Assessing Impacts of Metallic Contamination along the Tidal Gradient of a Riverine Mangrove: Multi-metal Bioaccumulation and Biomagnification of Filter-Feeding Bivalves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Collection and Sample Preparation
2.3. Determination of Metallic Concentrations
2.4. Stable Nitrogen Isotope Analysis of Bivalves
2.5. Data Analysis
3. Results
3.1. Inter-Site Variation of Metallic Concentrations of Surface Sediment along Riverine Mangrove
3.2. Inter-Site Variation of Metallic Concentrations of the Bivalves along Riverine Mangrove
3.3. BSAF of Metallic Contaminants in the Bivalves
3.4. Metallic TMF of the Bivalves
4. Discussion
4.1. Metal Concentrations of Sediment along the Tidal Gradient of Riverine Mangrove
4.2. Metal Concentrations of the Bivalves along the Tidal Gradient of Riverine Mangrove
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loomis, J.B.; Kent, P.; Strange, L.; Fausch, K.; Covich, A. Measuring the total economic value of restoring ecosystem services in an impaired river basin: Results from a contingent valuation survey. Ecol. Econ. 2000, 33, 103–117. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.J.; Koch, E.W.; Stier, A.; Silliman, B. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Goel, P.K. Water Pollution: Causes, Effects and Control; New Age International: New Delhi, India, 2006. [Google Scholar]
- Förstner, U.; Wittmann, G.T. Metal Pollution in the Aquatic Environment, 2rd ed.; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Day, J.W.; Yáñez-Arancibia, A.; Kemp, W.M. Human Impact and Management of Coastal and Estuarine Ecosystems. In Estuarine Ecology, 2nd ed.; Day, J.W., Crump, B.C., Kemp, W.M., Yáñez-Arancibia, A., Eds.; Wiley: Singapore, 2012; pp. 483–495. [Google Scholar]
- Bernhardt, E.S.; Palmer, M.A. Restoring streams in an urbanizing world. Freshw. Boil. 2007, 52, 738–751. [Google Scholar] [CrossRef]
- Brooker, M.P. The Ecological Effects of Channelization. Geogr. J. 1985, 151, 63. [Google Scholar] [CrossRef]
- Groffman, P.M.; Bain, D.J.; Band, L.E.; Belt, K.T.; Brush, G.S.; Grove, J.M.; Pouyat, R.V.; Yesilonis, I.C.; Zipperer, W.C. Down by the riverside: Urban riparian ecology. Front. Ecol. Environ. 2003, 1, 315–321. [Google Scholar] [CrossRef]
- Walter, M.T.; Archibald, J.A.; Buchanan, B.; Dahlke, H.; Easton, Z.M.; Marjerison, R.D.; Sharma, A.N.; Shaw, S.B. New Paradigm for Sizing Riparian Buffers to Reduce Risks of Polluted Storm Water: Practical Synthesis. J. Irrig. Drain. Eng. 2009, 135, 200–209. [Google Scholar] [CrossRef]
- Chapman, P.M.; Ho, K.T.; Munns, W.R.; Solomon, K.; Weinstein, M.P. Issues in sediment toxicity and ecological risk assessment. Mar. Pollut. Bull. 2002, 44, 271–278. [Google Scholar] [CrossRef]
- Pettigrove, V.; Hoffmann, A. Impact of urbanisation on heavy metal contamination in urban stream sediments: Influence of catchment geology. Australas. J. Ecotoxicol. 2003, 9, 119–128. [Google Scholar]
- Tam, N.F.; Wong, Y.-S. Accumulation and distribution of heavy metals in a simulated mangrove system treated with sewage. Hydrobiology 1997, 352, 67–75. [Google Scholar] [CrossRef]
- Valdés, J. Cu, Pb, and Zn content in sediments and benthic organisms from San Jorge Bay (northern Chile): Accumulation and biotransference in subtidal coastal systems. Cienc. Mar. 2014, 40, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Weber, P.; Behr, E.R.; Knorr, C.D.L.; Vendruscolo, D.S.; Flores, E.M.M.; Dressler, V.L.; Baldisserotto, B. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J. 2013, 106, 61–66. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S.-H. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- Norwood, W.; Borgmann, U.; Dixon, D.G.; Wallace, A. Effects of Metal Mixtures on Aquatic Biota: A Review of Observations and Methods. Hum. Ecol. Risk Assess. Int. J. 2003, 9, 795–811. [Google Scholar] [CrossRef]
