Allelopathic Effects of Cinnamomum migao on Seed Germination and Seedling Growth of its Associated Species Liquidambar formosana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
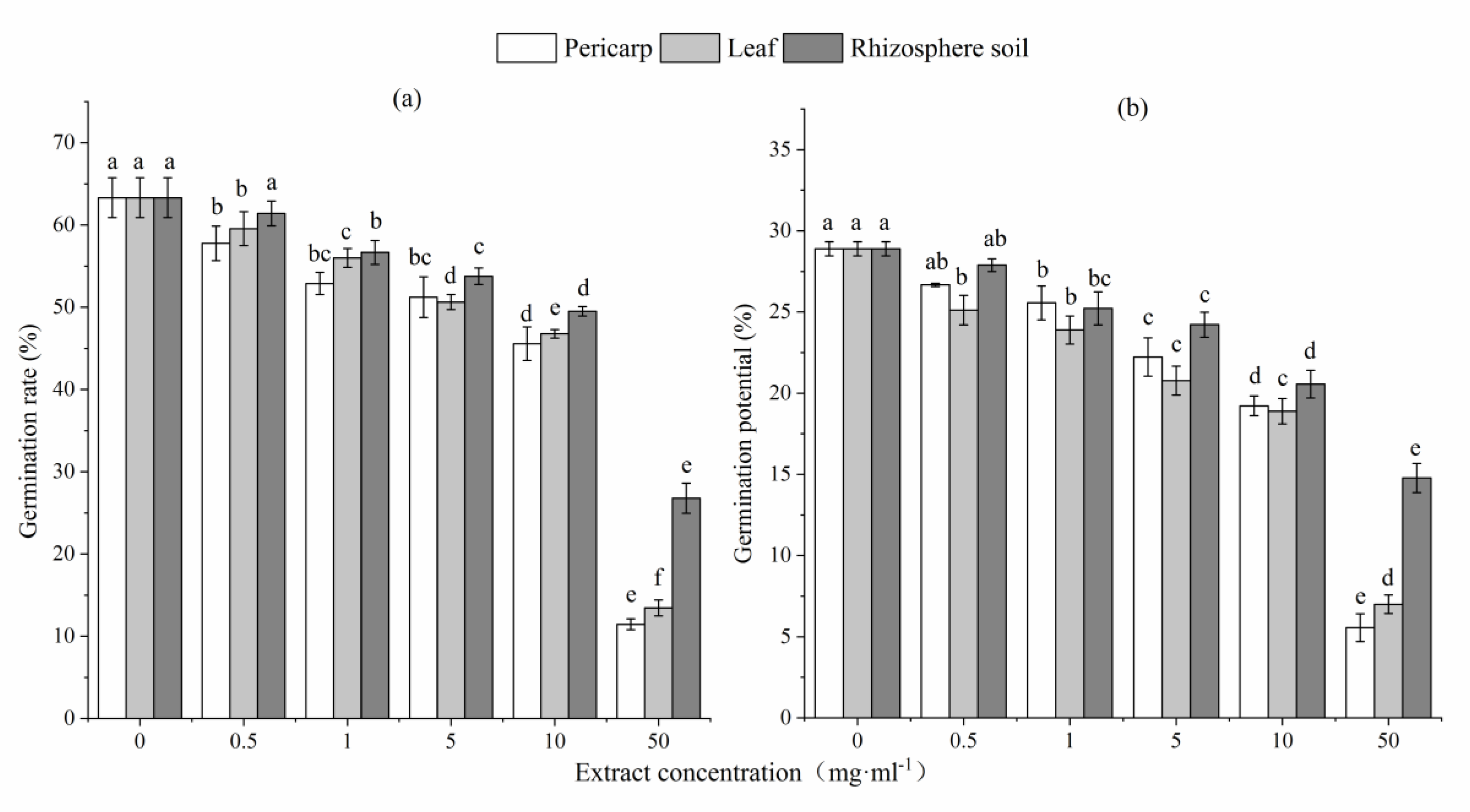
3.1. Effects of Aqueous Extracts of C. migao on L. formosana Seed Germination
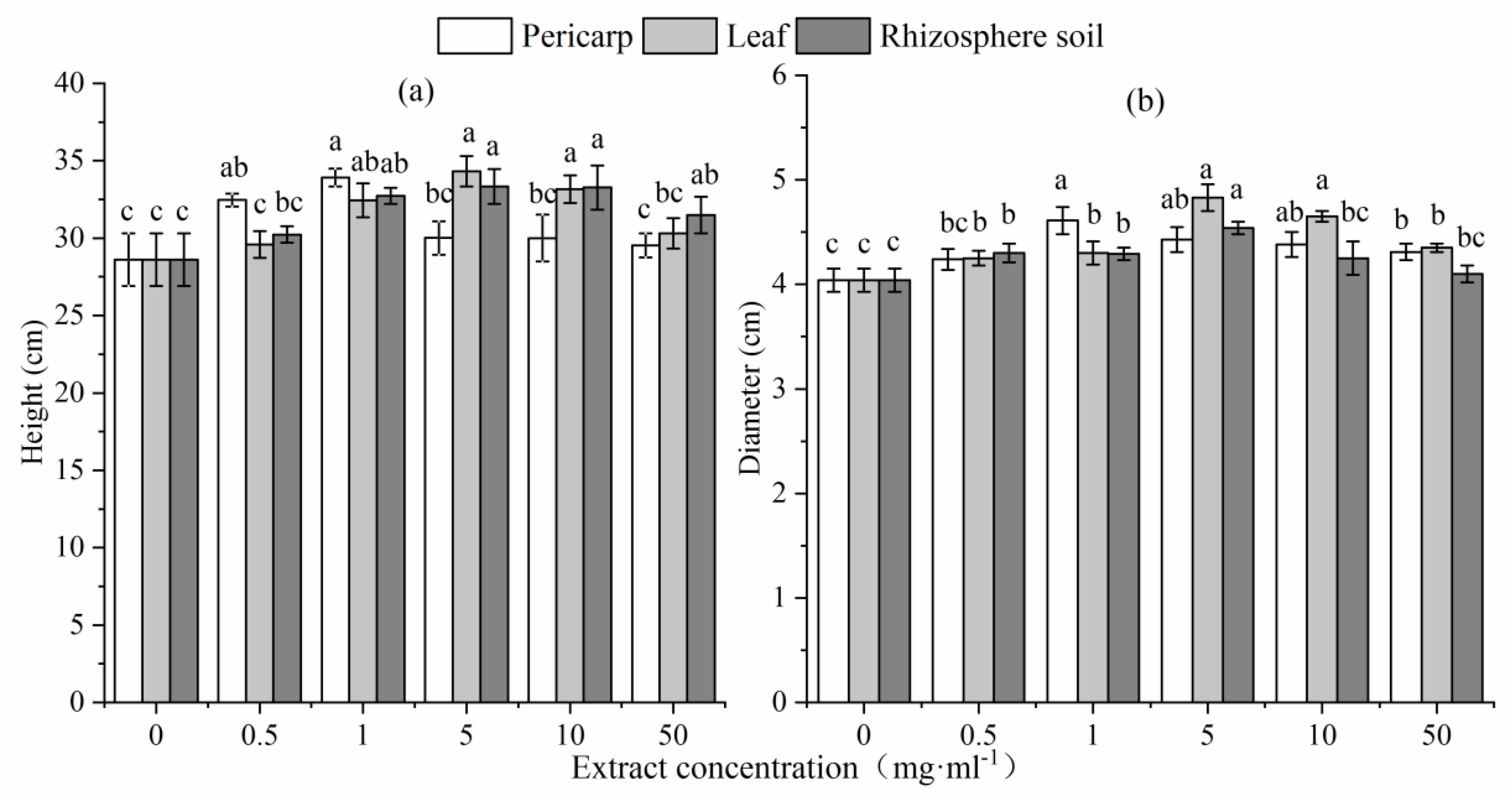
3.2. Effects of Aqueous Extracts of C. migao on the Morphology of L. formosana Seedlings
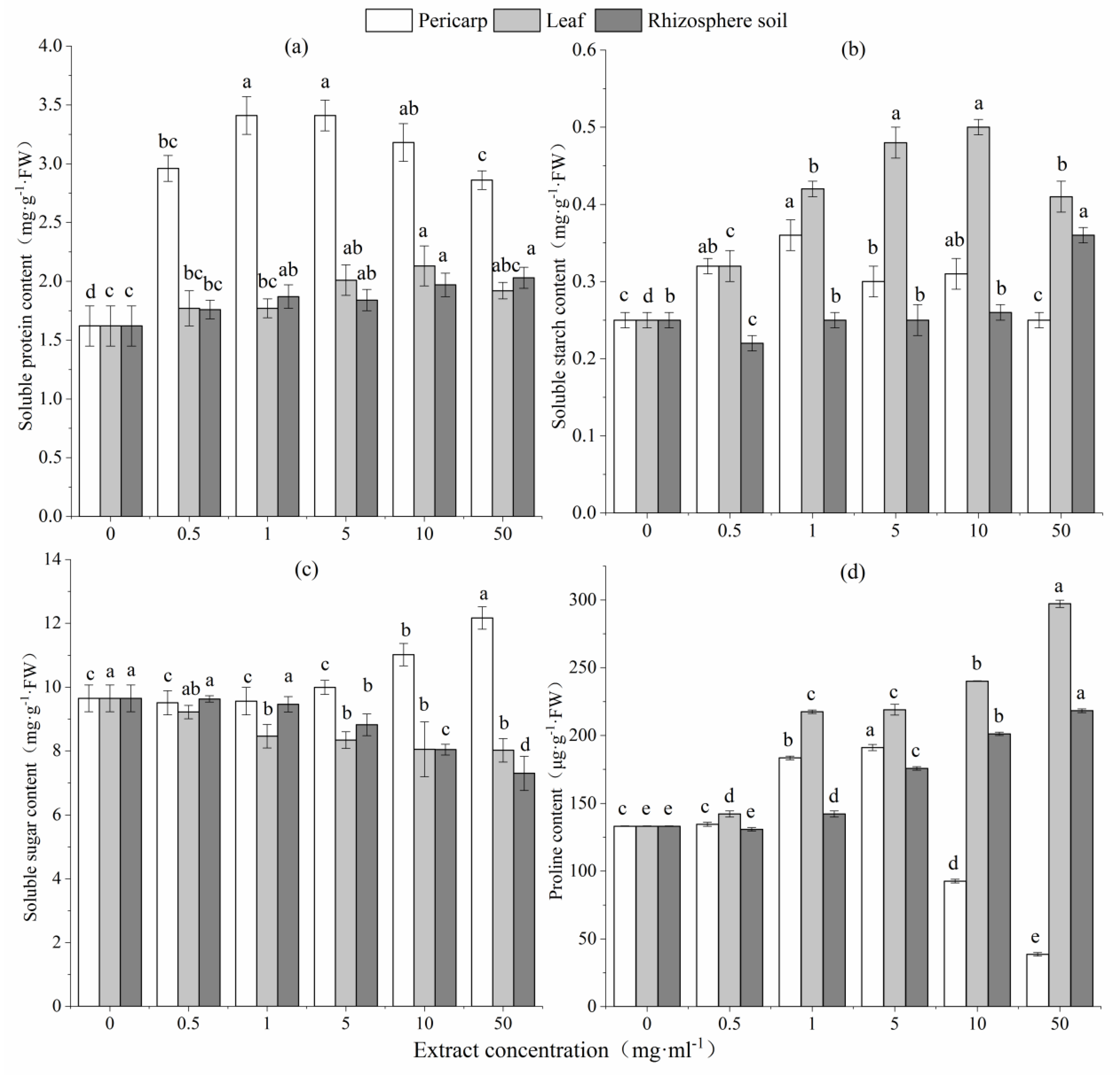
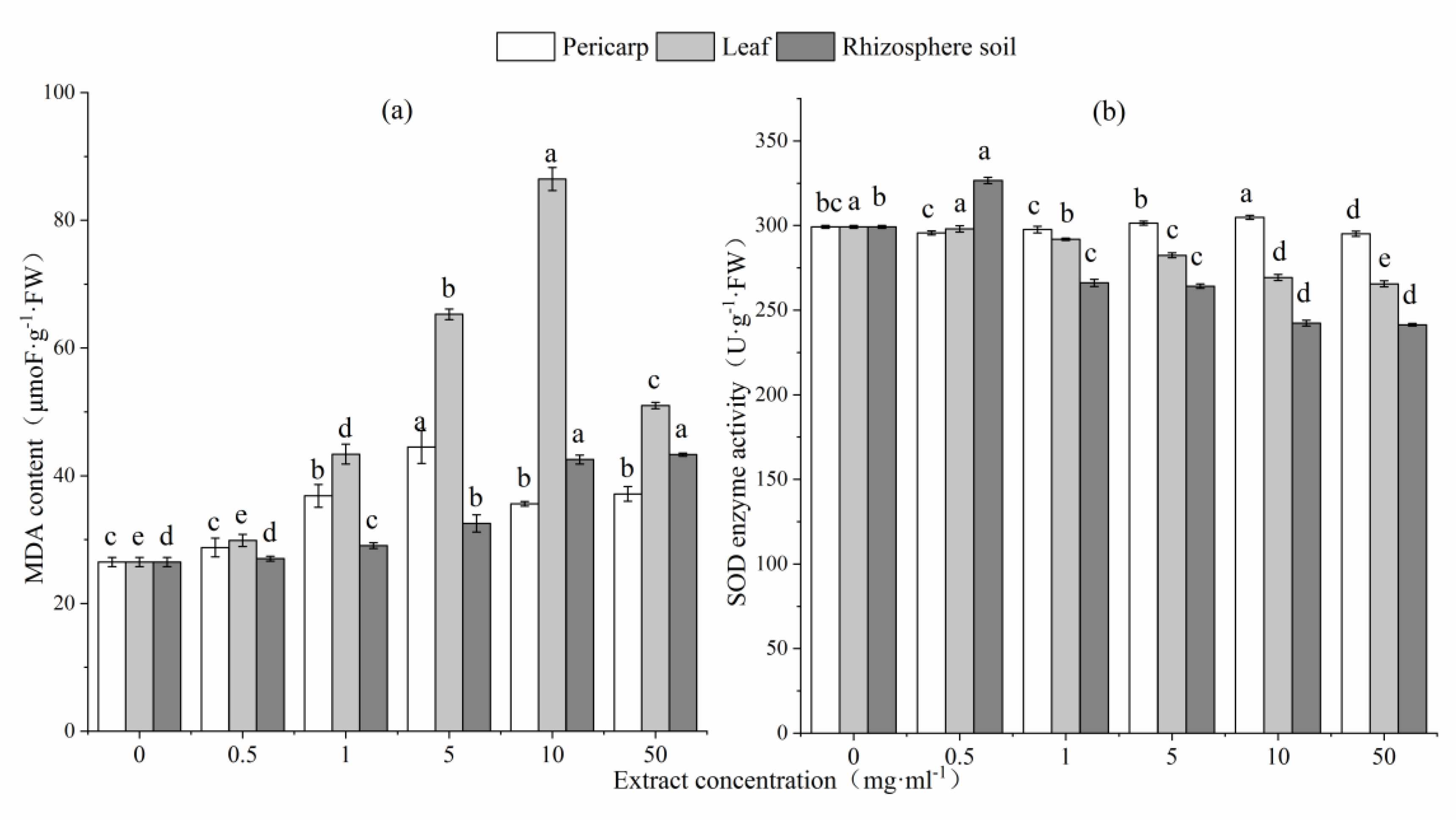
3.3. Effects of Aqueous Extracts C. Migao on Physiological and Biochemical Indices of L. formosana Seedlings
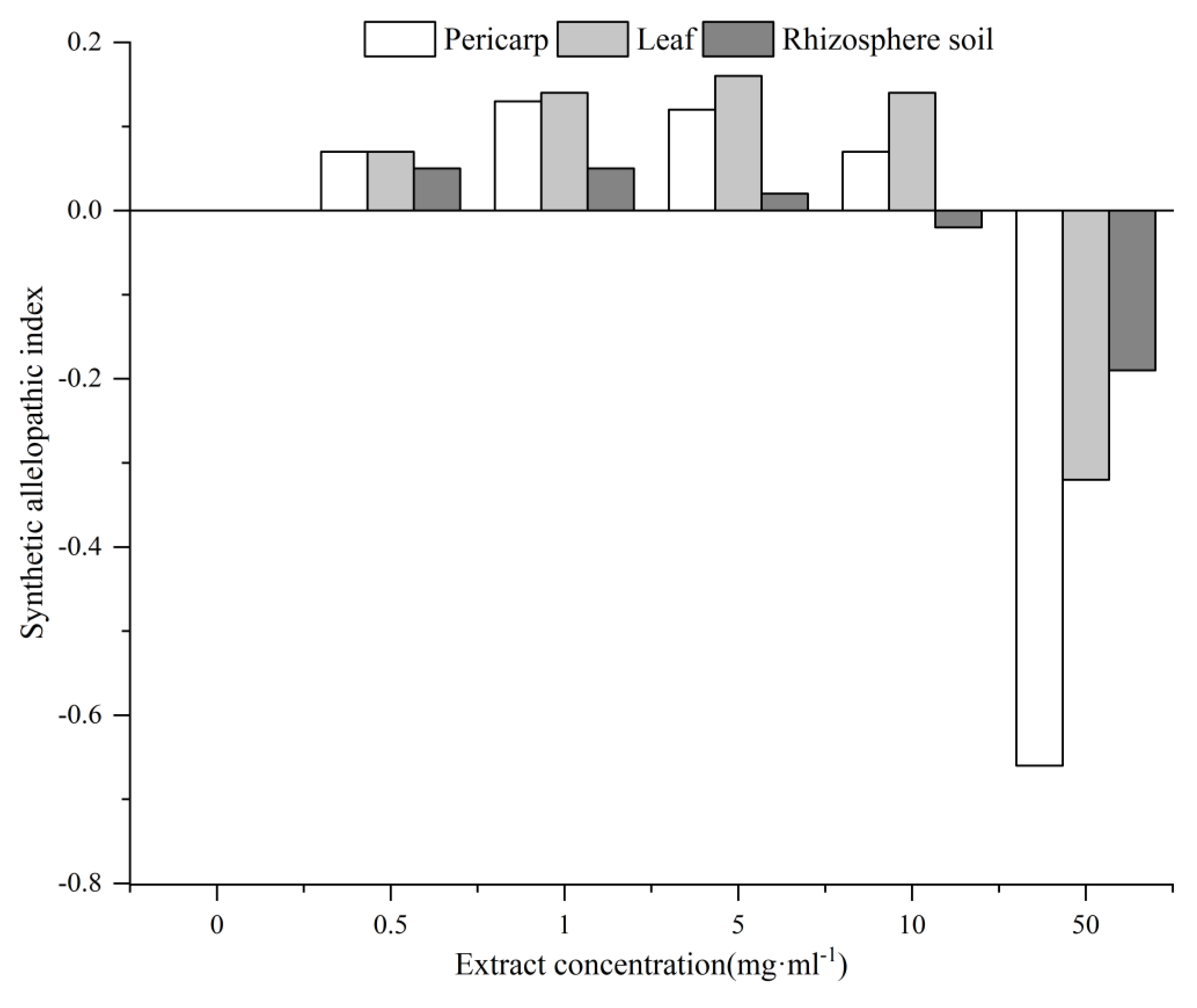
3.4. Allelopathic Effects of Aqueous Extracts of C. migao on L. formosana
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Einhellig, F.A. Allelopathy: Current status and future goals. In Allelopathy: Organisms, Processes, and Applications/Inderjit; Dakshini, K.M.M., Einhellig, F.A., Eds.; Acs. Sym. Ser. American Chemical Society: Washington, DC, USA, 1995; pp. 1–25. [Google Scholar]
- Das, R.; Geethangili, M.; Majhi, A.; Das, B.; Rao, Y.K.; Tzeng, Y.M. A new highly oxygenated pseudoguaianolide from a collection of the flowers of Parthenium hysterophorus. Chem. Pharm. Bull. 2005, 53, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: Amechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.J.; Kong, C.-H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Yu, L.Z.; Yu, L.F.; Li, K.Z.; Callaway, R.M.; Valiente-Banuet, A.; Du, Q.L. Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol. 2015, 205, 1350–1359. [Google Scholar]
