Modelling Post-Disturbance Successional Dynamics of the Canadian Boreal Mixedwoods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. SORTIE-ND Simulator
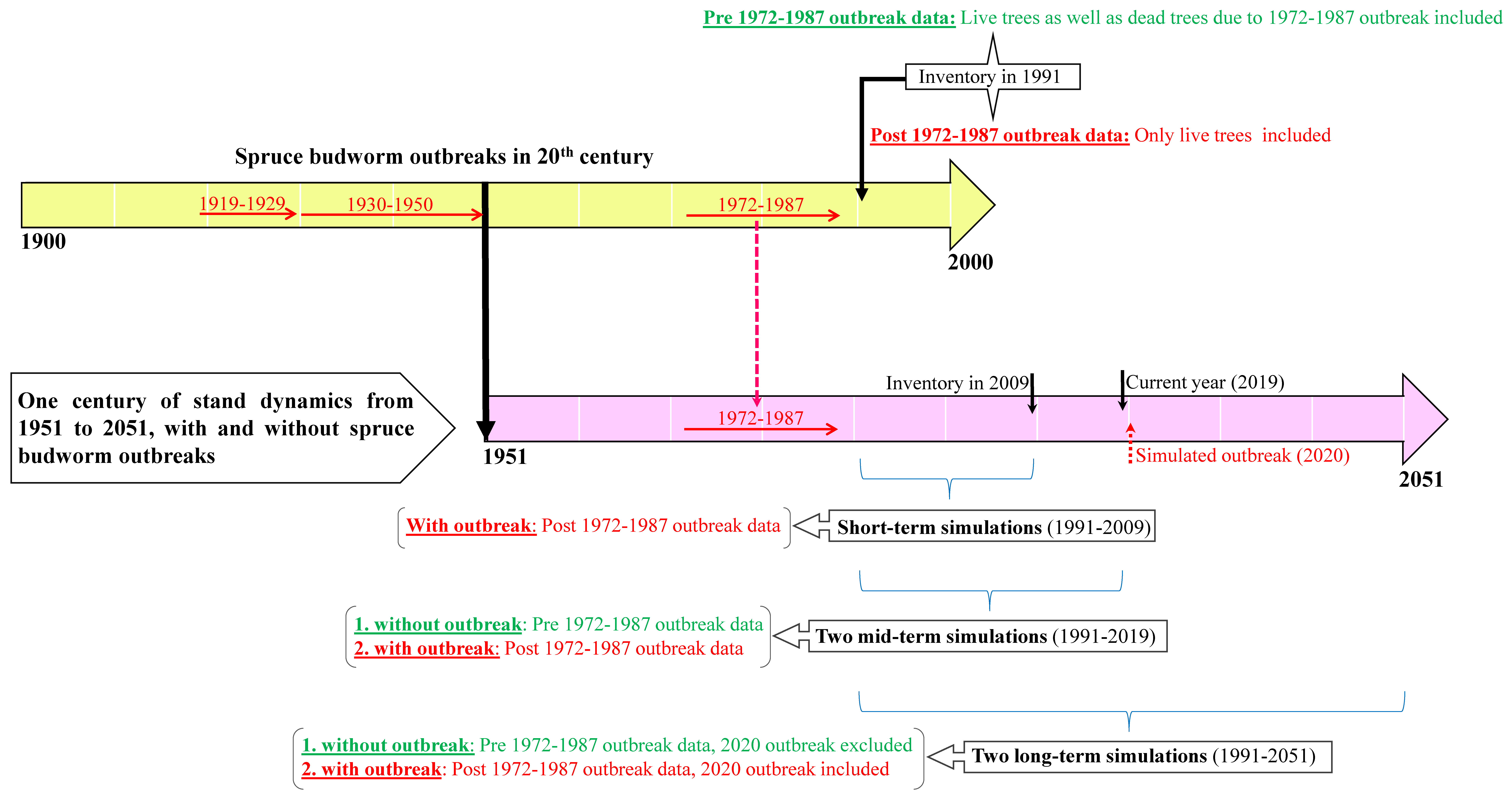
2.4. Simulation Runs
2.4.1. Model Parameterizations and Evaluations through Short-Term Simulations
2.4.2. Mid-Term Simulations
2.4.3. Long-Term Simulations
2.4.4. Assessing the Mid- to Long-Term Performance of the Model
2.4.5. Mid- to Long-Term Influence of Spruce Budworm Outbreak
3. Results
3.1. Model Evaluations
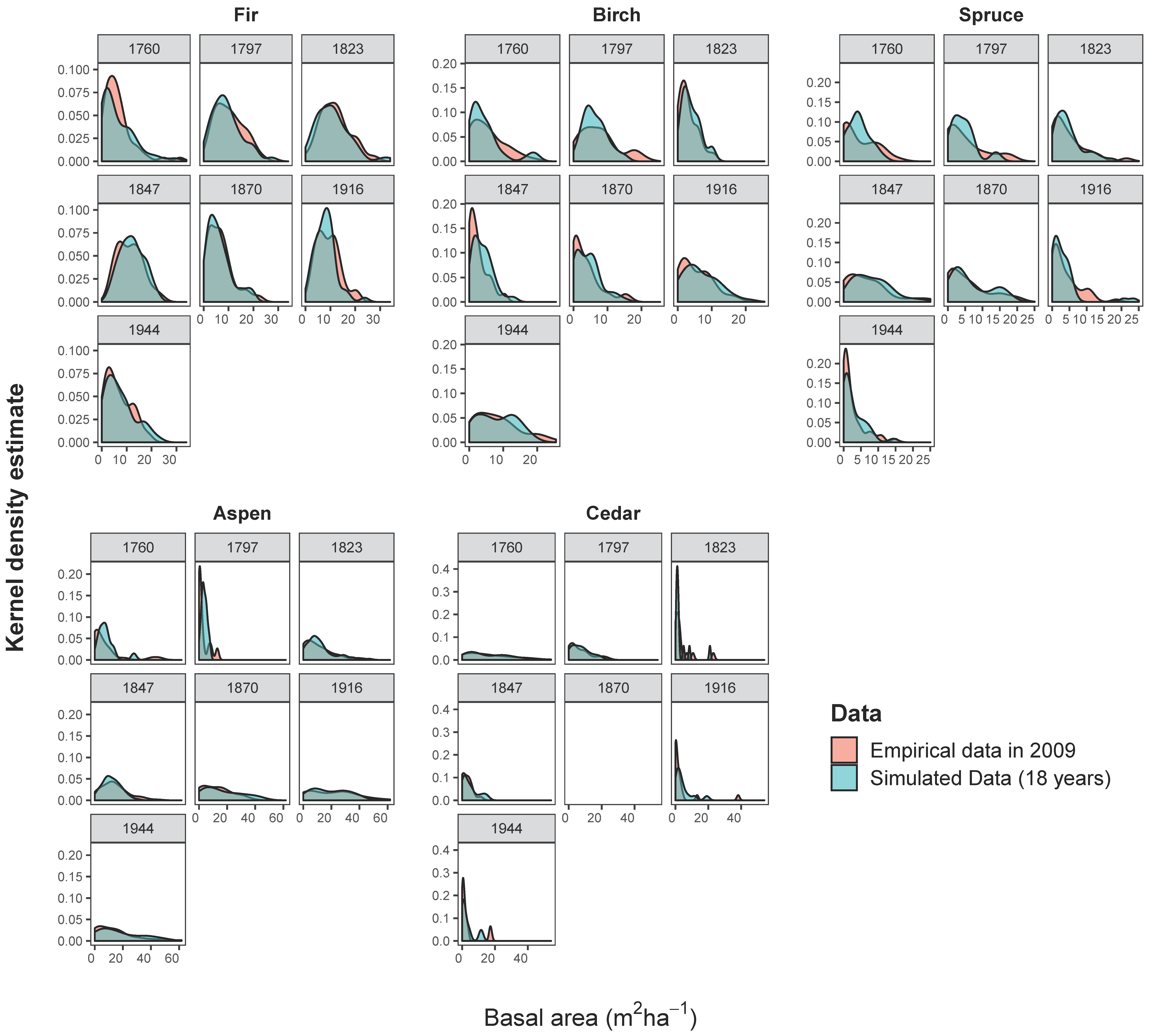
3.1.1. Short-Term Model Evaluation
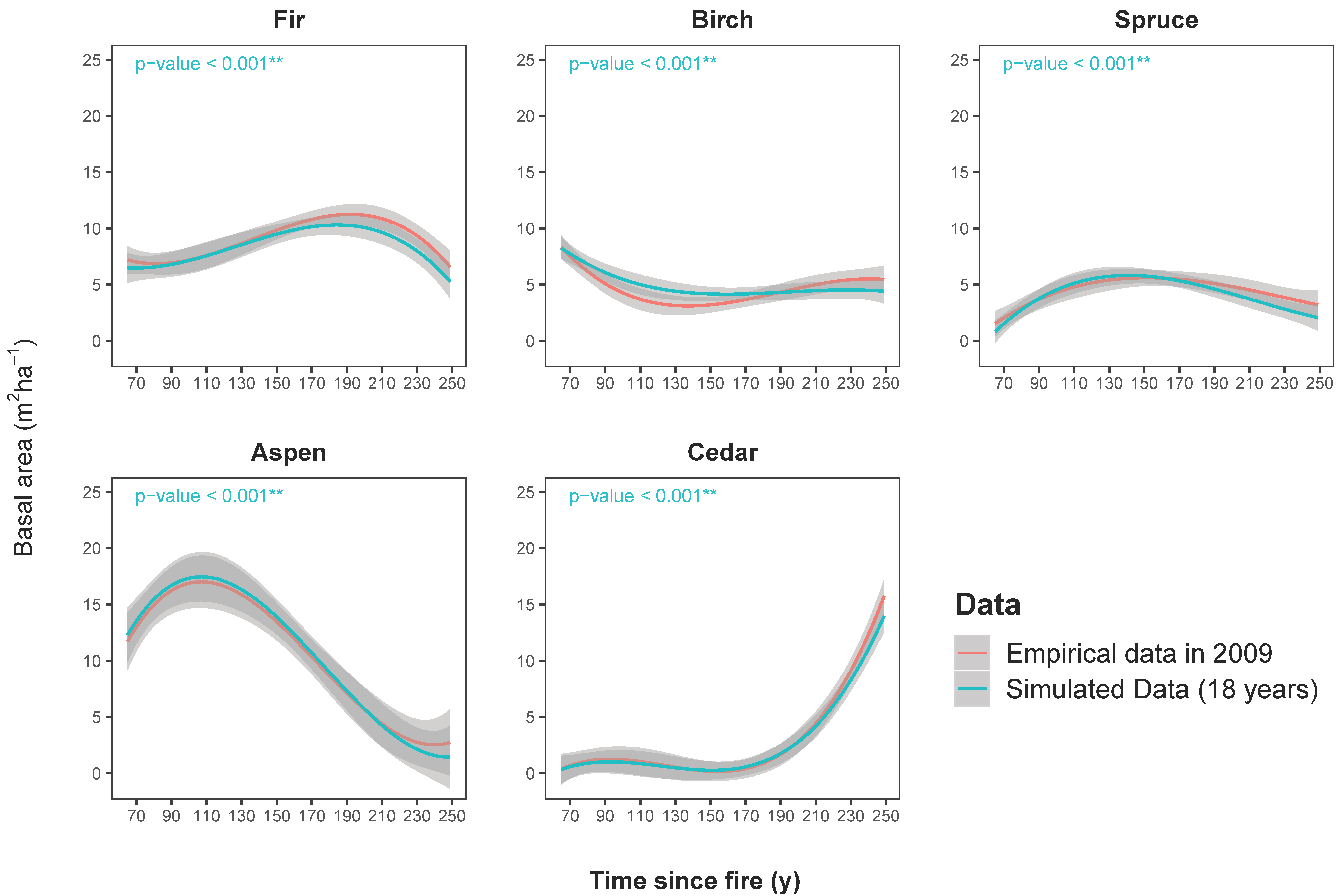
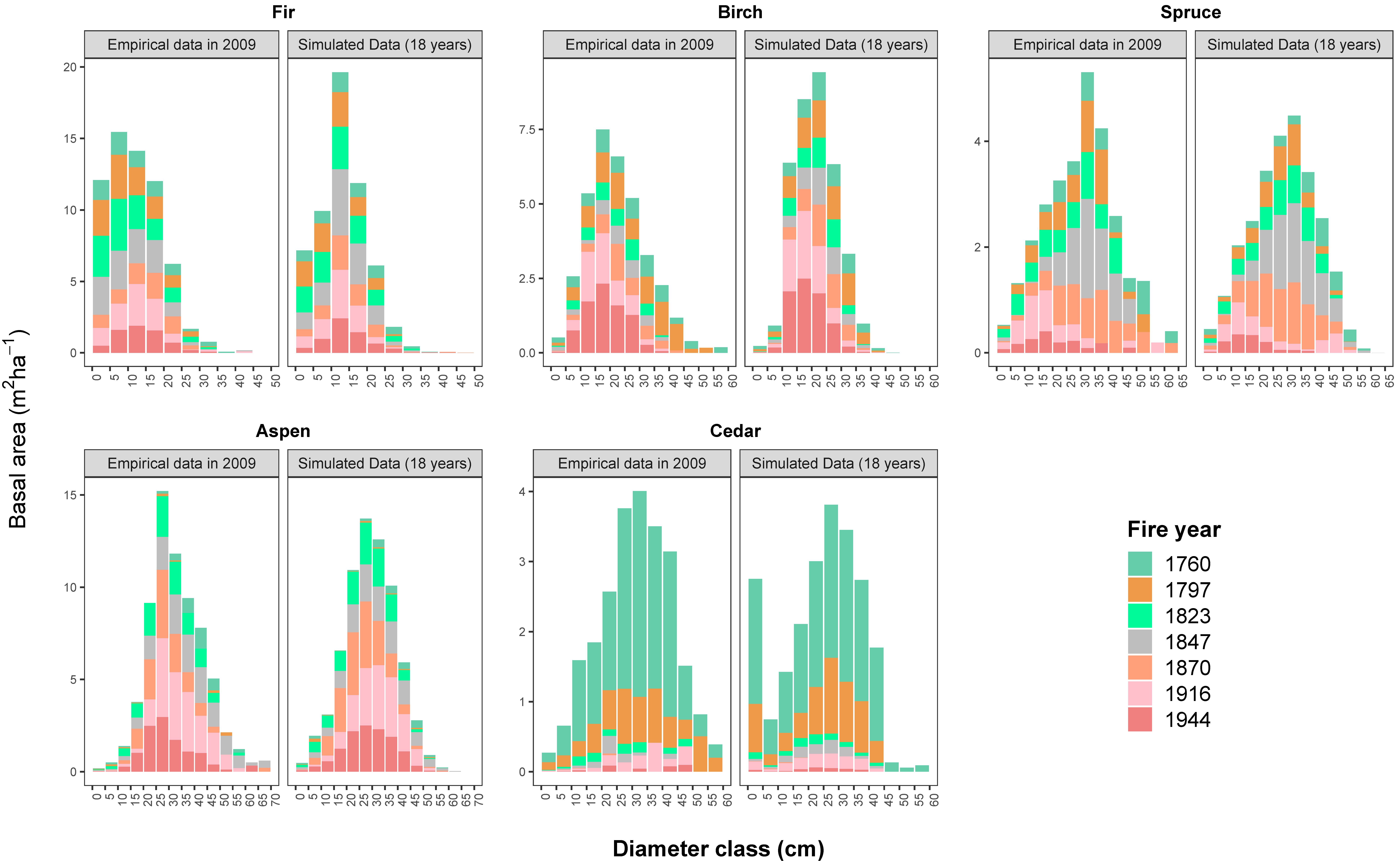
3.1.2. Mid- to Long-Term Model Evaluation
3.2. Mid- to Long-Term Influence of Spruce Budworm
4. Discussion
4.1. Short-Term Evaluation
Individual Species Dynamics within the Post-Fire Stands
4.2. Mid- to Long-Term Evaluation—Can We Reconstruct the Post-Fire Forest Succession?
4.3. Spruce Budworm Contribution to the Successional Dynamics of Post-Fire Stands
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Plot
Appendix A.2. Tree Population and Allometry Data
Appendix A.3. Light (GLI: Global Light Index)
Appendix A.4. Growth Sub-Models
Appendix A.4.1. Non-Limited Absolute Growth for Seedlings and Saplings
Appendix A.4.2. Neighborhood Competition Index Growth for Adult Trees
- , where DBH is the diameter of the target tree, X0 is the size effect mode, and Xb is the size effect variance.
- , where m is the shading effect coefficient, S is the amount of shadow that is cast by the neighborhood, and n is the shading effect exponent.
- , where C is the slope of the crowding effect, DBH is the diameter of the target tree, γ is the NCI size sensitivity to target tree species type, D is the NCI crowding effect steepness, and NCI is the individual-based tree NCI value.
Appendix A.4.3. Constant Radial Growth for Adult Trees
Appendix A.5. Mortality Sub-Models
Appendix A.5.1. Juvenile Mortality
Appendix A.5.2. Adult Stochastic Mortality
Appendix A.5.3. Weibull Snag Mortality
Appendix A.5.4. Competition Mortality
Appendix A.5.5. Senescence
Appendix A.6. Substrate
Appendix A.7. Gap Spatial Dispersal and Substrate Seed Survival
Appendix A.8. Output
Appendix B
| Evaluations * | Parameter | Old Value | New Value | Source of New Parameters |
|---|---|---|---|---|
| Overestimation for birch >5 cm DBH | NCI maximum potential growth | 0.692 | 0.593 | Figure 7 in [8] |
| DBH at onset of senescence | 50 cm | 30 cm | [100] | |
| Competition mortality shape parameter | 4.33E-8 | 4.33E-6 | [56] | |
| Competition mortality maximum parameter | 0.1 | 0.25 | ||
| Adult stochastic mortality | 0.00004 | 0.009 | ||
| Underestimation for aspen 5–15 cm DBH | Seedling stochastic mortality | 0.1 | 0.01 | |
| Underestimation for aspen >50 cm DBH | DBH at onset of senescence | 30 | 40 | [100] |
Appendix C
References
- Penner, M. Yield prediction for mixed species stands in boreal Ontario. For. Chron. 2008, 84, 46–52. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Popadiouk, R.V. Dynamics of North American boreal mixedwoods. Environ. Rev. 2002, 10, 137–166. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities and Acosystems, 2nd ed.; Macmillan: New York, NY, USA, 1975; ISBN 0024273902. [Google Scholar]
- Overpeck, J.T.; Rind, D.; Goldberg, R. Climate-induced changes in forest disturbance and vegetation. Nature 1990, 343, 51–53. [Google Scholar] [CrossRef]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes-Studies from Temperate Evergreen-Deciduous Forests; Cambridge University Press: Cambridge, UK, 2002; ISBN 0521650828. [Google Scholar]