- Tam, N.F.; Wong, Y. Spatial and temporal variations of heavy metal contamination in sediments of a mangrove swamp in Hong Kong. Mar. Pollut. Bull. 1995, 31, 254–261. [Google Scholar] [CrossRef]
- Chatterjee, M.; Massolo, S.; Sarkar, S.K.; Bhattacharya, A.K.; Bhattacharya, B.D.; Satpathy, K.K.; Saha, S. An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environ. Monit. Assess. 2008, 150, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Penha-Lopes, G.; Torres, P.; Cannicci, S.; Narciso, L.; Paula, J. Monitoring anthropogenic sewage pollution on mangrove creeks in southern Mozambique: A test of Palaemon concinnus Dana, 1852 (Palaemonidae) as a biological indicator. Environ. Pollut. 2011, 159, 636–645. [Google Scholar] [CrossRef]
- Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef]
- Estrada, E.S.; Juhel, G.; Han, P.; Kelly, B.; Lee, W.K.; Bayen, S. Multi-tool assessment of trace metals in mangroves combining sediment and clam sampling, DGT passive samplers and caged mussels. Sci. Total. Environ. 2017, 574, 847–857. [Google Scholar] [CrossRef]
- Alongi, D. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Ip, C.C.; Li, X.; Zhang, G.; Wai, O.W.; Li, Y.-S. Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environ. Pollut. 2007, 147, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Belabed, B.-E.; Laffray, X.; Dhib, A.; Fertouna-Belakhal, M.; Turki, S.; Aleya, L. Factors contributing to heavy metal accumulation in sediments and in the intertidal mussel Perna perna in the Gulf of Annaba (Algeria). Mar. Pollut. Bull. 2013, 74, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Rodrigues, P.P.; Mubiana, V.K.; Blust, R.; De Boeck, G. Linking environmental heavy metal concentrations and salinity gradients with metal accumulation and their effects: A case study in 3 mussel species of Vitória estuary and Espírito Santo bay, Southeast Brazil. Sci. Total. Environ. 2015, 523, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Rubí, J.R.; Luna-Acosta, A.; Etxebarria, N.; Soto, M.; Espinoza, F.; Ahrens, M.; Marigómez, I. Chemical contamination assessment in mangrove-lined Caribbean coastal systems using the oyster Crassostrea rhizophorae as biomonitor species. Environ. Sci. Pollut. Res. 2017, 25, 13396–13415. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Estrada, E.S.; Zhang, H.; Lee, W.K.; Juhel, G.; Smedes, F.; Kelly, B.C. Partitioning and Bioaccumulation of Legacy and Emerging Hydrophobic Organic Chemicals in Mangrove Ecosystems. Environ. Sci. Technol. 2019, 53, 2549–2558. [Google Scholar] [CrossRef]
- De Souza, M.; Windmölller, C.; Hatje, V. Shellfish from Todos os Santos Bay, Bahia, Brazil: Treat or threat? Mar. Pollut. Bull. 2011, 62, 2254–2263. [Google Scholar] [CrossRef] [Green Version]
- Shoults-Wilson, W.A.; Elsayed, N.; Leckrone, K.; Unrine, J.M. Zebra mussels (Dreissena polymorpha) as a biomonitor of trace elements along the southern shoreline of Lake Michigan. Environ. Toxicol. Chem. 2015, 34, 412–419. [Google Scholar] [CrossRef]
- Liu, J.; Cao, L.; Dou, S. Bioaccumulation of heavy metals and health risk assessment in three benthic bivalves along the coast of Laizhou Bay, China. Mar. Pollut. Bull. 2017, 117, 98–110. [Google Scholar] [CrossRef]
- Li, P.; Gao, X. Trace elements in major marketed marine bivalves from six northern coastal cities of China: Concentrations and risk assessment for human health. Ecotoxicol. Environ. Saf. 2014, 109, 1–9. [Google Scholar] [CrossRef]
- Environmental Protection Administration, Taiwan. Database for the National Water Quality Monitoring Project. Available online: https://wq.epa.gov.tw/Code/?Languages=en (accessed on 20 January 2020).