- Fernandez, C.; Monnier, Y.; Santonja, M.; Gallet, C.; Weston, L.A.; Bernard, P. The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Weiner, J. Plant allelochemical interference or soil chemical ecology? Perspect. Plant Ecol. 2001, 4, 3–12. [Google Scholar] [Green Version]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Sci. 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Farooq, M.; Wahid, A. Role of allelopathy during the invasion process by alien invasive plants in terrestrial ecosystems. In Allelopathy; Lorenzo, P., Hussain, M.I., Luís, G., Lorenzo, P., Hussain, M.I., González, Á.L., Eds.; Springer: Berlin, Germany, 2013; pp. 3–21. [Google Scholar]
- Nickerson, K.; Flory, S.L. Competitive and allelopathic effects of the invasive shrub Schinus terebinthifolius (Brazilian peppertree). Biol. Invasions. 2015, 17, 555–564. [Google Scholar] [CrossRef]
- Zhiqun, T.; Jian, Z.; Junli, Y.; Chunzi, W.; Danju, Z. Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere 2017, 173, 307–317. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increase antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yin, W.J.; Li, J.; Du, J.F.; Yang, Y.H.; Chen, X.J.; Lin, W.X. Physio-ecological properties of continuous cropping Rehmannia glutinosa. Chin. J. Plant Ecol. 2010, 34, 547–554. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.; Day, A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox. Sign. 2011, 15, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Kreslavskll, V.D.; Los, D.A.; Allakhverdiev, S.I.; Kuznetsov, V.V. Signaling role of reactive oxygen species in plants under stress. Russ. J. Plant. Physl. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Gimingham, C.H. Calluna and its associated species: Some aspects of co-existence in communities. Veg. 1978, 6, 179–186. [Google Scholar] [CrossRef]
- Longpré, M.H.; Bergeron, Y.; Paré, D.; Béland, M. Effect of companion species on the growth of jack pine (Pinus banksiana). Can. J. Forest. Res. 1994, 24, 1846–1853. [Google Scholar] [CrossRef]
- Legare, S.; Bergeron, Y.; Pare, D. Effect of aspen (Populus tremuloides) as a companion species on the growth of black spruce (Picea mariana) in the southwestern boreal forest of Quebec. For. Eco. Manag. 2005, 208, 211–222. [Google Scholar] [CrossRef]
- Li, L.X. Study on Cinnamomum migao Population Characteristics in Guizhou; Guizhou University: Guiyang, China, 2016. [Google Scholar]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonenei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Zhao, L.C. Study on the Chemieal Constituents of Cinnamomum migao; Guizhou University of Traditional Chinese Medicine: Guiyang, China, 2007. [Google Scholar]
- Wang, R.L.; Peng, S.L.; Zeng, R.S.; Ding, L.W.; Xu, Z.F. Cloning, expression and wounding induction of βcaryophyllene synthase gene from Mikania fnicrantha H.B.K. and allelopathic potential of β-caryophyllene. Allelopath. J. 2009, 24, 35–44. [Google Scholar]
- Chi, X.J.; Liu, M.; Ou, G.T.; Wen, A.H.; Liao, X.F. Allelopathy of aqueous extracts from Cinnamomum migao H.W.Li on Blumea balsamifera L.DC. Guangdong Agric. Sci. 2014, 41, 21–24. [Google Scholar]
