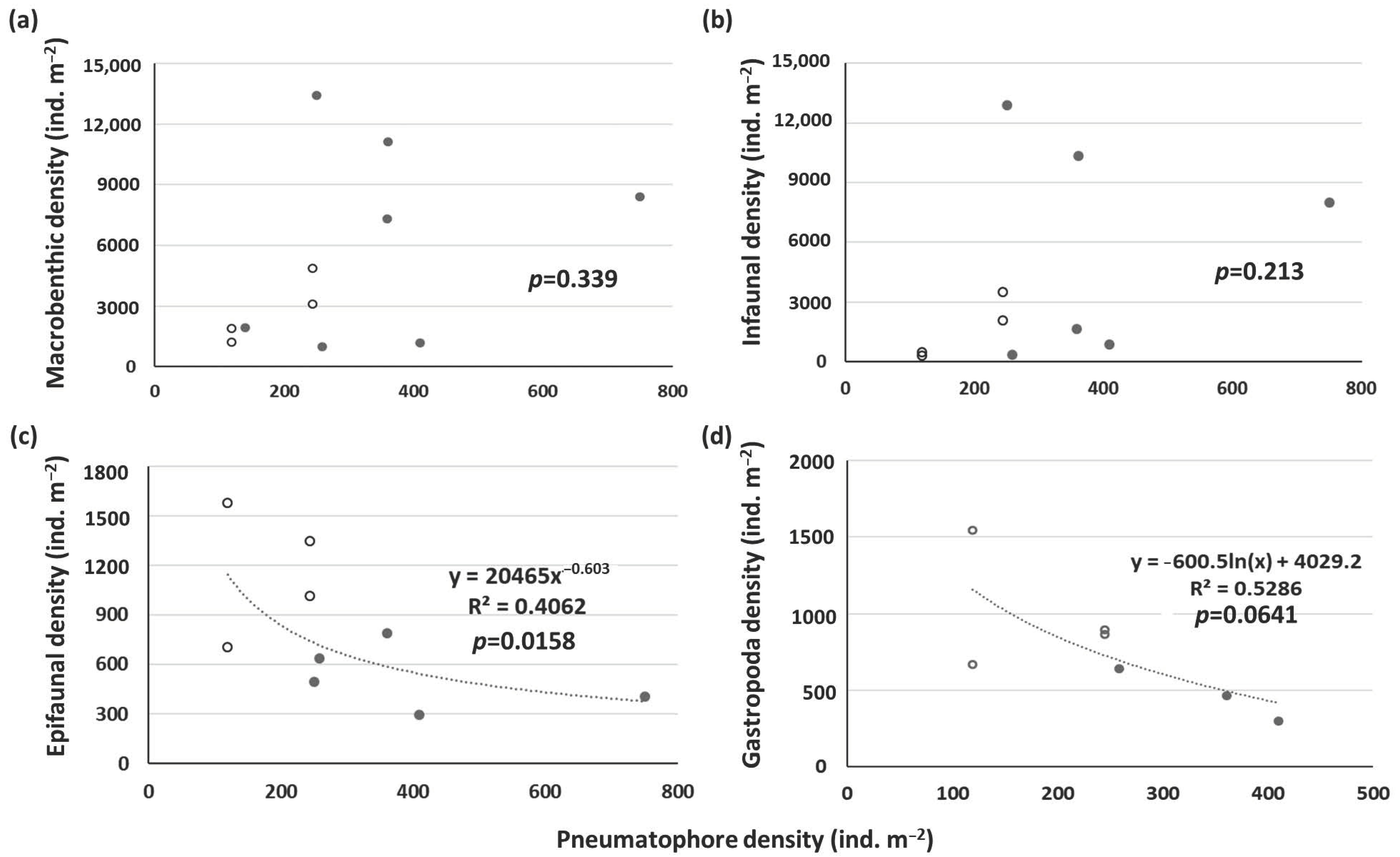
3.4. Environmental Variables Response
4. Discussion
4.1. Comparison between Mangrove Forests and Mudflats
4.2. Comparison among Mangrove Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y. Mangrove macrobenthos: Assemblages, services, and linkages. J. Sea Res. 2008, 59, 16–29. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish. 2015, 16, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Laegdsgaard, P.; Johnston, C. Why do juvenile fish utilize mangrove habitats? J. Exp. Mar. Biol. Ecol. 2001, 257, 229–253. [Google Scholar] [CrossRef] [Green Version]
- Sheaves, M.; Molony, B. Short-circuit in the mangrove food chain. Mar. Ecol. Prog. Ser. 2000, 199, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience 2001, 51, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C.E.; Cahoon, D.R.; Friess, D.A.; Guntenspergen, G.R.; Krauss, K.W.; Reef, R.; Rogers, K.; Saunders, M.L.; Sidik, F.; Swales, A.; et al. The vulnerability of Indo Pacific mangrove forests to sea-level rise. Nature 2015, 526, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Duke, N.C.; Kovacs, J.M.; Griffith, A.; Preece, L.; Hill, D.J.; van Oosterzee, P.; Mackenzie, J.; Morning, H.S.; Burrows, D. Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: A severe ecosystem response, coincidental with an unusually extreme weather event. Mar. Freshw. Res. 2017, 68, 1816–1829. [Google Scholar] [CrossRef]
- Feller, I.C.; Friess, D.A.; Krauss, K.W.; Lewis, R.R., III. The state of the world’s mangroves in the 21st century under climate change. Hydrobiologia 2017, 803, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Saintilan, N.; Khan, N.S.; Ashe, E.; Kelleway, J.J.; Rogers, K.; Woodroffe, C.D.; Horton, B.P. Thresholds of mangrove survival under rapid sea level rise. Science 2020, 368, 1118–1121. [Google Scholar] [CrossRef]
- Davidson, N.C.; Finlayson, C.M. Updating global coastal wetland areas presented in Davidson and Finlayson (2018). Mar. Freshw. Res. 2019, 70, 1195–1200. [Google Scholar] [CrossRef]
- Wilson, J.G.; Fleeger, J.W. Estuarine benthos. In Esturanie Ecology, 2nd ed.; Day, J.W., Kemp, W.M., Jr., Yáñez-Arancibia, A., Crump, B.C., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 303–325. [Google Scholar]
- Alongi, D.M. Impact of global change on nutrient dynamics in mangrove forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.W.; Kao, Y.C.; Chou, M.C.; Wu, H.H.; Ho, C.W.; Lin, H.J. Methane emissions from subtropical and tropical mangrove ecosystems in Taiwan. Forests 2020, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Shiau, Y.J.; Chiu, C.Y. Biogeochemical processes of C and N in the soil of mangrove forest ecosystems. Forests 2020, 11, 492. [Google Scholar] [CrossRef]
- Xenopoulos, M.A.; Downing, J.A.; Kumar, M.D.; Menden-Deuer, S.; Voss, M. Headwaters to oceans: Ecological and biogeochemical contrasts across the aquatic continuum. Limnol. Oceanogr. 2017, 62, S3–S14. [Google Scholar] [CrossRef]