- Environmental Protection Administration, Taiwan. Proposal for the Promotion of Remediation Actions for Pollution in Major Rivers; Environmental Protection Administration: Taipei, Taiwan, 2013.
- Central Weather Bureau, Taiwan. Climate Statistics. Available online: http://www.cwb.gov.tw/V7/service/publication.htm (accessed on 1 February 2018).
- McGeer, J.; Henningsen, G.; Lanno, R.; Fisher, N.; Sappington, K.; Drexler, J. Issue Paper on the Bioavailability and Bioaccumulation of Metals; U.S. Environmental Protection Agency Risk Assessment Forum: Washington DC, USA, 2004.
- Yarsan, E.; Yipe, M. The Important Terms of Marine Pollution “Biomarkers and Biomonitoring, Bioaccumulation, Bioconcentration, Biomagnification”. J. Mol. Biomarkers Diagn. 2013, S1, 003. [Google Scholar] [CrossRef]
- Lewis, M.A.; Pryor, R.; Wilking, L. Fate and effects of anthropogenic chemicals in mangrove ecosystems: A review. Environ. Pollut. 2011, 159, 2328–2346. [Google Scholar] [CrossRef]
- Fu, T.L. The Investigation of Heavy Metal Concentrations in Sediments of Dahan Creek in Taiwan and Their Potential Sources. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2016. [Google Scholar]
- Song, M.; Guan, Y. The electronic government performance of environmental protection administrations in Anhui province, China. Technol. Forecast. Soc. Chang. 2015, 96, 79–88. [Google Scholar] [CrossRef]
- Liu, W.C.; Chen, W.B.; Hsu, M.H. Influences of discharge reductions on salt water intrusion and residual circulation in Danshuei River. J. Mar. Sci. Technol. 2011, 19, 596–606. [Google Scholar]
