Arbuscular Mycorrhizal Fungi Mitigate Nitrogen Leaching under Poplar Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Materials
2.3. Poplar Planting and Treatment
2.4. Biochemical Measurements
2.4.1. Leached Fluid Collection and N Contents Determination
2.4.2. Seedling Growth and Physiological Indicators
2.4.3. AM Colonization Rate
2.4.4. Soil Physicochemical Properties
2.5. Data Processing
3. Results
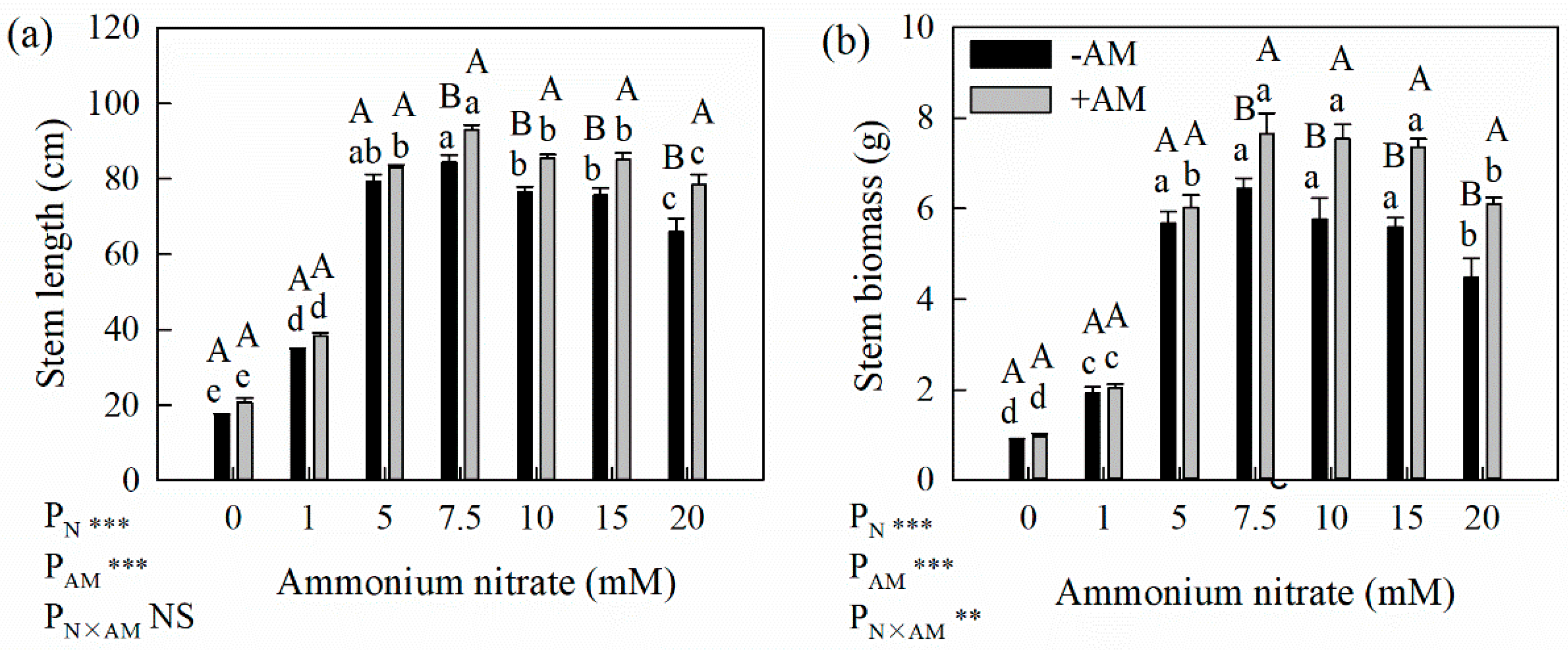
3.1. Effects of AM Fungi on Poplar Seedling Height and Stem Biomass Under Different N Levels
3.2. Effects of AM Fungi on Soil N Leaching
3.3. AM Colonization Rates under Different N Levels
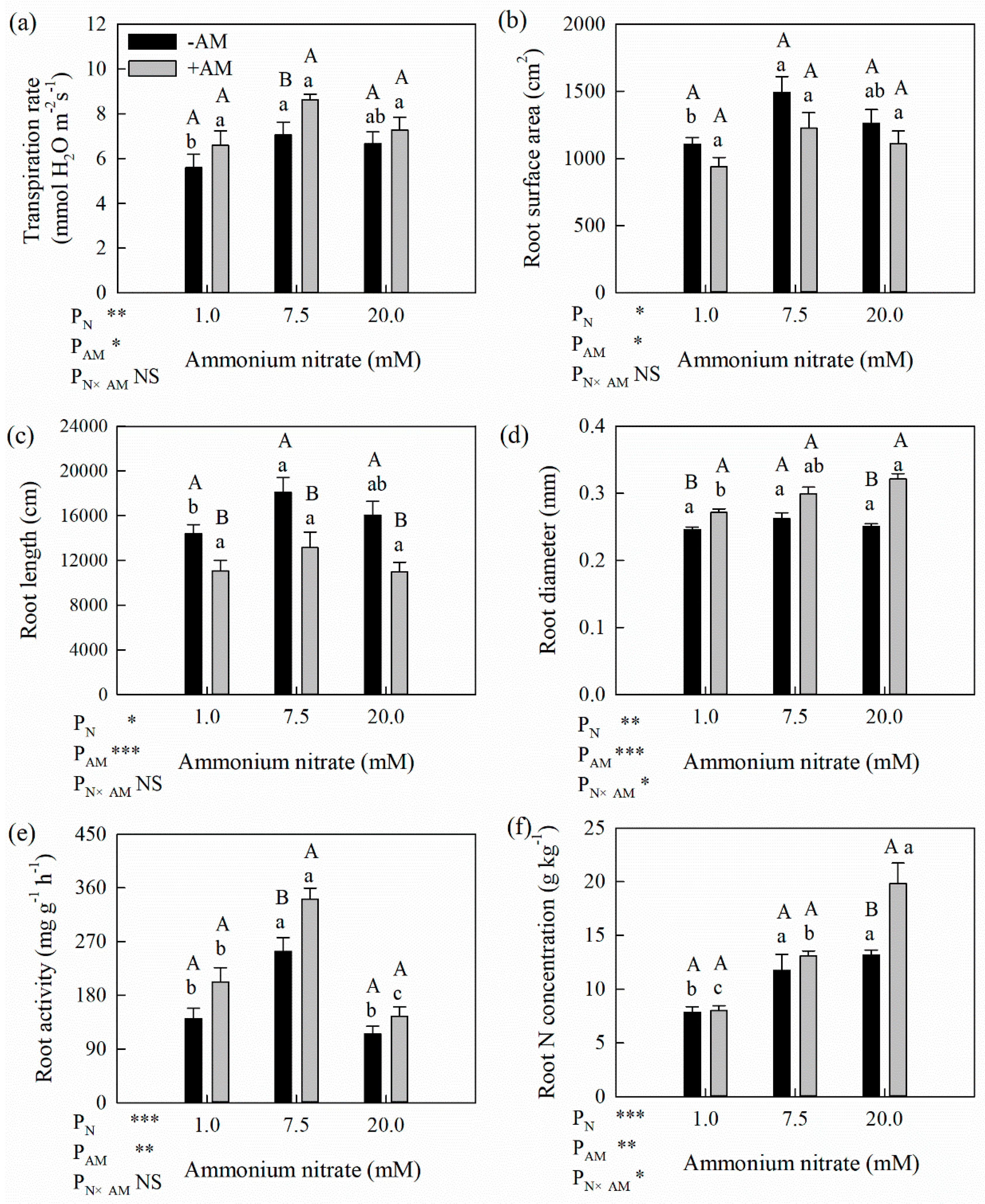
3.4. Effects of AM Fungi on Physiological and Root Morphological Variables of Poplar Seedlings
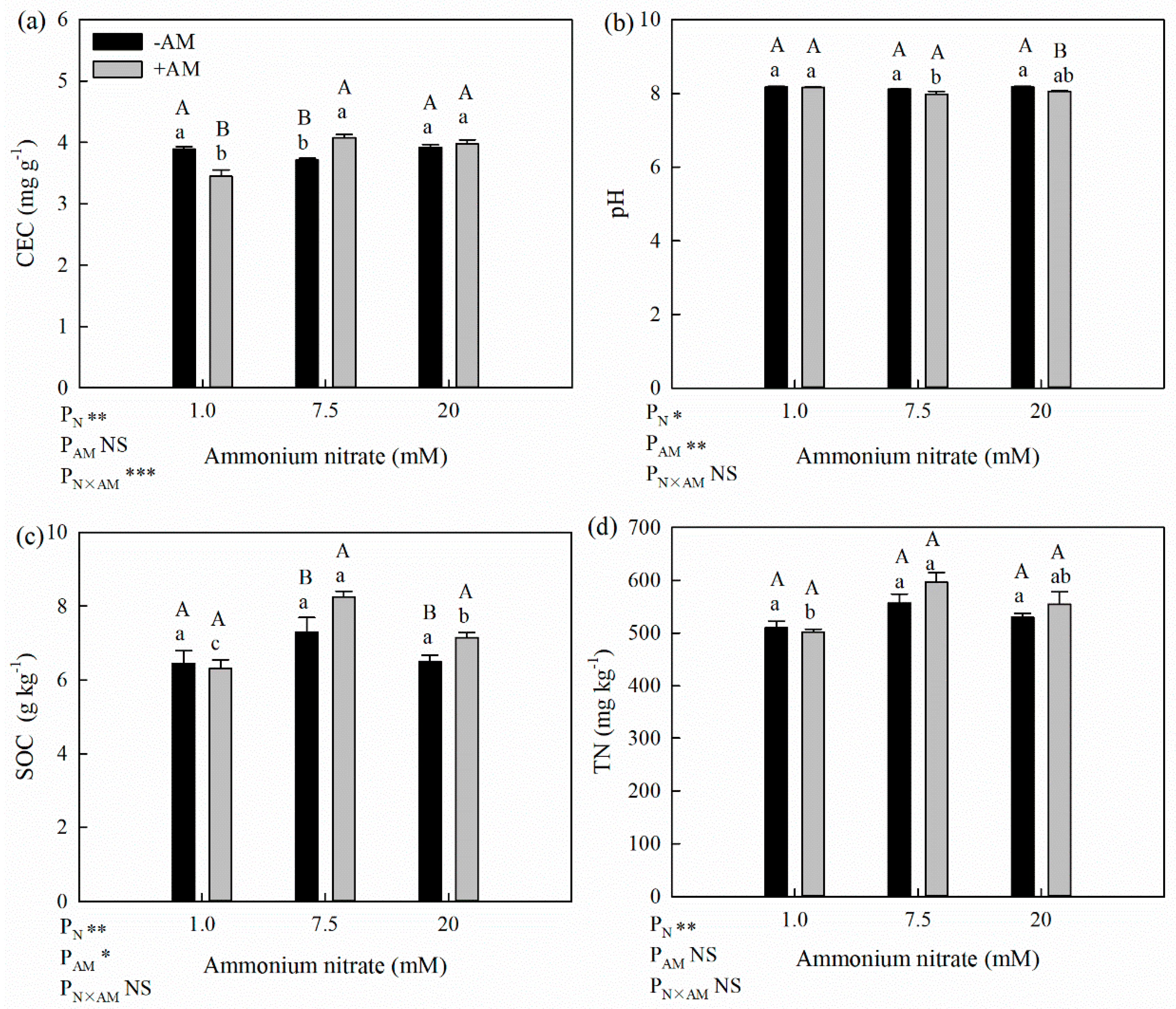
3.5. Response of Soil Properties to AM Inoculation
3.5.1. Soil CEC and pH
3.5.2. SOC and Soil Aggregates
3.5.3. Soil TN
3.6. The Relative Contributions of the Variables Affecting Soil N Leaching
4. Discussion
4.1. The Influence of Am Fungi on Soil N Leaching
4.2. Mechanism of AM Fungi on N Leaching in Poplar Microsystem
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; You, L.; Amini, M.; Obersteiner, M.; Herrero, M.; Zehnder, A.J.B.; Yang, H. A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. USA 2010, 107, 8035–8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohm, C.; Landgraf, D.; Makeschin, F. Effects of two contrasting agricultural land-use practices on nitrogen leaching in a sandy soil of Middle Germany. J. Plant Nutr. Soil Sci. 2009, 172, 408–417. [Google Scholar] [CrossRef]
- Farneselli, M.; Tosti, G.; Onofri, A.; Benincasa, P.; Guiducci, M.; Pannacci, E.; Tei, F. Effects of N sources and management strategies on crop growth, yield and potential N leaching in processing tomato. Eur. J. Agron. 2018, 98, 46–54. [Google Scholar] [CrossRef]
- Peng, S.Z.; He, Y.P.; Yang, S.H.; Xu, J.Z. Effect of controlled irrigation and drainage on nitrogen leaching losses from paddy fields. Paddy Water Environ. 2015, 13, 303–312. [Google Scholar] [CrossRef]
- Shaddox, T.W.; Kruse, J.K.; Miller, G.L.; Nkedi-Kizza, P.; Sartain, J.B. Surfactant-modified soil amendments reduce nitrogen and phosphorus leaching in a sand-based rootzone. J. Environ. Qual. 2016, 45, 1549–1556. [Google Scholar] [CrossRef]