- Holling, C.S. The role of forest insects in structuring the boreal landscape. In A Systems Analysis of the Global Boreal Forest; Shugart, H.H., Leemans, R., Bonan, G.B., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 170–191. ISBN 9780511565489. [Google Scholar]
- Payette, S. Fire as a controlling process in the North American boreal forest. In A Systems Analysis of the Global Boreal Forest; Shugart, H.H., Leemans, R., Bonan, G.B., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 144–169. ISBN 9780511565489. [Google Scholar]
- Bergeron, Y. Species and Stand Dynamics in the Mixed Woods of Quebec’s Southern Boreal Forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Nlungu-Kweta, P.; Leduc, A.; Bergeron, Y. Conifer Recruitment in Trembling Aspen (Populus Tremuloides Michx.) Stands along an East-West Gradient in the Boreal Mixedwoods of Canada. Forests 2014, 5, 2905–2928. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, Y.; Leduc, A.; Harvey, B.; Gauthier, S. Natural fire regime: A guide for sustainable management of the Canadian boreal forest. Silva Fenn. 2002, 36, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, Y.; Harper, K.A. Old-Growth Forests in the Canadian Boreal: The Exception Rather than the Rule. In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin, Germany, 2009; pp. 285–300. ISBN 978-3-540-92705-1. [Google Scholar]
- Bergeron, Y.; Chen, H.Y.H.; Kenkel, N.C.; Leduc, A.L.; Macdonald, S.E. Boreal mixedwood stand dynamics: ecological processes underlying multiple pathways. For. Chron. 2014, 90, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Ilisson, T.; Chen, H.Y.H. The direct regeneration hypothesis in northern forests. J. Veg. Sci. 2009, 20, 735–744. [Google Scholar] [CrossRef]
- Kurkowski, T.A.; Mann, D.H.; Rupp, T.S.; Verbyla, D.L. Relative importance of different secondary successional pathways in an Alaskan boreal forest. Can. J. For. Res. 2008, 38, 1911–1923. [Google Scholar] [CrossRef] [Green Version]
- de Römer, A.H.; Kneeshaw, D.D.; Bergeron, Y. Small gap dynamics in the southern boreal forest of eastern Canada: Do canopy gaps influence stand development? J. Veg. Sci. 2007, 18, 815–826. [Google Scholar] [CrossRef]
- Kneeshaw, D.; Bergeron, Y.; Kuuluvainen, T. Forest ecosystem structure and disturbance dynamics across the circumboreal forest. In The SAGE Handbook of Biogeography; SAGE: Thousand Oaks, CA, USA, 2011; pp. 263–280. [Google Scholar]
- Viereck, L.A.; Wein, R.W.; Maclean, D.A. The effects of fire in black spruce ecosystems of Alaska and northern Canada. In The Role of Fire in Northern Circumpolar Ecosystems; Wein, R.W., Maclean, D.A., Eds.; John Wiley & Sons: New York, NY, USA, 1983; pp. 201–220. [Google Scholar]
- Taylor, A.R.; Chen, H.Y.H. Multiple successional pathways of boreal forest stands in central Canada. Ecography 2011, 34, 208–219. [Google Scholar] [CrossRef]
- Colford-Gilks, A.K.; MacLean, D.A.; Kershaw, J.A.; Béland, M. Growth and mortality of balsam fir- and spruce-tolerant hardwood stands as influenced by stand characteristics and spruce budworm defoliation. Forest Ecol. Manag. 2012, 280, 82–92. [Google Scholar] [CrossRef]
- Stewart, J.D.; Hogg, E.H.; Hurdle, P.A.; Stadt, K.J.; Tollestrup, P.; Lieffers, V.J. Dispersal of white spruce seed in mature aspen stands. Can. J. Bot. 1998, 76, 181–188. [Google Scholar] [CrossRef]