- Yam, R.S.W.; Fan, Y.T.; Tan, Z.; Wang, T.D.; Chiu, C.Y. Assessing impacts of metallic contamination along the tidal gradient of a riverine mangrove: Multi-metal bioaccumulation and biomagnification of filter-feeding bivalves. Forests 2020, 11, 504. [Google Scholar] [CrossRef]
- Li, R.; Ye, Y.; Chen, G. Effect of Aegiceras corniculata mangrove rehabilitation on macrobenthic animals in Jiulongjiang River Estuary. J. Xiamen Univ. Nat. Sci. 2007, 46, 109–114. [Google Scholar]
- Leung, J.Y.S. Habitat heterogeneity determining the macrobenthic infaunal community in a mangrove swamp in South China: Implication for plantation and plant invasion. J. Coast. Res. 2015, 31, 624–633. [Google Scholar] [CrossRef]
- Levin, L.A.; Talley, T.S.; Hewitt, J. Macrobenthos of Spartina foliosa (Pacific cordgrass) salt marshes in southern California: Community structure and comparison to a Pacific mudflat and a Spartina alterniflora (Atlantic smooth cordgrass) marsh. Estuaries 1998, 21, 129–144. [Google Scholar] [CrossRef]
- Kawaida, S.; Nanjob, K.; Ohtsuchic, N.; Kohnod, H.; Sanoa, M. Cellulose digestion abilities determine the food utilization of mangrove estuarine crabs. Estuar. Coast. Shelf Sci. 2019, 222, 43–52. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Leung, J.; Tam, N. Influence of plantation of an exotic mangrove species, Sonneratia caseolaris (L.) Engl., on macrobenthic infaunal community in Futian Mangrove National Nature Reserve, China. J. Exp. Mar. Biol. Ecol. 2013, 448, 1–9. [Google Scholar] [CrossRef]
- Walden, G.; Noirot, C.; Nagelkerken, I. A future 1.2 °C increase in ocean temperature alters the quality of mangrove habitats for marine plants and animals. Sci. Total Environ. 2019, 690, 596–603. [Google Scholar] [CrossRef]
- Gleason, S.M.; Ewel, K.C.; Hue, N. Soil redox conditions and plant–soil relationships in a Micronesian mangrove forest. Estuar. Coast. Shelf Sci. 2003, 56, 1065–1074. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J.; Hogarth, P.J. A baseline study of the diversity and community ecology of crab and molluscan macrofauna in the Sematan mangrove forest, Sarawak, Malaysia. J. Trop. Ecol. 2003, 19, 127–142. [Google Scholar] [CrossRef]
- Dalfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuar. Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Morrisey, D.J.; Skilleter, G.A.; Ellis, J.I.; Burns, B.R.; Kemp, C.E.; Burt, K. Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar. Coast. Shelf Sci. 2003, 56, 581–592. [Google Scholar] [CrossRef]
- Zipperer, V.T. Ecological Effects of the Introduced Cordgrass, Spartina alterniflora, on the Benthic Community Structure of Willapa Bay, Washington. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1996. [Google Scholar]