- Yu, Q.G.; Chen, Y.X.; Ye, X.Z.; Zhang, Q.L.; Zhang, Z.J.; Tian, P. Evaluation of nitrification inhibitor 3,4-dimethyl pyrazole phosphate on nitrogen leaching in undisturbed soil columns. Chemosphere 2007, 67, 872–878. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Guether, M.; Balestrini, R.; Hannah, M.; He, J.; Udvardi, M.K.; Bonfante, P. Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol. 2009, 182, 200–212. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Bucking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy (Basel) 2015, 5, 587–612. [Google Scholar] [CrossRef] [Green Version]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Li, X.L.; George, E.; Marschner, H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.E.; Piatti, P.; Cheshire, M.V.; Watson, C.A. Polysaccharides and monosaccharides in the hyphosphere of the arbuscular mycorrhizal fungi Glomus E3 and Glomus tenue. Soil Biol. Biochem. 2007, 39, 680–683. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Chen, B.D.; Rillig, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol. Biochem. 2012, 46, 53–62. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology 2010, 91, 1163–1171. [Google Scholar] [CrossRef]
- Asghari, H.R.; Cavagnaro, T.R. Arbuscular mycorrhizas reduce nitrogen loss via leaching. PLoS ONE 2012, 7, e29825. [Google Scholar] [CrossRef] [Green Version]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang. Biol. 2018, 24, E171–E182. [Google Scholar] [CrossRef]
- Kucova, L.; Zahora, J.; Pokluda, R. Effect of mycorrhizal inoculation of leek Allium porrum L. on mineral nitrogen leaching. Hortic. Sci. 2016, 43, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Asghari, H.R.; Cavagnaro, T.R. Arbuscular mycorrhizas enhance plant interception of leached nutrients. Funct. Plant Biol. 2011, 38, 219–226. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; De Deyn, G.B.; Pugnaire, F.I.; Kothamasi, D.; van der Heijden, M.G.A. Symbiotic soil fungi enhance ecosystem resilience to climate change. Glob. Chang. Biol. 2017, 23, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Conen, F.; van der Heijden, M.G.A. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol. Biochem. 2015, 80, 283–292. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden, M.G.A. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Kohl, L.; van der Heijden, M.G.A. Arbuscular mycorrhizal fungal species differ in their effect on nutrient leaching. Soil Biol. Biochem. 2016, 94, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Kohl, L.; Oehl, F.; van der Heijden, M.G.A. Agricultural practices indirectly influence plant productivity and ecosystem services through effects on soil biota. Ecol. Appl. 2014, 24, 1842–1853. [Google Scholar] [CrossRef]
- Corkidi, L.; Merhaut, D.J.; Allen, E.B.; Downer, J.; Bohn, J.; Evans, M. Effects of mycorrhizal colonization on nitrogen and phosphorus leaching from nursery containers. HortScience 2011, 46, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- De Vries, F.T.; Thebault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjornlund, L.; Jorgensen, H.B.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [Green Version]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Francis, G.S.; Haynes, R.J.; Sparling, G.P.; Ross, D.J.; Williams, P.H. Nitrogen mineralization, nitrate leaching and crop growth following cultivation of a temporary leguminous pasture in autumn and winter. Fertil. Res. 1992, 33, 59–70. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Duffkova, R.; Fucik, P.; Jurkovska, L.; Janouskova, M. Experimental evaluation of the potential of arbuscular mycorrhiza to modify nutrient leaching in three arable soils located on one slope. Appl. Soil Ecol. 2019, 143, 116–125. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Wilson, B.R.; Lockwood, P.V.; Daniel, H.; Young, I.M. Soil organic carbon mineralization rates in aggregates under contrasting land uses. Geoderma 2014, 216, 10–18. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Lefevre, C.; Bonito, G. Characterizing root-associated fungal communities and soils of Douglas-fir (Pseudotsuga menziesii) stands that naturally produce Oregon white truffles (Tuber oregonense and Tuber gibbosum). Mycorrhiza 2016, 26, 367–376. [Google Scholar] [CrossRef]
- Manzone, M.; Bergante, S.; Facciotto, G. Energy and economic evaluation of a poplar plantation for woodchips production in Italy. Biomass Bioenerg. 2014, 60, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Baum, C.; Makeschin, F. Effects of nitrogen and phosphorus fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P. tremula × tremuloides). J. Plant Nutr. Soil Sci. 2000, 163, 491–497. [Google Scholar] [CrossRef]