- Liu, W.-C.; Chen, W.-B.; Hsu, M.-H. Using a three-dimensional particle-tracking model to estimate the residence time and age of water in a tidal estuary. Comput. Geosci. 2011, 37, 1148–1161. [Google Scholar] [CrossRef]
- Chapman, P.M.; Mann, G.S. Sediment Quality Values (SQVs) and Ecological Risk Assessment (ERA). Mar. Pollut. Bull. 1999, 38, 339–344. [Google Scholar] [CrossRef]
- Cuong, D.T.; Bayen, S.; Wurl, O.; Subramanian, K.; Wong, K.K.S.; Sivasothi, N.; Obbard, J.P. Heavy metal contamination in mangrove habitats of Singapore. Mar. Pollut. Bull. 2005, 50, 1732–1738. [Google Scholar] [CrossRef]
- Jara-Marini, M.; Soto-Jimenez, M.F.; Páez-Osuna, F. Trophic relationships and transference of cadmium, copper, lead and zinc in a subtropical coastal lagoon food web from SE Gulf of California. Chemosphere 2009, 77, 1366–1373. [Google Scholar] [CrossRef]
- Monikh, F.A.; Safahieh, A.; Savari, A.; Doraghi, A. Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa Estuary (Persian Gulf). Environ. Monit. Assess. 2012, 185, 215–222. [Google Scholar] [CrossRef]
- Veltman, K.; Huijbregts, M.A.J.; Van Kolck, M.; Wang, W.-X.; Hendriks, A.J. Metal Bioaccumulation in Aquatic Species: Quantification of Uptake and Elimination Rate Constants Using Physicochemical Properties of Metals and Physiological Characteristics of Species. Environ. Sci. Technol. 2008, 42, 852–858. [Google Scholar] [CrossRef]
- Mucha, A.P.; Vasconcelos, M.T.S.; Bordalo, A. Spatial and seasonal variations of the macrobenthic community and metal contamination in the Douro estuary (Portugal). Mar. Environ. Res. 2005, 60, 531–550. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, B.