- Ross, M.P. Macrofaunal loss and microhabitat destruction: The impact of trampling in a temperate mangrove forest, NSW Australia. Wetl. Ecol. Manag. 2006, 14, 167–184. [Google Scholar] [CrossRef]
- Nobbs, M. Effects of vegetation differ among three species of fiddler crabs (Uca spp.). J. Exp. Mar. Biol. Ecol. 2003, 284, 41–50. [Google Scholar] [CrossRef]
- Alongi, D.M. Bacterial productivity and microbial biomass in tropical mangrove sediment. Microb. Ecol. 1988, 15, 59–79. [Google Scholar] [CrossRef]
- Li, W.; Cui, L.; Zhang, M.; Wang, Y.; Zhang, Y.; Lei, Y.; Zhao, X. Effect of mangrove restoration on crab burrow density in Luoyangjiang Estuary, China. For. Ecosyst. 2015, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Hamilton, S.; Barbier, E.B.; Primavera, J.; Lewis, R.R., III. Better restoration policies are needed to conserve mangrove ecosystems. Nat. Ecol. Evol. 2019, 3, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Chen, P.O.; Huang, J.S.; Hsueh, M.L.; Hsieh, L.Y.; Lee, C.L.; Lin, H.J. Factors regulating carbon sinks in mangrove ecosystems. Global Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H. The Carbon Budgets of Kandelia obovata and Avicennia marina Mangroves. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2020. [Google Scholar]
- Yang, D.J.; Sun, R.P. Polychaetes in the Coastal China; Agriculture Publishing: Beijing, China, 1988. [Google Scholar]
- Al-Omari, N.H.A. Guide to Polychaetes Annelida in Qatar Marine Sediments; Qatar University Environmental Studies Center: Doha, Qatar, 2011. [Google Scholar]
- Shih, H.T.; Chan, B.K.; Shao, S.J.; Wong, K.J.H. Crustacean Fauna of Taiwan: Brachyuran Crabs. Volume II—Ocypodoidea; National Chung Hsing University: Taichung, Taiwan, 2015. [Google Scholar]
- Huang, S.; Shih, J.T.; Hsueh, M.L. Mangroves of Taiwan; Taiwan Endemic Species Research Institute: Nantou, Taiwan, 1998. [Google Scholar]
- Lai, G.Y. Guide to Taiwanese Shells; OWL Publishing House: Taipei, Taiwan, 2008. [Google Scholar]
- Skov, M.W.; Hartnoll, R.G. Comparative suitability of binocular observation, burrow counting and excavation for the quantification of the mangrove fiddler crab Uca annulipes (H. Milne Edwards). In Advances in Decapod Crustacean Research; Paula, J.P.M., Flores, A.A.V., Fransen, C.H.J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 201–212. [Google Scholar]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Hsieh, H.L.; Chang, K.H. Habitat characteristics and occurrence of the spionid Pseudopolydora sp. on the tube-caps of the onuphid Diopatra bilobata (Polychaeta: Spionidae, Onuphidae). Bull. Inst. Zool. Acad. Sin. 1991, 30, 331–339. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E: Plymouth, UK, 2001. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. An approach to statistical analysis and interpretation. Chang. Mar. Communities 1994, 2, 117–143. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual-Tutorial; Primer-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008. [Google Scholar]
