Root Responses of Five Japanese Afforestation Species to Waterlogging
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Waterial and Waterlogging Treatment
2.2. Aboveground Measurements
2.3. Root Measurements
2.4. Statistical Analysis
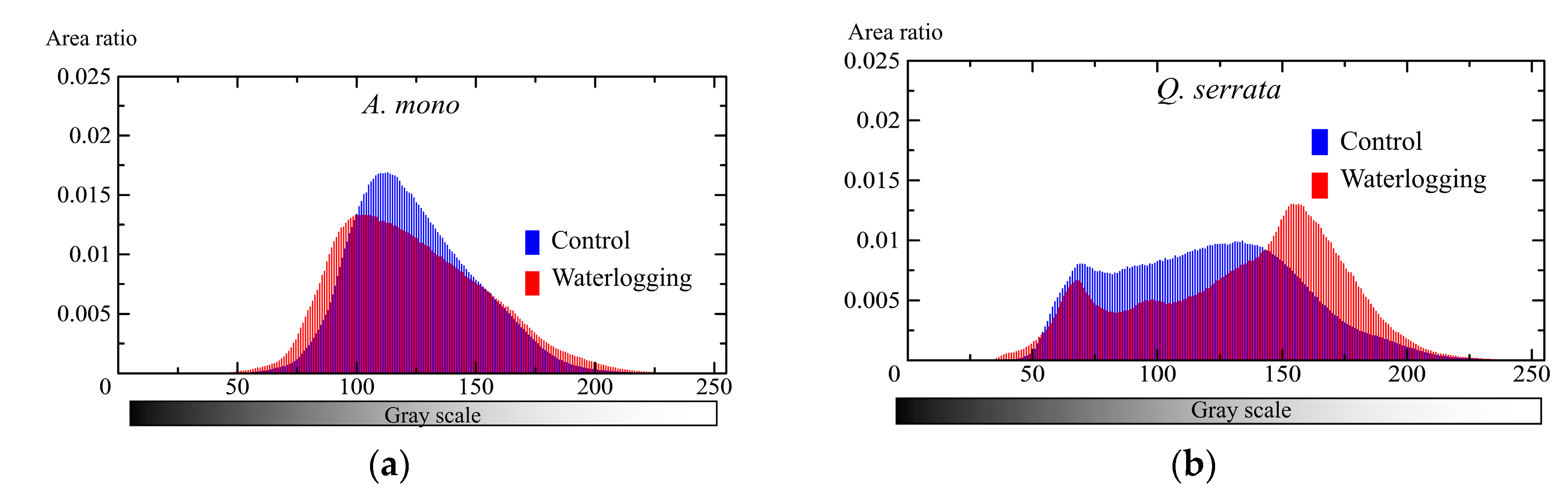
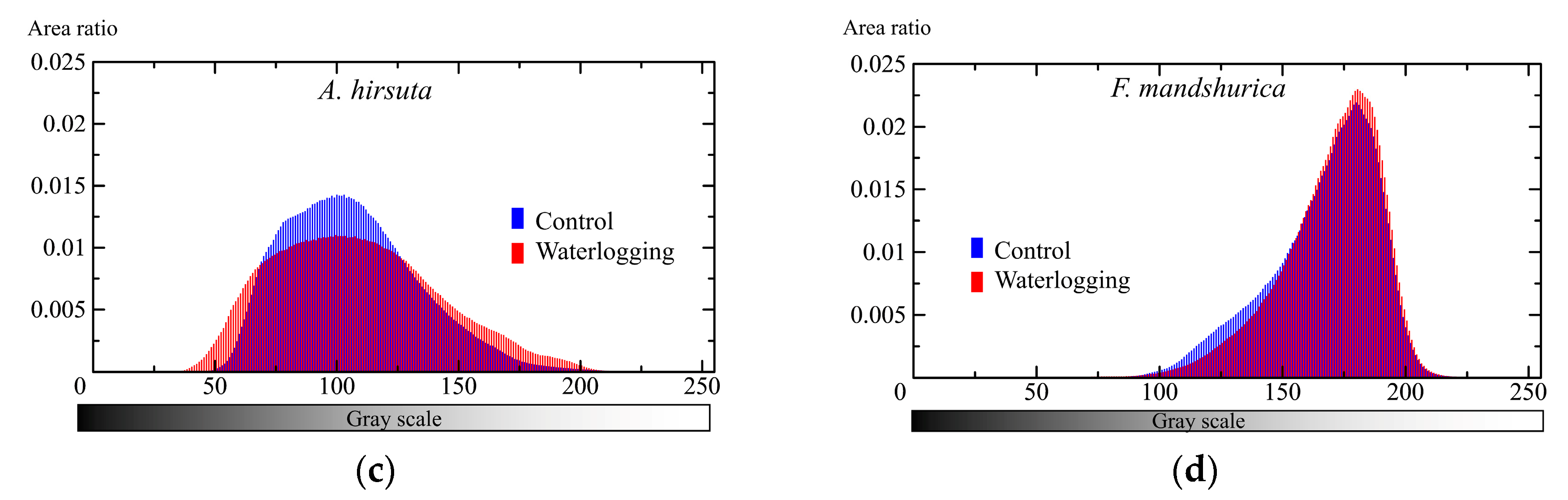
3. Results
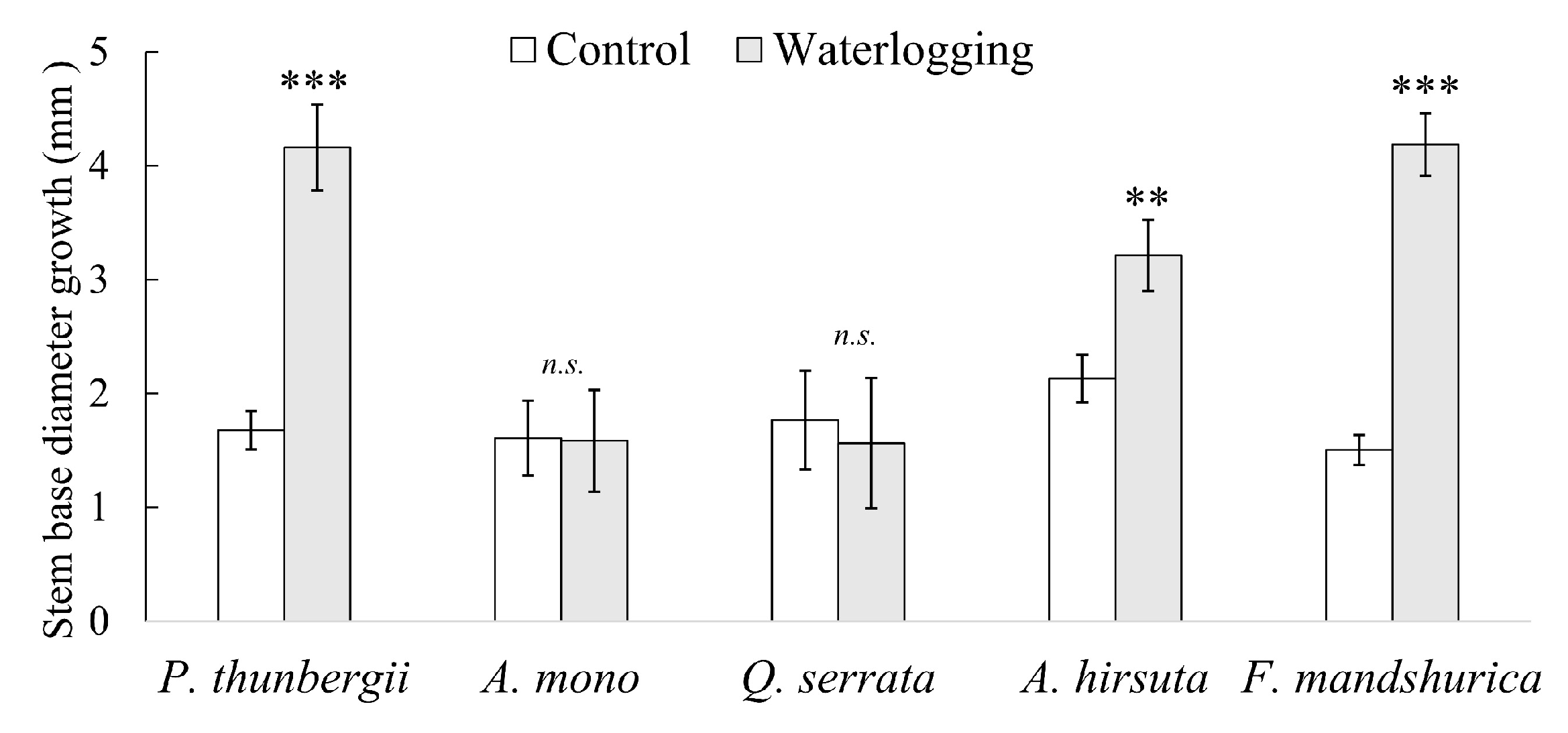
3.1. Aboveground
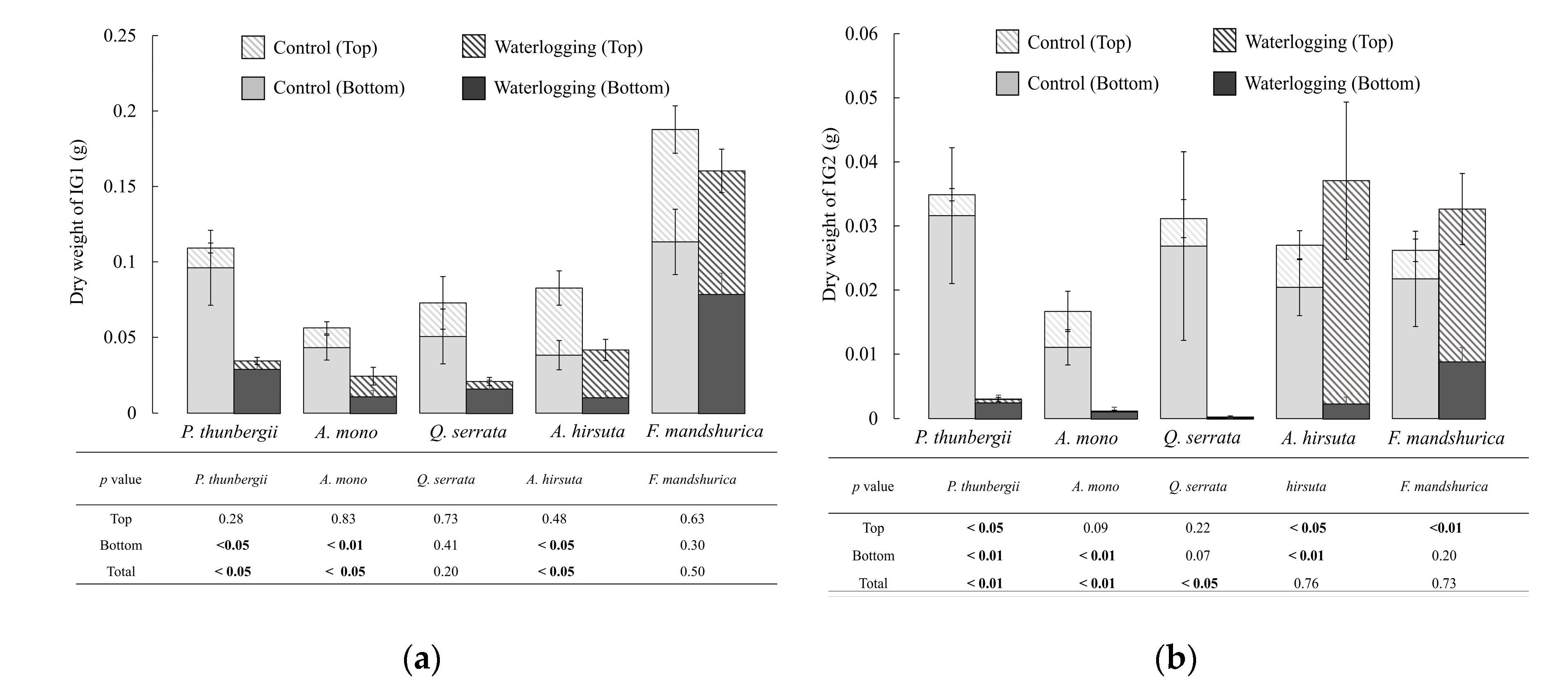