- Rooney, D.C.; Prosser, J.I.; Bending, G.D.; Baggs, E.M.; Killham, K.; Hodge, A. Effect of arbuscular mycorrhizal colonisation on the growth and phosphorus nutrition of Populus euramericana c.v. Ghoy. Biomass Bioenerg. 2011, 35, 4605–4612. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, H.Q.; Fang, F.R.; Wu, N.; Zhang, Y.X.; Tang, M. Effects of nitrogen and exogenous rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva’. J. Plant Growth Regul. 2017, 36, 824–835. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Zhao, L. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Artemisia ordosica Krasch. In Mu Us sandland, China. Soil Biol. Biochem. 2010, 42, 1313–1319. [Google Scholar] [CrossRef]
- Feng, C.; Ma, Y.; Fu, S.; Chen, H.Y.H. Soil carbon and nutrient dynamics following cessation of anthropogenic disturbances in degraded subtropical forests. Land Degrad. Dev. 2017, 28, 2457–2467. [Google Scholar] [CrossRef]
- Knoth, J.L.; Kim, S.H.; Ettl, G.J.; Doty, S.L. Effects of cross host species inoculation of nitrogen-fixing endophytes on growth and leaf physiology of maize. GCB Bioenergy 2013, 5, 408–418. [Google Scholar] [CrossRef]
- Liu, T.; Sheng, M.; Wang, C.Y.; Chen, H.; Li, Z.; Tang, M. Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 2015, 53, 250–258. [Google Scholar] [CrossRef]
- Chu, H.L.; Wang, C.Y.; Li, Z.M.; Wang, H.H.; Xiao, Y.G.; Chen, J.; Tang, M. The dark septate endophytes and ectomycorrhizal fungi effect on Pinus tabulaeformis carr. seedling growth and their potential effects to pine wilt disease resistance. Forests 2019, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Clemenssonlindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental-stress in coniferous forest stands—Applications and limitations. Plant Soil 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Schuman, G.E.; Stanley, M.A.; Knudsen, D. Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc. Am. J. 1973, 37, 480–481. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.; Miller, M.; Evans, D.; Fairchild, G.; Swan, J. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Qiu, X.C.; Zhu, Y.Q. Rapid analysis of cation-exchange properties in acidic soils. Soil Sci. 1993, 155, 301–308. [Google Scholar] [CrossRef]