- Peters, V.S.; Macdonald, S.E.; Dale, M.R.T. The interaction between masting and fire is key to white spruce regeneration. Ecology 2005, 86, 1744–1750. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin III, F.S.; Foote, J.; Kemmett, S.; Price, K.; Viereck, L. Decadal observations of tree regeneration following fire in boreal forests. Can. J. For. Res. 2004, 34, 267–273. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Substrate and litterfall effects on conifer seedling survivorship in southern boreal stands of Canada. Can. J. For. Res. 2003, 33, 672–681. [Google Scholar] [CrossRef]
- Gärtner, S.M.; Lieffers, V.J.; Macdonald, S.E. Ecology and management of natural regeneration of white spruce in the boreal forest. Environ. Rev. 2011, 19, 461–478. [Google Scholar] [CrossRef]
- McCullough, D.G.; Werner, R.A.; Neumann, D. Fire and insects in northern and boreal forest ecosystems of North America. Ann. Rev. Entomol. 1998, 43, 107–127. [Google Scholar] [CrossRef] [Green Version]
- Man, R.; Rice, J.A. Response of aspen stands to forest tent caterpillar defoliation and subsequent overstory mortality in northeastern Ontario, Canada. Forest Ecol. Manag. 2010, 260, 1853–1860. [Google Scholar] [CrossRef]
- Moulinier, J.; Lorenzetti, F.; Bergeron, Y. Effects of a forest tent caterpillar outbreak on the dynamics of mixedwood boreal forests of eastern Canada. Écoscience 2013, 20, 182–193. [Google Scholar] [CrossRef]
- Baskerville, G.L. Spruce Budworm: Super Silviculturist. For. Chron. 1975, 51, 138–140. [Google Scholar] [CrossRef] [Green Version]
- Pham, A.T.; de Grandpré, L.; Gauthier, S.; Bergeron, Y. Gap dynamics and replacement patterns in gaps of the northeastern boreal forest of Quebec. Can. J. For. Res. 2004, 34, 353–364. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy Gap Characteristics and Tree Replacement in the Southeastern Boreal Forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Taylor, A.R. A test of ecological succession hypotheses using 55-year time-series data for 361 boreal forest stands. Glob. Ecol. Biogeogr. 2012, 21, 441–454. [Google Scholar] [CrossRef]
- Bretfeld, M.; Doerner, J.P.; Franklin, S.B. Radial growth response and vegetative sprouting of aspen following release from competition due to insect-induced conifer mortality. For. Ecol. Manag. 2015, 347, 96–106. [Google Scholar] [CrossRef]
- Kneeshaw, D.; Gauthier, S. Old growth in the boreal forest: A dynamic perspective at the stand and landscape level. Environ. Rev. 2003, 11, S99–S114. [Google Scholar] [CrossRef]
- Murphy, L.E. SORTIE-ND User Manual, Version 6.11.; Institute of Ecosystem Studies: Millbrook, NY, USA, 2011. [Google Scholar]
- Stadt, K.J.; Huston, C.; Coates, K.D.; Feng, Z.; Dale, M.R.T.; Lieffers, V.J. Evaluation of competition and light estimation indices for predicting diameter growth in mature boreal mixed forests. Ann. For. Sci. 2007, 64, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, H.C.; Vanderwel, M.C.; Fuller, M.M.; Thomas, S.C.; Caspersen, J.P. Modelling stand development after partial harvests: An empirically based, spatially explicit analysis for lowland black spruce. Ecol. Model. 2010, 221, 256–267. [Google Scholar] [CrossRef]
- Beaudet, M.; Harvey, B.D.; Messier, C.; Coates, K.D.; Poulin, J.; Kneeshaw, D.D.; Brais, S.; Bergeron, Y. Managing understory light conditions in boreal mixedwoods through variation in the intensity and spatial pattern of harvest: A modelling approach. For. Ecol. Manag. 2011, 261, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.K.; Harvey, B.D.; Coates, K.D.; Brais, S.; Bergeron, Y. Modelling stand development after partial harvesting in boreal mixedwoods of eastern Canada. Ecol. Model. 2015, 300, 123–136. [Google Scholar] [CrossRef]