- Huang, S.C. Resource Investigation in the Mangrove Nature Reserve of the Danshui River; Technical Report; Luodong Forest District Office: Yilan, Taiwan, 2017. (In Chinese) [Google Scholar]
- Huang, S.C. Ecological Resources Monitoring in the Wazhiwei Nature Reserve; Technical Report; Agriculture Department: New Taipei City, Taiwan, 2019. (In Chinese) [Google Scholar]
- Kuo, C.C. Effects of Tree Thinning and Mangrove Species on Benthic Macrofaunal Community. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2016. [Google Scholar]
- Yu, S.P.; Lin, H.J. Relationships between bird foraging and benthic communities on intertidal flats. J. Wetl. 2015, 4, 65–80. Available online: https://www.airitilibrary.com/Publication/alDetailedMesh?docid=P20151104001-201512-201604010003-201604010003-65-80 (accessed on 13 October 2021).
- Chen, Y.C.; Chu, T.J.; Wei, J.D.; Shih, C.H. Effects of mangrove removal on benthic organisms in the Siangshan Wetland in Hsinchu, Taiwan. PeerJ 2018, 6, e5670. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.; Hwang, G.W.; Mayfield, A.B.; Chen, C.P.; Lin, H.J. The development of habitat suitability models for fiddler crabs residing in subtropical tidal flats. Ocean Coast Manag. 2019, 182, 104931. [Google Scholar] [CrossRef]
- Longonje, S.N.; Raffaelli, D. Feeding ecology of mangrove crabs in Cameroon. Appl. Ecol. Env. Res. 2014, 12, 959–973. [Google Scholar] [CrossRef]
- Ismail, N.S.; Ahmed, M.A.E. Macrobenthic invertebrates of mangrove, Avicennia marina (Forskal), and of intertidal flats of Khor Kalba, U.A.E., Gulf of Oman. In Towards the Rational Use of High Salinity Tolerant Plants; Lieth, H., Masoom, A.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 155–161. [Google Scholar]
- Bernardino, A.F.; Gomes, L.E.O.; Hadlich, H.L.; Andrades, R.; Correa, L.B. Mangrove clearing impacts on macrofaunal assemblages and benthic food webs in a tropical estuary. Mar. Pollut. Bull. 2018, 126, 228–235. [Google Scholar] [CrossRef]
- Checon, H.H.; Corte, G.N.; Silva, C.F.; Schaeffer-Novelli, Y.; Amaral, A.C.Z. Mangrove vegetation decreases density but does not affect species richness and trophic structure of intertidal polychaete assemblages. Hydrobiologia 2017, 795, 169–179. [Google Scholar] [CrossRef]
- Lin, J.; He, X.; Wang, J.; Lin, H.; Huang, Y.; Liu, K.; Mou, J.; Zhang, S.; Jiang, J. Macrobenthic diversity and seasonal changes in the mangrove swamp of Luoyangjiang Estuary, Fujian Province. Biodiv. Sci. 2016, 24, 791–801. [Google Scholar] [CrossRef]
- Chen, G.C.; Ye, Y.; Lu, C.Y. Changes of macrobenthic faunal community with stand age of rehabilitated Kandelia candel mangrove in Jiulongjiang Estuary. Ecol. Eng. 2007, 31, 215–224. [Google Scholar] [CrossRef]