- Li, H.S. Experimental Principles and Techniques of Plant. Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Zou, Q. Instruction for Plant Physiology Experiments; China Agricultural Publishing House: Beijing, China, 2000. [Google Scholar]
- Lu, R.K. Soil Argrochemistry Analysis Protocoes; China Agriculture Sc.: Beijing, China, 1999. [Google Scholar]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Abugho, S.B. Interaction of rice residue and PRE herbicides on emergence and biomass of four weed species. Weed Technol. 2012, 26, 627–632. [Google Scholar] [CrossRef]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; Gonzalez, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 445–453. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, L.; Long, F.L.; Liu, Y.; Zhao, D.H.; Li, L. Allelopathic effects of water extractions from two Veronica species on 6 kinds of receiving crops. J. Northwest A.F. Univ. 2013, 41, 178–190. [Google Scholar]
- Chon, S.U.; Choi, S.K.; Jung, S.; Jang, H.G.; Pyo, B.S.; Kim, S.M. Effects of Alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of Alfalfa and Barnyard grass. Crop. Prot. 2002, 21, 1077–1082. [Google Scholar] [CrossRef]
- Kaushal, R.; Verma, K.S.; Singh, K.N. Effects of Grewia optiva and Populus deltoids leachates on field crops. Allelopath. J. 2003, 11, 229–234. [Google Scholar]
- Dai, Z.C.; Wang, X.Y.; Qi, S.S.; Du, D.L. Effects of leaf litter on inter-specific competitive ability of the invasive plant Wedelia trilobata. Ecol. Res. 2016, 31, 367–374. [Google Scholar] [CrossRef]