- Canham, C.D.; Thompson, J.; Zimmerman, J.K.; Uriarte, M. Variation in Susceptibility to Hurricane Damage as a Function of Storm Intensity in Puerto Rican Tree Species. Biotropica 2010, 42, 87–94. [Google Scholar] [CrossRef]
- Canham, C.D.; Murphy, L. The demography of tree species response to climate: Seedling recruitment and survival. Ecosphere 2016, 7, e01424. [Google Scholar] [CrossRef]
- Evans, M.R.; Moustakas, A. A comparison between data requirements and availability for calibrating predictive ecological models for lowland UK woodlands: Learning new tricks from old trees. Ecol. Evol. 2016, 6, 4812–4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, B.S.; Buckley, H.L.; Barker-Plotkin, A.A.; Orwig, D.A.; Ellison, A.M. When a foundation crumbles: Forecasting forest dynamics following the decline of the foundation species Tsuga canadensis. Ecosphere 2017, 8, e01893. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, J.C.; Canham, C.D.; Barten, P.K. Predicting long-term forest development following hemlock mortality. In Proceedings of the Symposium on sustainable management of hemlock ecosystems in eastern North America; McManus, Katherine, A., Shields, Kathleen, S., Souto, Dennis, R., Eds.; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Delaware, OH, USA, 2000; pp. 62–75. [Google Scholar]
- Bergeron, Y.; Leduc, A.; Joyal, C.; Morin, H. Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can. J. For. Res. 1995, 25, 1375–1384. [Google Scholar] [CrossRef]
- Saucier, J.P.; Bergeron, J.F.; Grondin, P.; Robitaille, A. Les Régions Écologiquesdu Québec Méridional (3iéme Version): Un des Éléments du Systéme Hiérar-Chique de Classification Écologique du Territoire Mis au Point par le Ministére desRessources Naturelles du Québec, L’Aubelle, February–March 1998; Gouvernement du Québec, Ministère des Ressources naturelles: Québec, QC, Canada, 1998. [Google Scholar]
- Environment Canada. Canadian Climate Normals 1981–2010. Available online: http://www.climate.weather.gc.ca/climate_normals/index_e.html (accessed on 15 August 2017).
- Dansereau, P.-R.; Bergeron, Y. Fire history in the southern boreal forest of northwestern Quebec. Can. J. For. Res. 1993, 23, 25–32. [Google Scholar] [CrossRef]
- Bergeron, Y.; Gauthier, S.; Kafka, V.; Lefort, P.; Lesieur, D. Natural fire frequency for the eastern Canadian boreal forest: Consequences for sustainable forestry. Can. J. For. Res. 2001, 31, 384–391. [Google Scholar] [CrossRef]
- Morin, H.; Laprise, D.; Bergeron, Y. Chronology of spruce budworm outbreaks near Lake Duparquet, Abitibi region, Quebec. Can. J. For. Res. 1993, 23, 1497–1506. [Google Scholar] [CrossRef]
- Campbell, E.M.; MacLean, D.A.; Bergeron, Y. The Severity of Budworm-Caused Growth Reductions in Balsam Fir/Spruce Stands Varies with the Hardwood Content of Surrounding Forest Landscapes. For. Sci. 2008, 54, 195–205. [Google Scholar] [CrossRef]
- Cooke, B.J.; Lorenzetti, F. The dynamics of forest tent caterpillar outbreaks in Québec, Canada. For. Ecol. Manag. 2006, 226, 110–121. [Google Scholar] [CrossRef]
- Cooke, B.J.; Lorenzetti, F.; Roland, J. On the duration and distribution of forest tent caterpillar outbreaks in east-central Canada. J. Entomol. Soc. Ont. 2009, 140, 3–18. [Google Scholar]
- Gouvernement du Québec. Available online: https://mffp.gouv.qc.ca/publications/forets/fimaq/insectes/livree/Liv_2018_P.pdf (accessed on 5 December 2018).