-G. Effects of salinity, temperature and food type on the uptake and elimination rates of cd, cr, and zn in the asiatic clamcorbicula fluminea. Ocean Sci. J. 2005, 40, 79–89. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Wang, D.-X.; Liu, Y.; Shen, Z. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: Relative role of sediments’ properties and metal speciation. Chemosphere 2013, 91, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Rumisha, C.; Elskens, M.; Leermakers, M.; Kochzius, M. Trace metal pollution and its influence on the community structure of soft bottom molluscs in intertidal areas of the Dares Salaam coast, Tanzania. Mar. Pollut. Bull. 2012, 64, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Wang, W.-X. Analyzing biomagnification of metals in different marine food webs using nitrogen isotopes. Mar. Pollut. Bull. 2008, 56, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ward, D.; Williams, J.J.; Fisher, N.S. Metal Bioaccumulation by Estuarine Food Webs in New England, USA. J. Mar. Sci. Eng. 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, E.A.; Miller, S.W. Bioindicators: Using organisms to measure environmental impacts. Nat. Educ. Knowl. 2010, 3, 8–13. [Google Scholar]
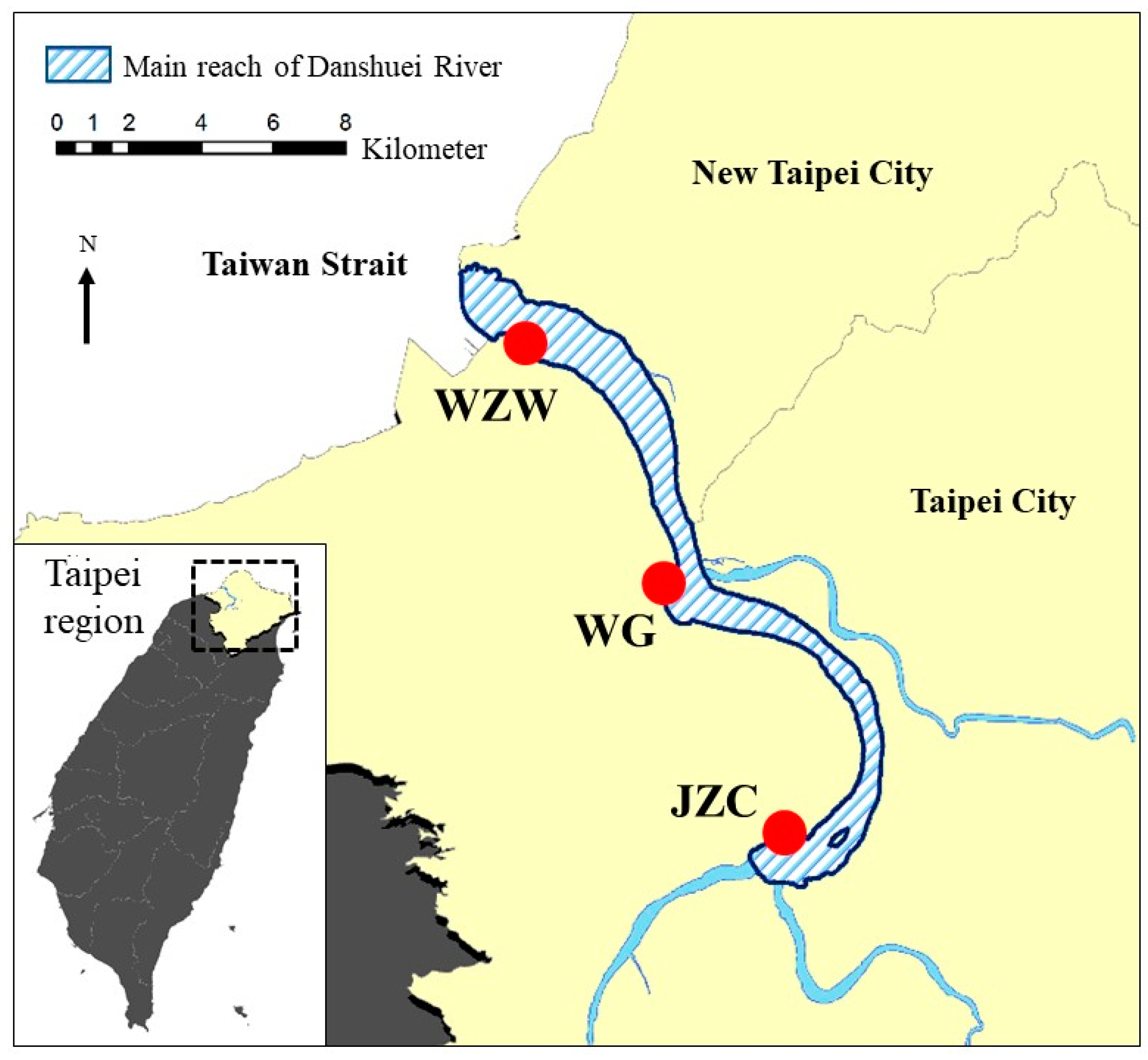
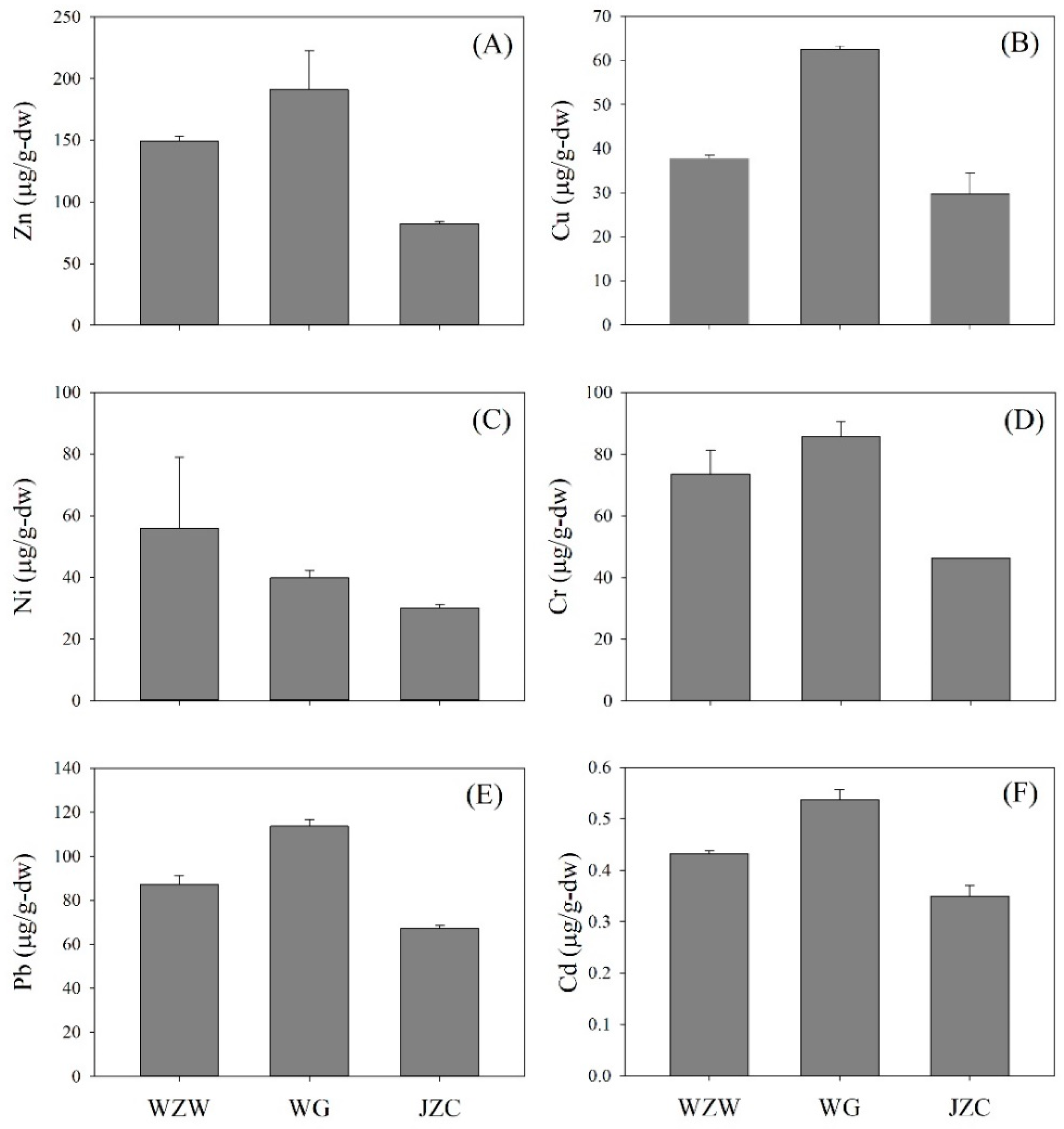


| Study Site | |||
|---|---|---|---|
| WZW | WG | JZC | |
| Geographical location | 25°09′ N, 121°25′ E | 25°06′ N, 121°27′ E | 25°02′ N, 121°29′ E |
| Distance from sea (km) | 1.11 | 8.51 | 19.02 |
| Salinity | 18.69 ± 2.75 | 6.48 ± 2.77 | 1.17 ± 0.66 |
| pH | 7.76 ± 0.03 | 7.42 ± 0.09 | 7.18 ± 0.14 |
| Dissolved oxygen (mg/L) | 4.76 ± 0.38 | 3.30 ± 0.47 | 2.98 ± 1.47 |