- Blum, K. Effects of ferulie acid and allelopathic compound on net P, K, and water uptake by cumuber seedlings in a split--root system. J. Chem. Ecol. 1990, 16, 455–463. [Google Scholar]
- Hossain, M.K.; Dhali, M.A.H.; Hossain, M.S. Effects of forest soil and leaf--litter on germination and initial seedling growth of Leucaena leucocephala. Allelopath. J. 2002, 10, 13–20. [Google Scholar]
- Weidenhamer, J.D. New approaches for the analyse of allelochemicals in soil. Allelopath. J. 2007, 19, 135–142. [Google Scholar]
- Shikanai, T. Regulation of photosynthetic electron transport. BBA-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Q.; Li, J.; Hu, Y.; Chen, J. Growth response and nutrient uptake of Eriobotrya japonica plants inoculated with three isolates of arbuscular mycorrhizal fungi under water stress condition. J. Plant. Nutr. 2014, 37, 690–730. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of Cucumber and Figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Reactive Oxygen Intermediates. Cell 2010, 140, 951. [Google Scholar] [CrossRef]
- Zhang, F.J.; Guo, J.Y.; Liu, W.X.; Wan, F.H. Influence of coastal plain yellowtops (Flaveria bidentis) residues on growth of cotton seedlings and soil fertility. Arch. Agron. Soil Sci. 2012, 58, 1117–1128. [Google Scholar] [CrossRef]