- Walker, L.R.; Wardle, D.A.; Bardgett, R. d.; Clarkson, B. d. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Leduc, A.; Coates, K.D. Parameterization Changes to the lac Duparquet SORTIE-ND Model; A Report Carried Out as a Part of Master Thesis; Département dessciences biologiques, Université du Québec à Montréal: Montréal, QC, Canada, 2013. [Google Scholar]
- Poulin, J.; Messier, C. Rapport de Paramétrisation du Modèle de Simulationde la Dynamique Forestière SORTIE-ND Pour la Forêt Boréale et Sub-Boréale Del’ouest du Québec; 2008. Available online: http://www.cef-cfr.ca/uploads/CEF/parametrisation.pdf (accessed on 1 October 2008).
- MacLean, D.A.; Ostaff, D.P. Patterns of balsam fir mortality caused by an uncontrolled spruce budworm outbreak. Can. J. For. Res. 1989, 19, 1087–1095. [Google Scholar] [CrossRef]
- Blais, J.R. Mortality of balsam fir and white spruce following a spruce budworm outbreak in the Ottawa River watershed in Quebec. Can. J. For. Res. 1981, 11, 620–629. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 5 December 2018).
- Harvey, B.D.; Brais, S. Partial cutting as an analogue to stem exclusion and dieback in trembling aspen (Populus tremuloides ) dominated boreal mixedwoods: Implications for deadwood dynamicsThis article is one of a selection of papers published in the Special Forum IUFRO 1.05 Uneven-Aged Silvicultural Research Group Conference on Natural Disturbance-Based Silviculture: Managing for Complexity. Can. J. For. Res. 2007, 37, 1525–1533. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Flint, A.; Huang, C.-y.; Flint, L.; Berry, J.A.; Davis, F.W.; Sperry, J.S.; Field, C.B. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.J. Within-stand variation in windthrow in southern boreal forests of Minnesota: Is it predictable? Can. J. For. Res. 2004, 34, 365–375. [Google Scholar] [CrossRef]
- Bouchard, M.; Pothier, D.; Ruel, J.-C. Stand-replacing windthrow in the boreal forests of eastern Quebec. Can. J. For. Res. 2009, 39, 481–487. [Google Scholar] [CrossRef]
- Rowe, J.S. Critique of some vegetational concepts as applied to forests of northwestern Alberta. Can. J. Bot. 1961, 39, 1007–1017. [Google Scholar] [CrossRef]
- Caners, R.T.; Kenkel, N.C. Forest stand structure and dynamics at Riding Mountain National Park, Manitoba, Canada. Community Ecol. 2003, 4, 185–204. [Google Scholar] [CrossRef]
- Bergeron, Y.; Charron, D. Postfire stand dynamics in a southern boreal forest (Québec): A dendroecological approach. Ecoscience 1994, 1, 173–184. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Conifer seedling recruitment in a southeastern Canadian boreal forest: the importance of substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar] [CrossRef]
- Robert, E.; Brais, S.; Harvey, B.D.; Greene, D. Seedling establishment and survival on decaying logs in boreal mixedwood stands following a mast year 1 This article is one of a selection of papers from the International Symposium on Dynamics and Ecological Services of Deadwood in Forest Ecosystems. Can. J. For. Res. 2012, 42, 1446–1455. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Messier, C.; Burton, P.J.; Ruel, J.C.; Grover, B.E. Naturebased silviculture for sustaining a variety of boreal forest values (Chapter 13). In Towards Sustainable Management of the Boreal Forest; Burton, P.J., Messier, C., Smith, D.W., Adamowicz, W.L., Eds.; NRC Research Press: Ottawa, ON, Canada, 2003; pp. 480–530. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Palik, B.J.; Pregitzer, K.S. The relative influence of establishment time and height-growth rates on species vertical stratification during secondary forest succession. Can. J. For. Res. 1991, 21, 1481–1490. [Google Scholar] [CrossRef]
- Frelich, L.E.; Reich, P.B. Spatial Patterns and Succession in a Minnesota Southern-Boreal Forest. Ecol. Monogr. 1995, 65, 325–346. [Google Scholar] [CrossRef]
- Cumming, S.G.; Schmiegelow, F.K.A.; Burton, P.J. Gap dynamics in boreal aspen stands: Is the forest older than we think? Ecol. Appl. 2000, 10, 744–759. [Google Scholar] [CrossRef]
- Sims, R.A.; Kershaw, H.M.; Wickware, G.M. The Autecology of Major Tree Species in the North Central Region of Ontario. COFRDA Report 3302; Canada-Ontario Forest Resource Development Agreement; Canadian Forestry Service: Sault-Ste-Marie, ON, Canada, 1990. [Google Scholar]
- Brassard, B.W.; Chen, H.Y.H. Stand Structural Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 115–137. [Google Scholar] [CrossRef]