| Ammonium–nitrogen (mg/L) | 0.92 ± 0.05 | 1.90 ± 0.06 | 1.47 ± 0.35 |
| Nitrate–nitrogen (mg/L) | 0.21 ± 0.01 | 0.38 ± 0.04 | 0.39 ± 0.01 |
| Phosphate–phosphorus (mg/L) | 0.12 ± 0.04 | 0.20 ± 0.12 | 0.43 ± 0.22 |
| Chlorophyll a (µg/L) | 10.96 ± 0.42 | 39.39 ± 15.50 | 81.15 ± 6.70 |
| Turbidity (NTU) | 13.00 ± 5.66 | 82.00 ± 22.63 | 94.5 ± 17.68 |
| Site | Studied Metallic Contaminants | |||||
|---|---|---|---|---|---|---|
| Zn | Cu | Ni | Cr | Pb | Cd | |
| WZW | 10.30 | 2.40 | 0.25 | 0.36 | 0.04 | 0.42 |
| WG | 8.10 | 0.89 | 0.50 | 0.34 | 0.07 | 0.31 |
| JZC | 0.39 | 1.9 | 0.66 | 0.01 | 0 | 0.26 |
| F | 65.97 *** | 20.56 *** | 14.67 *** | 18.48 *** | 86.60 *** | 3.86 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yam, R.S.W.; Fan, Y.-T.; Tan, Z.; Wang, T.-D.; Chiu, C.-Y. Assessing Impacts of Metallic Contamination along the Tidal Gradient of a Riverine Mangrove: Multi-metal Bioaccumulation and Biomagnification of Filter-Feeding Bivalves. Forests 2020, 11, 504. https://doi.org/10.3390/f11050504
Yam RSW, Fan Y-T, Tan Z, Wang T-D, Chiu C-Y. Assessing Impacts of Metallic Contamination along the Tidal Gradient of a Riverine Mangrove: Multi-metal Bioaccumulation and Biomagnification of Filter-Feeding Bivalves. Forests. 2020; 11(5):504. https://doi.org/10.3390/f11050504
Chicago/Turabian StyleYam, Rita S. W., Yen-Tzu Fan, Zhehan Tan, Tzu-Dan Wang, and Chiu-Yu Chiu. 2020. "Assessing Impacts of Metallic Contamination along the Tidal Gradient of a Riverine Mangrove: Multi-metal Bioaccumulation and Biomagnification of Filter-Feeding Bivalves" Forests 11, no. 5: 504. https://doi.org/10.3390/f11050504