- Zhang, K.M.; Shen, Y.; Fang, Y.M.; Liu, Y. Changes in gametophyte physiology of Pteris multifida induced by the leaf leachate treatment of the invasive Bidens pilosa. Environ. Sci. Pollut. R. 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Giallfreda, L.; Salmino, F.; Vtoallte, A. Pesticide effects on the activity of free, immobillized and intertase. Soil Biol. Bicohem. 1995, 27, 1201–1208. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Dick, W.A. Research and developments in measuring activities. In Enzymes in Soil; Burns, R.G., Dick, R.P., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 567–596. [Google Scholar]
- Yakushev, A.V.; Kuznetsova, I.N.; Blagodatskaya, E.V.; Blagodatsky, S.A. Temperature dependence of the activity of polyphenol peroxidases and polyphenol oxidases in modern and buried soils. Eurasian Soil Sci. 2014, 47, 459–465. [Google Scholar] [CrossRef]
- Fan, D.; Fan, K.; Zhang, D.; Zhang, M.; Wang, X. Impact of fertilization on soil polyphenol dynamics and carbon accumulation in a tea plantation, Southern China. J. Soil Sediment. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Pandher, J.K.; Arora, V.; Kohli, R.K. Phytotoxic effect of Parthenium residues on the selected soil properties and growth of chickpea and radish. Weed Biol Manag. 2002, 2, 73–78. [Google Scholar]
- Zhou, L.C.; Chen, X.M.; Li, X.L.; Yang, X.Q.; Xia, W. Study on Variation of Available Phosphorus of Soil in Karst Regions Under Rocky Desertification, Southwest China. J. Earth Sci. Environ. 2009, 31, 418–422. [Google Scholar]
- Inderjit, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends. Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Rout, M.E.; Chrzanowski, T.H.; Smith, W.K.; Gough, L. Ecological impacts of the invasive grasssorghum halepenseon native tallgrass prairie. Biol. Invasions. 2013, 15, 327–339. [Google Scholar] [CrossRef]