- Fratini, S.; Cannicci, S.; Vannini, M. Feeding clusters and olfaction in the mangrove snail Terebralia palustris (Linnaeus) (Potamididae: Gastropoda). J. Exp. Mar. Biol. Ecol. 2001, 261, 173–183. [Google Scholar] [CrossRef]
- Nishijima, W.; Nakano, Y.; Nakai, S.; Okuda, T.; Imai, T.; Okada, M. Impact of flood events on macrobenthic community structure on an intertidal flat developing in the Ohta river estuary. Mar. Pollut. Bull. 2013, 74, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, A.C. Effects of mangrove removal on benthic communities and sediment characteristics at Mangawhai Harbour, northern New Zealand. J. Mar. Sci. 2010, 67, 1087–1104. [Google Scholar] [CrossRef] [Green Version]
- Ysebaert, T.; Meire, P.; Maes, D.; Buijs, J. The benthic macrofauna along the estuarine gradient of the Schelde Estuary. Neth. J. Aquat. Ecol. 1993, 27, 327–341. [Google Scholar] [CrossRef]
- LaSalle, M.W.; Bishop, T.D. Food habits of two larval flies (Dolichopodidae: Diptera) in two gulf coast oligohaline tidal marshes. Estuaries 1990, 13, 341–348. [Google Scholar] [CrossRef]
- Penha-Lopes, G.; Bouillon, S.; Mangion, P.; Macia, A.; Paula, J. Population structure, density and food sources of Terebralia palustris (Potamididae: Gastropoda) in a low intertidal Avicennia marina mangrove stand (Inhaca Island, Mozambique). Estuar. Coast. Shelf Sci. 2009, 84, 318–325. [Google Scholar] [CrossRef]
- Thilagavathi, B.; Varadharajan, D.; Babu, A.; Manoharan, J.; Vijayalakshmi, S.; Balasubramanian, T. Distribution and diversity of macrobenthos in different mangrove ecosystems of Tamil Nadu Coast, India. J. Aquac. Res. Dev. 2013, 4, 199. [Google Scholar] [CrossRef] [Green Version]
- Bishop, M.J.; Byers, J.E.; Marcek, B.J.; Gribben, P.E. Density-dependent facilitation cascades determine epifaunal community structure in temperate Australian mangroves. Ecology 2012, 93, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.B.K.; Ramanathan, A.L. Sedimentary nutrient dynamics in a tropical estuarine mangrove ecosystem. Estuar. Coast. Shelf Sci. 2008, 80, 60–66. [Google Scholar] [CrossRef]
- Farooq, S.; Siddiqui, P.J.A. Assessment of three mangrove forest systems for future management through benthic community structure receiving anthropogenic influences. Ocean Coast. Manag. 2020, 190, 105126. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, Y.; Jiang, Z.; Ma, X.; Inyang, A.I.; Cheng, H. Effects of salt on root aeration, nitrification, and nitrogen uptake in mangroves. Forests 2019, 10, 1131. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.S. Spatial habitat suitability models of mangroves with Kandelia obovata. Forests 2020, 11, 477. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, X.; Wang, M. Propagule dispersal determines mangrove zonation at intertidal and estuarine scales. Forests 2019, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Cannicci, S.; Lee, S.Y.; Bravo, H.; Cantera-Kintz, J.R.; Dahdouh-Guebas, F.; Fratini, S.; Fusi, F.; Jimenez, P.J.; Nordhaus, I.; Porri, F.; et al. A functional analysis reveals extremely low redundancy in global mangrove invertebrate fauna. Proc. Natl. Acad. Sci. USA 2021, 118, e2016913118. [Google Scholar] [CrossRef]