- Zasada, J.; Sharik, T.L.; Nygren, M. The reproductive process in boreal forest trees. In A Systems Analysis of the Boreal Forest; Shugart, H.H., Leemans, R., Bonan, G.B., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 85–125. [Google Scholar]
- Scott, M.L.; Murphy, P.G. Regeneration Patterns of Northern White Cedar, an Old-Growth Forest Dominant. Am. Midl. Nat. 1987, 117, 10. [Google Scholar] [CrossRef]
- Grandpré, L.; Gagnon, D.; Bergeron, Y. Changes in the understory of Canadian southern boreal forest after fire. J. Veg. Sci. 1993, 4, 803–810. [Google Scholar] [CrossRef]
- Grandpré, L.; Morissette, J.; Gauthier, S. Long-term post-fire changes in the northeastern boreal forest of Quebec. J. Veg. Sci. 2000, 11, 791–800. [Google Scholar] [CrossRef]
- Archambault, S.; Bergeron, Y. An 802-year tree-ring chronology from the Quebec boreal forest. Can. J. For. Res. 1992, 22, 674–682. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Sylvics of North America; Department of Agriculture: Washington, DC, USA, 1990. [Google Scholar]
- Lavertu, D.; Mauffette, Y.; Bergeron, Y. Suckering success of aspen (Populus tremuloides Michx.) in relation to stand age and soil disturbance. J. Veg. Sci. 1994, 5, 561–568. [Google Scholar] [CrossRef]
- Loehle, C. Strategy Space and the Disturbance Spectrum: A Life-History Model for Tree Species Coexistence. Am. Nat. 2000, 156, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.F.; Zasada, J.C.; Sirois, L.; Kneeshaw, D.; Morin, H.; Charron, I.; Simard, M.-J. A review of the regeneration dynamics of North American boreal forest tree species. Can. J. For. Res. 1999, 29, 824–839. [Google Scholar] [CrossRef]
- Greene, D.F.; Johnson, E.A. Tree recruitment from burn edges. Can. J. For. Res. 2000, 30, 1264–1274. [Google Scholar] [CrossRef]
- D’Aoust, V.; Kneeshaw, D.; Bergeron, Y. Characterization of canopy openness before and after a spruce budworm outbreak in the southern boreal forest. Can. J. For. Res. 2004, 34, 339–352. [Google Scholar] [CrossRef]
- Bouchard, M.; Kneeshaw, D.; Bergeron, Y. Mortality and stand renewal patterns following the last spruce budworm outbreak in mixed forests of western Quebec. For. Ecol. Manag. 2005, 204, 297–313. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Neau, M.; Marchand, M.; de Grandpré, L.; Kneeshaw, D. Phenological synchrony between eastern spruce budworm and its host trees increases with warmer temperatures in the boreal forest. Ecol. Evol. 2019, 9, 576–586. [Google Scholar] [CrossRef]
- MacLean, D.A.; Andersen, A.R. Impact of a spruce budworm outbreak in balsam fir and subsequent stand development over a 40-year period. For. Chron. 2008, 84, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Ghent, A.W.; Fraser, D.A.; Thomas, J.B. Studies of regeneration in forest stands devastated by the spruce budworm. For. Sci. 1957, 3, 184–208. [Google Scholar]
- Nyland, R.D. Silviculture: Concepts and Applications, 2nd ed.; Reissued; Waveland Press: Long Grove, IL, USA, 2007; ISBN 1577665279. [Google Scholar]
- Morin, H. Dynamics of balsam fir forests in relation to spruce budworm outbreaks in the Boreal Zone of Quebec. Can. J. For. Res. 1994, 24, 730–741. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Ecological factors affecting the abondance of advance regeneration in Quebec’s southwestern boreal forest. Can. J. For. Res. 1996, 26, 888–898. [Google Scholar] [CrossRef]
- Bergeron, Y.; Dubuc, M. Succession in the southern part of the Canadian boreal forest. Vegetatio 1989, 79, 51–63. [Google Scholar] [CrossRef]
- Zoladeski, C.A.; Maycock, P.F. Dynamics of the Boreal Forest in Northwestern Ontario. Am. Midl. Nat. 1990, 124, 289. [Google Scholar] [CrossRef]
- Landhausser, S.M.; Mulak, T.L.; Lieffers, V.J. The effect of roots and litter of Calamagrostis canadensis on root sucker regeneration of Populus tremuloides. Forestry 2007, 80, 481–488. [Google Scholar] [CrossRef]
- Wright, E.F.; Coates, K.D.; Bartemucci, P. Regeneration from seed of six tree species in the interior cedar-hemlock forests of British Columbia as affected by substrate and canopy gap position. Can. J. For. Res. 1998, 28, 1352–1364. [Google Scholar] [CrossRef]