- Alam, S.M.; Ala, S.A.; Azmi, A.R. Influence of aqueous leaf extract of purple nutsedge (Cyperus rotundus L.) and Nacl on germination and seeding growth of wheat (Triticum aestivum L.). Pak. J. Sci. Ind. Res. 1999, 42, 372–373. [Google Scholar]
- Padhy, B.; Patnaik, P.K.; Tripathy, A.K. Allelopathic potential of Eucalyptus leaf litter leachates on germination and seedling growth of Fingemillet. Allelopath. J. 2000, 7, 69–78. [Google Scholar]
- Benvenuti, S.; Dinelli, G.; Bonetti, A. Germination ecology of Leptochloa chinensis: A new weed in the Italian rice agro-environment. Weed Res. 2004, 44, 87–96. [Google Scholar] [CrossRef]
- Kong, C.H.; Wang, M.L.; Wang, P.; Han, W.N.; Xiang, R.M. Reproduction allocation and potential mechanism of individual allelopathic rice plants in the presence of competing Barnyardgrass. Pest. Manag. Sci. 2013, 69, 142–148. [Google Scholar] [CrossRef]
- Christoph, K.; Markus, S.; Roland, G. Weed suppression and early sugar beet development under different cover crop mulches. Plant. Prot. Sci. 2017, 52, 1–7. [Google Scholar] [CrossRef]

| Part | Concentrations (mg mL−1) | AN/mg kg−1 | AP/mg kg−1 | AK/mg kg−1 |
|---|---|---|---|---|
| Pericarp | 0 | 74.91 ± 3.24 a | 7.91 ± 0.38 a | 58.70 ± 2.94 a |
| 0.5 | 69.81 ± 0.04 a | 7.99 ± 1.05 a | 52.00 ± 2.56 a | |
| 1 | 66.20 ± 4.67 a | 8.32 ± 2.31 a | 62.50 ± 0.85 a | |
| 5 | 76.34 ± 2.82 a | 8.03 ± 2.15 a | 60.20 ± 0.28 a | |
| 10 | 74.32 ± 2.30 a | 7.89 ± 1.80 a | 58.65 ± 1.05 a | |