- Wei, S.H.; Zhou, Q.X.; Koval, P.V. Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci. Total Environ. 2006, 369, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bieganowski, A.; Ryzak, M.; Witkowska-Walczak, B. Determination of soil aggregate disintegration dynamics using laser diffraction. Clay Min. 2010, 45, 23–34. [Google Scholar] [CrossRef]
- Bago, B.; Cano, C.; Azcon-Aguilar, C.; Samson, J.; Coughlan, A.P.; Piche, Y. Differential morphogenesis of the extraradical mycelium of an arbuscular mycorrhizal fungus grown monoxenically on spatially heterogeneous culture media. Mycologia 2004, 96, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.L.; Hartley, A.E.; Vogelsang, K.M.; Bever, J.D.; Schultz, P.A. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005, 167, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819. [Google Scholar] [CrossRef]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glornus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Toussaint, J.P.; St-Arnaud, M.; Charest, C. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can. J. Microbiol. 2004, 50, 251–260. [Google Scholar]
- Curtin, D.; Rostad, H.P.W. Cation exchange and buffer potential of Saskatchewan soils estimated from texture, organic matter and pH. Can. J. Soil Sci. 1997, 77, 621–626. [Google Scholar] [CrossRef]
- Gai, X.P.; Wang, H.Y.; Liu, J.; Zhai, L.M.; Liu, S.; Ren, T.Z.; Liu, H.B. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.X.; Meng, S.; Li, Y.M.; Su, L.; Zhao, Z. Nitrogen uptake and allocation in Populus simonii in different seasons supplied with isotopically labeled ammonium or nitrate. Trees Struct. Funct. 2016, 30, 2011–2018. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.N.; Yang, G.W.; Yang, H.J.; Chen, Y.L.; Liu, N.; Zhang, Y.J. Arbuscular mycorrhizal fungi affect seedling recruitment: A potential mechanism by which N deposition favors the dominance of grasses over forbs. Plant Soil 2014, 375, 127–136. [Google Scholar] [CrossRef]