- Coates, K.D.; Canham, C.D.; LePage, P.T. Above- versus below-ground competitive effects and responses of a guild of temperate tree species. J. Ecol. 2009, 97, 118–130. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A.; Kobe, R.K.; Ribbens, E. Forest Models Defined by Field Measurements: Estimation, Error Analysis and Dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Pothier, D.; Savard, F. Actualisation des Tables de Production Pour les Principales Espèces Forestières du Québec; Gouvernement du Québec, Ministère des Ressources naturelles: Québec, QC, Canada, 1998; Publication No. RN98–3054. [Google Scholar]
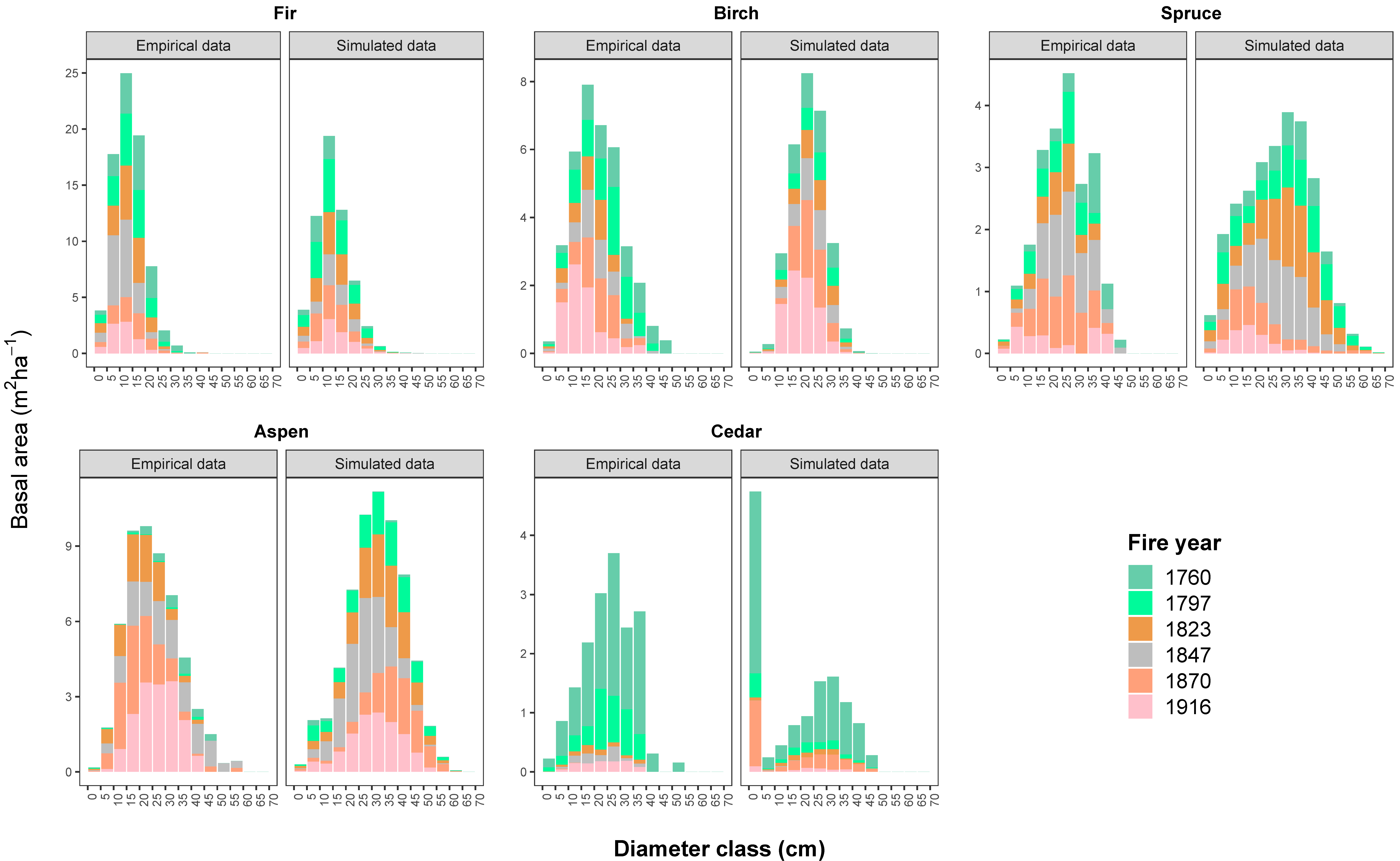

| Fire Date | Fire Area * (ha) | Stand Age (year) | Number of Transects | Plots Count | Mean Basal Area (m2ha−1, ±SE) of Standing Live Trees (DBH > 1 cm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fir | Birch | Spruce | Aspen | Cedar | |||||
| 1760 | >7760 | 231 | 6 | 54 | 2.60 (0.55) | 6.69 (0.86) | 2.04 (0.40) | 2.44 (0.84) | 10.89 (1.46) |
| 1797 | 178 | 194 | 4 | 50 | 2.77 (0.42) | 7.92 (0.83) | 2.08 (0.39) | 0.50 (0.20) | 3.84 (0.62) |
| 1823 | 288 | 168 | 5 | 66 | 4.32 (0.47) | 3.88 (0.37) | 2.46 (0.38) | 8.03 (1.03) | 0.53 (0.26) |
| 1847 | 122 | 144 | 4 | 74 | 6.84 (0.50) | 4.62 (0.45) | 5.40 (0.58) | 11.88 (1.00) | 0.77 (0.29) |
| 1870 | 555 | 121 | 6 | 64 | 3.46 (0.36) | 5.86 0.60) | 4.46 (0.56) | 12.78 (1.51) | 0.02 (0.02) |
| 1916 | 35 | 75 | 3 | 52 | 4.54 (0.40) | 7.63 (0.83) | 1.99 (0.57) | 16.74 (2.01) | 0.97 (0.36) |
| 1944 | >298 | 47 | 4 | 71 | 3.81 (0.48) | 9.34 (0.76) | 0.81 (0.19) | 11.57 (1.72) | 0.22 (0.14) |
| Source of Empirical Data Used for Setting Initial Conditions | Number of Initial Conditions * | Simulation Period | Purpose of Simulation |
|---|---|---|---|
| Short-Term Simulations | |||
| Empirical data in 1991 following spruce budworm outbreak | 431 | 18 years | Model parameterization to reproduce empirical data in 2009. |
| Mid-Term Simulations | |||
| Empirical data in 1991 following spruce budworm outbreak | 431 | 28 years | (1) Evaluating the mid-term effect of the recent budworm outbreak on stand dynamics. (2) Estimating stand composition and species proportion to define mortality rate of spruce budworm event for the long-term simulations. |
| Empirical data in 1991 prior to spruce budworm outbreak | 431 | 28 years | |
| Long-Term Simulations | |||
| Empirical data in 1991 following spruce budworm outbreak | 431 | 60 years | (1) Evaluating the long-term performance of the model. (2) Evaluating the long-term effect of the recent budworm outbreak on stand dynamics. |
| Empirical data in 1991 prior to spruce budworm outbreak | 431 | 60 years | |
| Fire Date | Balsam Fir | White Spruce | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Basal Area | Number | Basal Area | |||||||||
| Prior | Post | Mort | Prior | Post | Mort | Prior | Post | Mort | Prior | Post | Mort | |
| 1760 | 1560 (179) | 489 (109) | 68.65 | 15.16 (0.88) | 2.60 (0.55) | 82.85 | 67 (11) | 43 (8) | 35.82 | 2.77 (0.50) | 2.04 (0.40) | 26.35 |
| 1797 | 2303 (221) | 1117 (192) | 51.50 | 14.55 (1.04) | 2.77 (0.42) | 80.96 | 159 (24) | 118 (20) | 25.79 | 3.03 (0.42) | 2.08 (0.39) | 31.35 |
| 1823 | 2434 (211) | 1485 (206) | 38.99 | 13.87 (0.70) | 4.32 (0.47) | 68.85 | 209 (38) | 188 (36) | 10.05 | 2.90 (0.41) | 2.46 (0.38) | 15.17 |
| 1847 | 3289 (231) | 1523 (174) | 53.69 | 17.45 (1.06) | 6.84 (0.50) | 60.80 | 159 (16) | 132 (14) | 16.98 | 6.06 (0.65) | 5.40 (0.58) | 10.89 |
| 1870 | 1311 (132) | 801 (95) | 38.90 | 7.94 (0.61) | 3.46 (0.36) | 56.42 | 242 (27) | 218 (25) | 9.92 | 5.02 (0.61) | 4.46 (0.56) | 11.16 |
| 1916 | 1757 (144) | 1319 (138) | 24.93 | 7.72 (0.56) | 4.54 (0.40) | 41.19 | 254 (40) | 246 (40) | 3.15 | 2.04 (0.58) | 1.99 (0.57) | 2.45 |
| 1944 | 1272 (198) | 1096 (174) | 13.84 | 4.69 (0.66) | 3.81 (0.48) | 18.76 | 179 (50) | 167 (47) | 6.70 | 0.95 (0.23) | 0.81 (0.19) | 14.74 |
| Fire Date | Species Mean Basal Area for Standing Live Sapling and Adult Trees | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fir | Birch | Spruce | Aspen | Cedar | ||||||
| Emp. | Sim. | Emp. | Sim. | Emp. | Sim. | Emp. | Sim. | Emp. | Sim. | |
| 1760 | 6.49 (0.81) | 5.61 (0.88) | 4.88 (0.67) | 4.03 (0.68) | 3.28 (0.61) | 2.37 (0.43) | 3.45 (1.21) | 2.21 (0.68) | 15.73 (1.89) | 13.80 (1.77) |
| 1797 | 10.38 (0.79) | 8.43 (0.86) | 7.40 (0.79) | 5.88 (0.54) | 4.53 (0.78) | 3.04 (0.52) | 0.73 (0.34) | 0.67 (0.24) | 5.11 (0.93) | 5.09 (0.81) |
| 1823 | 11.93 (0.74) | 10.54 (0.84) | 3.07 (0.34) | 3.63 (0.35) | 4.35 (0.60) | 3.84 (0.50) | 9.99 (1.30) | 10.74 (1.25) | 0.86 (0.40) | 0.71 (0.35) |