- Egawa, R.; Sharma, S.; Nadaoka, K.; MacKenzie, R.A. Burrow dynamics of crabs in subtropical estuarine mangrove forest. Estuar. Coast. Shelf Sci. 2021, 252, 107244. [Google Scholar] [CrossRef]
| Site | XF | ZN | BD | BM |
|---|---|---|---|---|
| Latitude | 24°54′ N | 24°40′ N | 23°21′ N | 23°17′ N |
| Longitude | 120°58′ E | 120°50′ E | 120°07′ E | 120°06′ E |
| Dominant tree species | Kandelia obovata (S., L.) | Kandelia obovata | Avicennia marina (Forsk.) | Avicennia marina |
| Forest age (year) | 70 | 40 | 120 | 100 |
| Forest condition | Intact, eroded | Intact, expanded | Intact, expanded | Intact, threatened |
| Cover area (ha) | 8.25 | 14.5 | 19.5 | 18.9 |
| Distance to the sea (km) | 0.70 | 1.68 | 0.40 | 0.62 |
| Submersion time during a high tide in June 2020 (minutes) | 145 | 35 | 502 | 452 |
| NO2− + NO3− (μM) # | 320 ± 66 c | 28.3 ± 5.8 b | 6.36 ± 1.50 a | 15.3 ± 3.6 b |
| NH4+ (μM) # | 66.3 ± 14.1 b | 100 ± 22 b | 6.80 ± 1.38 a | 16.1 ± 3.9 a |
| PO4−3 (μM) # | 30.4 ± 7.4 b | 24.5 ± 9.8 b | 2.83 ± 0.56 a | 2.92 ± 0.65 a |
| Tree height (m) | 5.20 ± 2.09 c | 5.04 ± 1.58 c | 4.02 ± 2.86 b | 3.21 ± 1.95 a |
| Diameter at breast height (DBH, cm) | 6.00 ± 0.10 b | 6.13 ± 0.07 b | 5.36 ± 0.05 a | 7.16 ± 0.08 b |
| Tree density (ind. m−2) | 2.30 ± 0.27 b | 1.80 ± 0.11 b | 0.60 ± 0.16 a | 0.33 ± 0.07 a |
| Pneumatophore density (ind. m−2) | NA | NA | 119.11 ± 13.83 a | 244.81 ± 30.44 b |
| Sediment light intensity (µmol photon m−2 s−1) | 65.2 ± 82.6 a | 124.2 ± 109.8 b | 185.6 ± 260.3 b | 189.6 ± 104.6 b |
| Sediment salinity | 0.93 ± 0.17 a | 2.64 ± 0.26 b | 3.43 ± 0.47 c | 4.25 ± 0.61 c |
| Sediment water content (%) | 28.99 ± 5.21 a | 26.08 ± 5.32 a | 50.38 ± 8.03 c | 34.60 ± 3.22 b |
| Sediment organic content (%) | 4.78 ± 0.76 c | 4.08 ± 0.13 b | 7.60 ± 0.66 d | 3.49 ± 0.37 a |
| Sediment median grain size (mm) | 0.061 ± 0.013 c | 0.024 ± 0.003 a | 0.032 ± 0.003 b | 0.037 ± 0.009 b |
| Sediment sorting coefficient | 1.90 ± 0.10 c | 1.40 ± 0.05 a | 2.25 ± 0.06 d | 1.58 ± 0.12 b |
| Sediment bulk density (g cm−3) | 1.03 ± 0.08 b | 1.28 ± 0.09 c | 0.57 ± 0.15 a | 0.96 ± 0.13 b |
| Sediment silt/clay content (%) | 41.69 ± 6.74 a | 88.72 ± 2.66 d | 61.73 ± 1.26 b | 66.68 ± 5.78 c |
| Sediment ORP (mV) | 174.9 ± 73.9 b | 69.3 ± 83.6 b | −258.5 ± 68.4 a | −162.1 ± 45.1 a |
| Benthic chlorophyll a concentration (mg m−2) | 65.60 ± 5.40 b | 30.28 ± 14.61 a | 131.19 ± 54.48 c | 68.08 ± 21.25 b |
| XF | ZN | BD | BM |
|---|---|---|---|
| Mangrove forests | |||
| Oligochaeta | Phascolosoma arcuatum | Stenothyridae | Capitellidae |