- Vogeler, I.; Green, S.R.; Mills, T.; Clothier, B.E. Modelling nitrate and bromide leaching from sewage sludge. Soil Till. Res. 2006, 89, 177–184. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.L.; Zhang, S.B.; Chen, B.D. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, M.; Sandmann, M.; Graefe, J. Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Aronsson, P.G.; Bergström, L.F. Nitrate leaching from lysimeter-grown short-rotation willow coppice in relation to N-application, irrigation and soil type. Biomass Bioenerg. 2001, 21, 155–164. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.W.; Zhang, F.F.; Huang, Y.H. Influence of arbuscular mycorrhizae on the root system of maize plants under salt stress. Can. J. Microbiol. 2009, 55, 879–886. [Google Scholar] [CrossRef]
- Guo, R.Y.; Qin, W.; Jiang, C.G.; Kang, L.Y.; Nendel, C.; Chen, Q. Sweet corn significantly increases nitrogen retention and reduces nitrogen leaching as summer catch crop in protected vegetable production systems. Soil Till. Res. 2018, 180, 148–153. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M. Distribution characteristics of soil pH, CEC and organic matter in a small watershed of the Loess Plateau. Yingyong Shengtai Xuebao 2009, 20, 2710–2715, (Chinese with English abstract). [Google Scholar]
- Curtin, D.; Fraser, P.M.; Beare, M.H. Loss of soil organic matter following cultivation of long-term pasture: Effects on major exchangeable cations and cation exchange capacity. Soil Res. 2015, 53, 377–385. [Google Scholar] [CrossRef]
- Daynes, C.N.; Field, D.J.; Saleeba, J.A.; Cole, M.A.; McGee, P.A. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol. Biochem. 2013, 57, 683–694. [Google Scholar] [CrossRef]
- Sarapatka, B.; Alvarado-Solano, D.P.; Cizmar, D. Can glomalin content be used as an indicator for erosion damage to soil and related changes in organic matter characteristics and nutrients? Catena 2019, 181, 8. [Google Scholar] [CrossRef]
- Sharifi, Z.; Azadi, N.; Rahimi, S.; Certini, G. The response of glomalin-related soil proteins to fire or tillage. Geoderma 2018, 329, 65–72. [Google Scholar] [CrossRef]
- Bergeron, M.; Lacombe, S.; Bradley, R.L.; Whalen, J.; Cogliastro, A.; Jutras, M.F.; Arp, P. Reduced soil nutrient leaching following the establishment of tree-based intercropping systems in eastern Canada. Agrofor. Syst. 2011, 83, 321–330. [Google Scholar] [CrossRef]
- Kanthle, A.K.; Lenka, N.K.; Lenka, S.; Tedia, K. Biochar impact on nitrate leaching as influenced by native soil organic carbon in an Inceptisol of central India. Soil Tillage Res. 2016, 157, 65–72. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [Green Version]