| 1847 | 11.36 (0.60) | 11.97 (0.58) | 2.58 (0.32) | 3.88 (0.35) | 6.47 (0.73) | 6.85 (0.67) | 13.67 (1.15) | 11.56 (0.80) | 0.63 (0.22) | 0.85 (0.32) |
| 1870 | 6.69 (0.64) | 6.42 (0.62) | 3.72 (0.53) | 4.33 (0.46) | 5.52 (0.74) | 6.10 (0.71) | 11.56 (1.33) | 15.33 (1.72) | 0.04 (0.04) | 0.03 (0.03) |
| 1916 | 9.13 (0.64) | 7.98 (0.59) | 5.51 (0.64) | 6.52 (0.72) | 3.33 (0.56) | 2.56 (0.59) | 17.80 (2.28) | 16.20 (1.86) | 1.37 (0.77) | 1.22 (0.46) |
| 1944 | 6.60 (0.62) | 6.26 (0.71) | 8.04 (0.78) | 8.01 (0.68) | 1.71 (0.36) | 1.28 (0.31) | 11.50 (1.66) | 12.20 (1.78) | 0.33 (0.25) | 0.27 (0.17) |
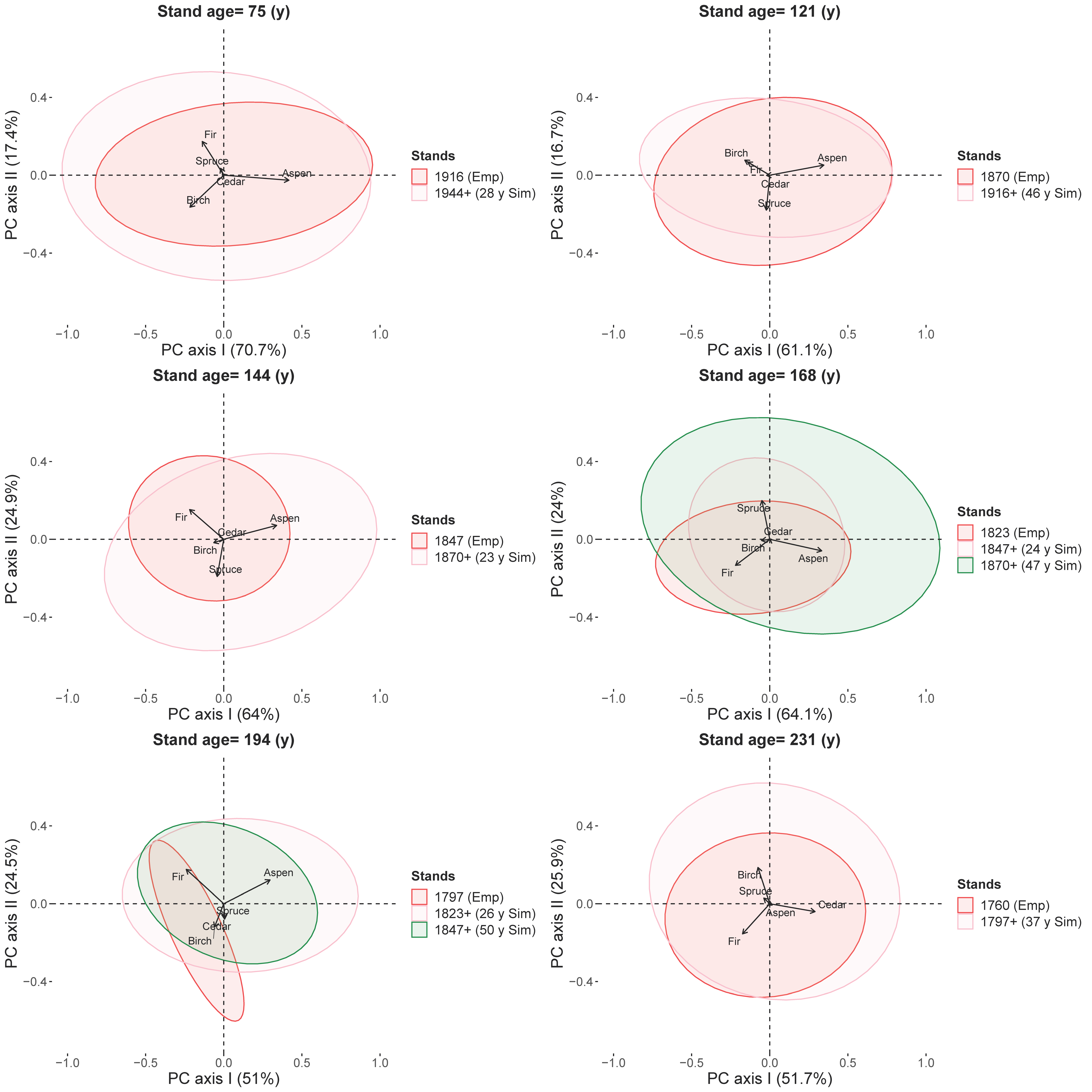
| Empirical Data Fire (Stand Age) | Simulation Data | |
|---|---|---|
| Fire Year + Length of Simulation (years) | ||
| 1760 (231 y) | 1797 + 37 (Total−) | |
| Fir− Birch Spruce Aspen Cedar | ||
| 1797 (194 y) | 1823 + 26 | 1847 + 50 (Total) |
| Fir Birch− Spruce+ Aspen+ Cedar− | Fir Birch− Spruce+ Aspen+ Cedar− | |
| 1823 (168 y) | 1847 + 24 (Total+) | 1870 + 47 (Total) |
| Fir Birch Spruce+ Aspen+ Cedar | Fir−Birch−Spruce+Aspen+ Cedar | |
| 1847 (144 y) | 1870 + 23 (Total) | |
| Fir− Birch Spruce Aspen Cedar− | ||
| 1870 (121 y) | 1916 + 46 (Total) | |
| Fir+ Birch Spruce− Aspen Cedar+ | ||
| 1916 (75 y) | 1944 + 28 (Total) | |
| Fir Birch Spruce Aspen Cedar | ||
| Fire Date | Simulation Length (years) | Species Mean Basal Area for Standing Live Sapling and Adult Trees | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fir | Birch | Spruce | Aspen | Cedar | |||||||
| No-SBW | SBW | No-SBW | SBW | No-SBW | SBW | No-SBW | SBW | No-SBW | SBW | ||
| 1760 | 28 | 23.69 (0.96) | 7.98 (1.09) | 2.92 (0.61) | 3.64 (0.65) | 3.36 (0.56) | 2.68 (0.48) | 1.57 (0.52) | 1.94 (0.58) | 15.06 (1.91) | 16.87 (2.07) |
| 60 | 32.97 (1.26) | 9.25 (1.06) | 1.26 (0.4) | 2.92 (0.56) | 3.54 (0.66) | 3.93 (0.78) | 0.53 (0.17) | 1.26 (0.35) | 19.46 (2.22) | 25.88 (2.63) | |
| 1797 | 28 | 25.73 (1.01) | 12.65 (1.15) | 4.52 (0.52) | 5.26 (0.53) | 4.89 (0.65) | 3.72 (0.61) | 0.56 (0.20) | 0.70 (0.26) | 5.71 (0.92) | 6.52 (1.02) |
| 60 | 35.74 (1.05) | 15.02 (1.24) | 1.93 (0.39) | 3.53 (0.55) | 6.7 (0.89) | 5.57 (0.93) | 0.49 (0.2) | 0.82 (0.37) | 8.24 (1.27) | 12.04 (1.74) | |
| 1823 | 28 | 23.85 (0.96) | 14.77 (1.04) | 3.16 (0.32) | 3.35 (0.33) | 5.18 (0.60) | 4.73 (0.57) | 10.71 (1.31) | 11.35 (1.29) | 0.80 (0.39) | 0.88 (0.42) |
| 60 | 32.68 (1.18) | 18.72 (1.09) | 1.59 (0.26) | 1.91 (0.28) | 7.17 (0.80) | 6.07 (0.68) | 7.40 (1.06) | 8.76 (1.03) | 1.13 (0.52) | 1.52 (0.66) | |
| 1847 | 28 | 23.60 (0.73) | 15.56 (0.68) | 3.13 (0.29) | 3.47 (0.32) | 8.47 (0.78) | 7.71 (0.72) | 10.76 (0.86) | 11.14 (0.84) | 0.90 (0.33) | 0.97 (0.35) |
| 60 | 31.79 (1.03) | 20.46 (0.86) | 1.32 (0.17) | 1.82 (0.27) | 9.31 (0.85) | 7.24 (0.66) | 6.25 (0.71) | 7.33 (0.7) | 1.28 (0.46) | 1.72 (0.59) | |
| 1870 | 28 | 14.11 (0.95) | 8.68 (0.83) | 3.58 (0.39) | 3.72 (0.40) | 7.71 (0.86) | 7.20 (0.83) | 15.72 (1.76) | 15.80 (1.74) | 0.03 (0.03) | 0.03 (0.03) |
| 60 | 23.29 (1.32) | 12.68 (1.13) | 1.60 (0.2) | 1.73 (0.23) | 11.3 (1.15) | 9.05 (1.01) | 11.41 (1.32) | 12.41 (1.36) | 0.04 (0.04) | 0.05 (0.05) | |
| 1916 | 28 | 14.29 (0.85) | 10.70 (0.72) | 6.42 (0.71) | 6.47 (0.71) | 3.12 (0.63) | 3.09 (0.63) | 14.67 (1.77) | 14.73 (1.76) | 1.43 (0.54) | 1.60 (0.61) |
| 60 | 24.13 (1.09) | 15.04 (0.91) | 4.90 (0.57) | 5.12 (0.58) | 5.67 (0.84) | 4.62 (0.74) | 6.99 (1.15) | 7.40 (1.15) | 2.33 (0.82) | 3.01 (1.07) | |
| 1944 | 28 | 9.08 (1.02) | 8.22 (0.88) | 7.86 (0.64) | 7.93 (0.65) | 1.86 (0.45) | 1.65 (0.41) | 12.11 (1.73) | 12.23 (1.73) | 0.34 (0.22) | 0.34 (0.22) |
| 60 | 16.69 (1.50) | 11.75 (1.17) | 6.19 (0.54) | 6.40 (0.55) | 3.47 (0.79) | 2.49 (0.61) | 8.26 (1.27) | 8.64 (1.28) | 0.57 (0.36) | 0.67 (0.41) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleki, K.; Gueye, M.A.; Lafleur, B.; Leduc, A.; Bergeron, Y. Modelling Post-Disturbance Successional Dynamics of the Canadian Boreal Mixedwoods. Forests 2020, 11, 3. https://doi.org/10.3390/f11010003
Maleki K, Gueye MA, Lafleur B, Leduc A, Bergeron Y. Modelling Post-Disturbance Successional Dynamics of the Canadian Boreal Mixedwoods. Forests. 2020; 11(1):3. https://doi.org/10.3390/f11010003
Chicago/Turabian StyleMaleki, Kobra, Mohamadou Alpha Gueye, Benoit Lafleur, Alain Leduc, and Yves Bergeron. 2020. "Modelling Post-Disturbance Successional Dynamics of the Canadian Boreal Mixedwoods" Forests 11, no. 1: 3. https://doi.org/10.3390/f11010003