| 29.16% | 85.28% | 43.27% | 21.62% |
| Decapoda juveniles | Assimineidae | Iravadia quadrasi | Ampharetidae |
| 25.36% | 8.98% | 26.84% | 16.89% |
| Dolichopodidae larvae | Ampharetidae | Nemertina | |
| 20.39% | 7.91% | 16.64% | |
| Corophiidae | Oligochaeta | Iravadia quadrasi | |
| 7.84% | 6.44% | 12.67% | |
| Capitellidae | Dolichopodidae larvae | Oligochaeta | |
| 6.76% | 5.56% | 10.76% | |
| Nonvegetated mudflats | |||
| Corophiidae | Capitellidae | Mictyris brevidactylus | Capitellidae |
| 25.10% | 21.21% | 20.77% | 37.66% |
| Capitellidae | Assimineidae | Pirenella cingulata | Naticidae |
| 24.52% | 20.57% | 19.91% | 14.75% |
| Assimineidae | Metaplax longipes | Oligochaeta | Nemertina |
| 18.78% | 13.43% | 15.79% | 11.59% |
| Dolichopodidae larvae | Phascolosoma arcuatum | Pirenella alata | Cossuridae |
| 12.76% | 9.52% | 14.79% | 10.91% |
| Oligochaeta | Corophiidae | Decapoda juveniles | Spionidae |
| 5.87% | 9.13% | 10.50% | 8.25% |
| Species | XF | ZN | BD | BM | ||||
|---|---|---|---|---|---|---|---|---|
| v | nv | v | nv | v | nv | v | nv | |
| Mictyris brevidactylus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.36 | 0.00 | 0.00 |
| Sesarmidae | 1.75 | 1.36 | 1.75 | 1.00 | 0.58 | 0.00 | 0.50 | 0.09 |
| Helice formosensis | 0.00 | 0.64 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Macrophthalmus sp. | 0.00 | 1.09 | 0.00 | 14.36 | 0.00 | 0.00 | 0.17 | 18.00 |
| Austruca lactea | 0.33 | 17.82 | 0.08 | 0.55 | 0.00 | 8.64 | 0.00 | 0.00 |
| Austruca triangularis | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 |
| Austruca perplexa | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gelasimus borealis | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.09 | 0.00 | 0.00 |
| Tubuca arcuata | 0.50 | 3.45 | 0.08 | 1.00 | 0.00 | 1.82 | 0.00 | 0.00 |
| Xeruca formosensis | 0.08 | 0.18 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ilyoplax sp. | 0.00 | 0.00 | 0.00 | 0.45 | 0.00 | 0.00 | 0.00 | 0.00 |
| Scopimera sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 0.00 |
| Tmethypocoelis ceratophora | 0.00 | 0.00 | 0.00 | 7.09 | 0.00 | 0.45 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.-H.; Ho, C.-W.; Lin, C.-W.; Huang, S.-C.; Lin, H.-J. Differential Response of Macrobenthic Abundance and Community Composition to Mangrove Vegetation. Forests 2021, 12, 1403. https://doi.org/10.3390/f12101403
Pan S-H, Ho C-W, Lin C-W, Huang S-C, Lin H-J. Differential Response of Macrobenthic Abundance and Community Composition to Mangrove Vegetation. Forests. 2021; 12(10):1403. https://doi.org/10.3390/f12101403
Chicago/Turabian StylePan, Sin-He, Chuan-Wen Ho, Chiao-Wen Lin, Shou-Chung Huang, and Hsing-Juh Lin. 2021. "Differential Response of Macrobenthic Abundance and Community Composition to Mangrove Vegetation" Forests 12, no. 10: 1403. https://doi.org/10.3390/f12101403