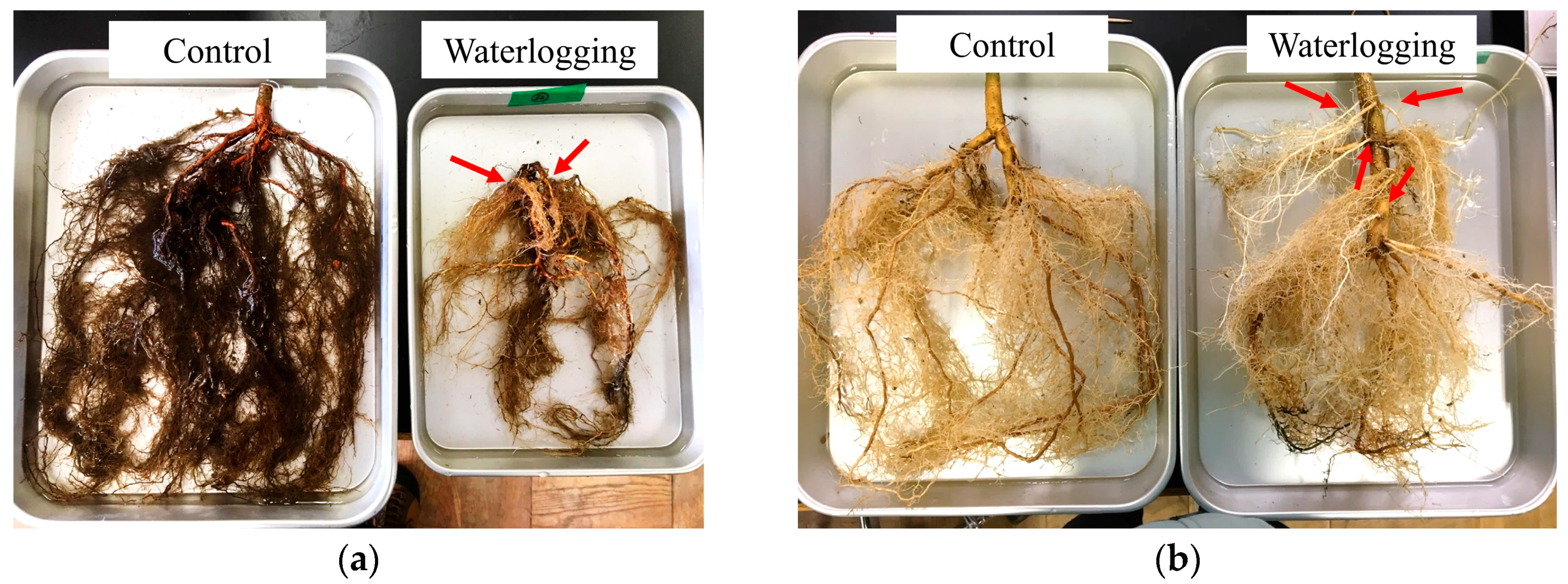
3.2. Belowground
4. Discussion
5. Conclusions
- (1)
- Inhabits of non-waterlogged environments: P. thunbergii, A. mono and Q. serrata. Inhibition of root growth + damage and loss of pre-existing roots (top and bottom of root system).
- (2)
- Inhabits of waterlogged environments (with very slow water movement): A. hirsuta. Continuous root growth (only at top) + damage and loss of pre-existing roots (bottom half).