| N Levels (mM) | Inoculation | Volume (mL) | Nitrate N (mg L−1) | Ammonium N (mg L−1) | Nitrate & Ammonium (mg) |
|---|---|---|---|---|---|
| 1 | −AM | 66.8 ± 5.1 | 0.75 ± 0.19 Ab | 0.35 ± 0.04 Ab | 0.07 ± 0.02 Ab |
| +AM | 67.0 ± 3.8 | 0.57 ± 0.13 Ab | 0.36 ± 0.01 Ab | 0.07 ± 0.01 Ab | |
| 7.5 | −AM | 58.8 ± 6.8 | 4.71 ± 0.47 Ab | 0.63 ± 0.22 Ab | 0.31 ± 0.05 Ab |
| +AM | 60.9 ± 6.9 | 3.75 ± 0.67 Ab | 0.45 ± 0.04 Ab | 0.26 ± 0.05 Ab | |
| 20 | −AM | 63.8 ± 2.1 | 31.71 ± 2.38 Aa | 4.56 ± 0.70 Aa | 2.31 ± 0.15 Aa |
| +AM | 68.5 ± 10.2 | 25.36 ± 2.57 Ba | 1.48 ± 0.23 Ba | 1.79 ± 0.12 Ba | |
| Significance | N | NS | *** | *** | *** |
| AM | NS | * | ** | * | |
| N × AM | NS | * | ** | * |
| N Levels (mM) | Inoculation | Aggregates Distribution (%) | ||||
|---|---|---|---|---|---|---|
| <0.002 mm | 0.002–0.01 mm | 0.01–0.25 mm | 0.25–0.5 mm | 0.5–1 mm | ||
| 1 | −AM | 13.37 ± 1.04 | 12.31 ± 0.98 | 70.42 ± 2.09 | 3.12 ± 0.14 Aa | 0.79 ± 0.01 Aa |
| +AM | 14.49 ± 0.13 | 13.24 ± 0.33 | 68.61 ± 0.37 | 3.03 ± 0.06 Ab | 0.63 ± 0.03 Ac | |
| 7.5 | −AM | 14.48 ± 0.40 | 13.23 ± 0.65 | 68.57 ± 0.89 | 2.88 ± 0.02 Bab | 0.83 ± 0.01 Ba |
| +AM | 14.18 ± 0.51 | 13.04 ± 1.09 | 67.29 ± 1.60 | 4.32 ± 0.02 Aa | 1.17 ± 0.01 Ab | |
| 20 | −AM | 14.05 ± 0.10 | 12.86 ± 1.40 | 69.70 ± 1.46 | 2.74 ± 0.09 Bb | 0.65 ± 0.02 Bb |
| +AM | 14.49 ± 1.20 | 12.73 ± 0.44 | 66.92 ± 0.63 | 4.45 ± 0.25 Aa | 1.41 ± 0.01 Aa | |
| Significance | N | NS | NS | NS | ** | *** |
| AM | NS | NS | NS | *** | *** | |
| N × AM | NS | NS | NS | *** | *** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, F.; Wang, C.; Wu, F.; Tang, M.; Doughty, R. Arbuscular Mycorrhizal Fungi Mitigate Nitrogen Leaching under Poplar Seedlings. Forests 2020, 11, 325. https://doi.org/10.3390/f11030325
Fang F, Wang C, Wu F, Tang M, Doughty R. Arbuscular Mycorrhizal Fungi Mitigate Nitrogen Leaching under Poplar Seedlings. Forests. 2020; 11(3):325. https://doi.org/10.3390/f11030325
Chicago/Turabian StyleFang, Fengru, Chunyan Wang, Fei Wu, Ming Tang, and Russell Doughty. 2020. "Arbuscular Mycorrhizal Fungi Mitigate Nitrogen Leaching under Poplar Seedlings" Forests 11, no. 3: 325. https://doi.org/10.3390/f11030325