| 50 | 64.46 ± 2.61 a | 7.53 ± 2.53 a | 56.70 ± 0.14 a | |
| Leaf | 0 | 74.91 ± 3.24 a | 7.91 ± 0.38 a | 58.70 ± 2.94 a |
| 0.5 | 73.33 ± 0.00 a | 7.10 ± 0.57 a | 69.90 ± 0.99 a | |
| 1 | 63.98 ± 5.33 a | 9.48 ± 1.83 a | 65.00 ± 4.79 a | |
| 5 | 61.16 ± 2.52 a | 9.88 ± 1.63 a | 65.70 ± 5.23 a | |
| 10 | 60.00 ± 3.26 a | 9.12 ± 1.02 a | 73.25 ± 3.26 a | |
| 50 | 59.35 ± 4.74 a | 8.06 ± 0.96 a | 81.40 ± 3.79 a | |
| Rhizosphere soil | 0 | 74.91 ± 3.24 a | 7.91 ± 0.38 a | 58.70 ± 2.94 a |
| 0.5 | 73.37 ± 3.10 a | 8.20 ± 1.05 a | 64.50 ± 2.45 a | |
| 1 | 67.98 ± 3.57 ab | 9.02 ± 0.79 a | 70.50 ± 1.86 a | |
| 5 | 67.77 ± 2.46 ab | 7.65 ± 2.01 a | 63.80 ± 0.28 a | |
| 10 | 62.65 ± 2.86 ab | 7.76 ± 1.23 a | 60.56 ± 0.86 a | |
| 50 | 52.38 ± 3.98 b | 7.51 ± 0.87 a | 55.80 ± 4.84 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Chen, J.; Xiong, X.; Wang, S.; Liu, J. Allelopathic Effects of Cinnamomum migao on Seed Germination and Seedling Growth of its Associated Species Liquidambar formosana. Forests 2019, 10, 535. https://doi.org/10.3390/f10070535
Wang D, Chen J, Xiong X, Wang S, Liu J. Allelopathic Effects of Cinnamomum migao on Seed Germination and Seedling Growth of its Associated Species Liquidambar formosana. Forests. 2019; 10(7):535. https://doi.org/10.3390/f10070535
Chicago/Turabian StyleWang, Deng, Jingzhong Chen, Xue Xiong, Shu Wang, and Jiming Liu. 2019. "Allelopathic Effects of Cinnamomum migao on Seed Germination and Seedling Growth of its Associated Species Liquidambar formosana" Forests 10, no. 7: 535. https://doi.org/10.3390/f10070535