- (3)
- Inhabits of waterlogged environments (stagnant water): F. mandshurica. Continuous root growth (top and bottom) + no damage of pre-existing roots.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Valipour, M. Drainage, waterlogging, and salinity. Arch. Agron. Soil Sci. 2014, 60, 1625–1640. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Jackson, M.B. Plant Adaptations to Anaerobic Stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Blom, C.W.P.M. Adaptations to Flooding Stress: From Plant Community to Molecule. Plant Biol. 1999, 1, 261–273. [Google Scholar] [CrossRef]
- Armstrong, W. Aeration in Higher Plants. Adv. Bot. Res. 1980, 7, 225–332. [Google Scholar]
- Kundzewicz, Z.W.; Kanae, S.; Seneviratne, S.I.; Handmer, J.; Nicholls, N.; Peduzzi, P.; Mechler, R.; Bouwer, L.M.; Arnell, N.; Mach, K.; et al. Flood risk and climate change: Global and regional perspectives. Hydrol. Sci. J. 2014, 59, 2014. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Gessler, A. Global climate change and tree nutrition: Influence of water availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef]
- Horn, R.; Domzzał, H.; Słowińska-Jurkiewicz, A.; van Ouwerkerk, C. Soil compaction processes and their effects on the structure of arable soils and the environment. Soil Tillage Res. 1995, 35, 23–36. [Google Scholar] [CrossRef]
- Frey, B.; Kremer, J.; Rüdt, A.; Sciacca, S.; Matthies, D.; Lüscher, P. Compaction of forest soils with heavy logging machinery affects soil bacterial community structure. Eur. J. Soil Biol. 2009, 45, 312–320. [Google Scholar] [CrossRef]
- Ono, K.; Komoriya, A.; Tachibana, R.; Imaya, A.; Suzuki, S.; Noguchi, H.; Noguchi, K.; Hagino, H. Effects of row deep tillage for the growth base formed by piling up soil in damp lowlands behind coastal sand dunes to construct coastal disaster prevention forest belts on the Kujukuri coastline, Japan. Soil Sci. Plant Nutr. 2018, 64, 1–13. [Google Scholar] [CrossRef]
- Plane, E.; Hill, K.; May, C. A Rapid Assessment Method to Identify Potential Groundwater Flooding Hotspots as Sea Levels Rise in Coastal Cities. Water 2019, 11, 2228. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Plant responses to flooding of soil. Bioscience 1980, 30, 88–93. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Shimamura, S.; Yamamoto, R.; Nakamura, T.; Shimada, S.; Komatsu, S. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann. Bot. 2010, 106, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Li, S.; Pezeshki, S.R.; Douglas, F. Partial flooding enhances aeration in adventitious roots of black willow (Salix nigra) cuttings. J. Plant Physiol. 2006, 163, 619–628. [Google Scholar] [CrossRef]
- Dreyer, E. Compared sensitivity of seedlings from 3 woody species (Quercus robur L., Quercus rubra L. and Fagus silvatica L.) to water-logging and associated root hypoxia: Effects on water relations and photosynthesis. Ann. Sci. 1994, 51, 417–429. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Yelenosky, G. Photosynthetic responses of citrus trees to soil flooding. Physiol. Plant 1991, 81, 7–14. [Google Scholar] [CrossRef]
- Argus, R.E.; Colmer, T.D.; Grierson, P.F. Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef]
- Yamamoto, F.; Sakata, T.; Terazawa, K. Growth, morphology, stem anatomy, and ethylene production in flooded Alnus japonica seedlings. IAWA J. 1995, 16, 47–59. [Google Scholar] [CrossRef]
- Colin-Belgrand, M.; Dreyer, E.; Biron, P. Sensitivity of seedlings from different oak species to waterlogging: Effects on root growth and mineral nutrition. In Annales des sciences forestières; EDP Sciences: Les Ulis, France, 1991; Volume 48, pp. 193–204. [Google Scholar]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Asai, T.; Masaka, K. Biomass and net production of a natural coastal forest mainly composed by Kashiwa-oak (Quercus dentata T HUNB.) in northern Hokkaido . Bull. Hokkaido For. Res. Inst. 1988, 11–19, (In Japanese with English summary). [Google Scholar]
- Kanno, H.; Hirabuki, Y.; Sugiyama, T.; Tomita, M.; Hara, K. Vegetation change in various coastal forest habitats after a huge tsunami: A three-year study (in Japanese with English abstract). Jpn. J. Conserv. Ecol. 2014, 19, 201–220. [Google Scholar]
- Xu, X.N.; Shibata, H. Landscape patterns of overstory litterfall and related nutrient fluxes in a cool-temperate forest watershed in northern Hokkaido, Japan. J. For. Res. 2007, 18, 249–254. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, F.; Sakata, T.; Terazawa, K. Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol. 1995, 15, 713–719. [Google Scholar] [CrossRef]
- Nagasaka, A. Effects of flooding on growth and leaf dynamics of two-year-old deciduous tree seedlings under different flooding treatments (in Japanese). Bull. Hokkaido For. Res. Inst. 2001, 38, 101–105. [Google Scholar]
- Nagakura, J.; Shigenaga, H.; Akama, A.; Takahayshi, M. Efficient water use is essential for biomass production and for drought tolerance in plants. Tree Physiol. 2004, 24, 1203–1208. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M.; Lakso, A.N. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 2000, 147, 171–178. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Marked differences in survivorship among apple roots of different diameters. Ecology 2001, 82, 882–892. [Google Scholar] [CrossRef]
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Wang, A.F.; Roitto, M.; Sutinen, S.; Lehto, T.; Heinonen, J.; Zhang, G.; Repo, T. Waterlogging in late dormancy and the early growth phase affected root and leaf morphology in Betula pendula and Betula pubescens seedlings. Tree Physiol. 2015, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Levan, M.A.; Riha, S.J. The precipitation of black oxide coatings on flooded conifer roots of low internal porosity. Plant Soil 1986, 95, 33–42. [Google Scholar] [CrossRef]
- Dreyer, E.; Colin-Belgrand, M.; Biron, P. Photosynthesis and shoot water status of seedlings from different oak species submitted to waterlogging. Ann. Sci. 1991, 48, 205–214. [Google Scholar] [CrossRef]
- Fukuju, Y. Effects of depth of flooding on growth and anatomy of stem and knee roots of Taxoduim distichum. IAWA Bull. 1992, 13, 93–104. [Google Scholar]
- Gomes, A.R.S.; Kozlowski, T.T. Responses of Pinus halepensis seedlings to flooding. Can. J. For. Res. 1980, 10, 308–311. [Google Scholar] [CrossRef]
- Tang, Z.C.; Kozlowski, T.T. Responses of Pinus banksiana and Pinus resinosa seedlings to flooding. Can. J. For. Res. 1983, 13, 633–639. [Google Scholar] [CrossRef]
- Hirano, Y.; Todo, C.; Yamase, K.; Tanikawa, T.; Dannoura, M.; Ohashi, M.; Doi, R.; Wada, R.; Ikeno, H. Quantification of the contrasting root systems of Pinus thunbergii in soils with different groundwater levels in a coastal forest in Japan. Plant Soil 2018, 1–11. [Google Scholar] [CrossRef]
- Oda, T. Study on the reaction of planted tree root systems to water-logging and its application to developing forests in damp lowlands of coastal sand dunes. Spec. Bull. Chiba. Pref. Res. Cent. 2001, 3, 1–78, (In Japanese with English summary). [Google Scholar]
- Hodge, A. Root decisions. Plant Cell Environ. 2009, 32, 628–640. [Google Scholar] [CrossRef]
- Dresbøll, D.B.; Thorup-Kristensen, K.; McKenzie, B.M.; Dupuy, L.X.; Bengough, A.G. Timelapse scanning reveals spatial variation in tomato (Solanum lycopersicum L.) root elongation rates during partial waterlogging. Plant Soil 2013, 369, 467–477. [Google Scholar] [CrossRef]
- Haase, K.; De Simone, O.; Junk, W.J.; Schmidt, W. Internal oxygen transport in cuttings from flood-adapted varzea tree species. Tree Physiol. 2003, 23, 1069–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sou, H.-D.; Masumori, M.; Kurokochi, H.; Tange, T. Histological observation of primary and secondary aerenchyma formation in adventitious roots of Syzygium kunstleri (King) Bahadur and R.C.Gaur grown in hypoxic medium. Forests 2019, 10, 137. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Señorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.J.W.; Colmer, T.D.; Blom, C.W.P.M.; Voesenek, L.A.C.J. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ. 2000, 23, 1237–1245. [Google Scholar] [CrossRef]
- Karizumi, N. The Latest Illistrations of Tree Roots (General Remarks); Seibundoshinko-syo: Tokyo, Japan, 2010. (In Japanese) [Google Scholar]
- Yamamoto, F.; Kozlowski, T.T. Effects of flooding, tilting of stems, and ethrel application on growth, stem anatomy and ethylene production of Pinus densiflora seedlings. J. Exp. Bot. 1987, 38, 293–310. [Google Scholar] [CrossRef]


| Species | Position | Treatment | Root Diameter | SRL | RTD |
|---|---|---|---|---|---|
| P. thunbergii | Top | C (n = 9) | 0.47 ± 0.04 | 27.7 ± 2.6 | 0.23 ± 0.01 |
| WL (n = 7) | 0.53 ± 0.03 | 29.7 ± 4.8 | 0.19 ± 0.03 * | ||
| Bottom | C (n = 9) | 0.53 ± 0.05 | 22.0 ± 2.2 | 0.28 ± 0.02 | |
| WL (n = 9) | 0.65 ± 0.04 *** | 20.5 ± 1.8 | 0.16 ± 0.01 *** | ||
| A. mono | Top | C (n = 8) | 0.35 ± 0.01 | 39.9 ± 4.3 | 0.29 ± 0.02 |
| WL (n = 8) | 0.37 ± 0.01 | 47.6 ± 5.7 | 0.21 ± 0.02 ** | ||
| Bottom | C (n = 9) | 0.37 ± 0.01 | 33.7 ± 3.1 | 0.30 ± 0.02 | |
| WL (n = 7) | 0.36 ± 0.02 | 50.5 ± 8.6 | 0.23 ± 0.03 * | ||
| Q. serrata | Top | C (n = 4) | 0.21 ± 0.03 | 69.5 ± 20.0 | 0.59 ± 0.14 |
| WL (n = 4) | 0.18 ± 0.02 | 207.5 ± 76.9 | 0.26 ± 0.05 | ||
| Bottom | C (n = 5) | 0.22 ± 0.04 | 100.9 ± 34.1 | 0.56 ± 0.13 | |
| WL(n = 4) | 0.27 ± 0.07 | 152.37 ± 65.8 | 0.24 ± 0.04 | ||
| A. hirsuta | Top | C (n = 9) | 0.32 ± 0.01 | 34.9 ± 5.0 | 0.40 ± 0.04 |
| WL (n = 9) | 0.36 ± 0.03 | 101.4 ± 35.1 | 0.18 ± 0.02 *** | ||
| Bottom | C (n = 9) | 0.32 ± 0.03 | 50.1 ± 5.2 | 0.32 ± 0.02 | |
| WL (n = 8) | 0.36 ± 0.03 | 84.1 ± 17.7 | 0.17 ± 0.01 *** | ||
| F. mandshurica | Top | C (n = 9) | 0.38 ± 0.02 | 28.0 ± 5.1 | 0.46 ± 0.09 |
| WL (n = 9) | 0.35 ± 0.04 | 40.0 ± 4.6 | 0.36 ± 0.04 | ||
| Bottom | C (n = 9) | 0.42 ± 0.05 | 29.1 ± 6.5 | 0.38 ± 0.04 | |
| WL (n = 9) | 0.43 ± 0.05 | 34.4 ± 4.7 | 0.32 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, S.; Noguchi, K.; Tange, T. Root Responses of Five Japanese Afforestation Species to Waterlogging. Forests 2020, 11, 552. https://doi.org/10.3390/f11050552
Fujita S, Noguchi K, Tange T. Root Responses of Five Japanese Afforestation Species to Waterlogging. Forests. 2020; 11(5):552. https://doi.org/10.3390/f11050552
Chicago/Turabian StyleFujita, Saki, Kyotaro Noguchi, and Takeshi Tange. 2020. "Root Responses of Five Japanese Afforestation Species to Waterlogging" Forests 11, no. 5: 552. https://doi.org/10.3390/f11050552
APA StyleFujita, S., Noguchi, K., & Tange, T. (2020). Root Responses of Five Japanese Afforestation Species to Waterlogging. Forests, 11(5), 552. https://doi.org/10.3390/f11050552





