Woody Litter Increases Headwater Stream Metal Export Ratio in an Alpine Forest
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site and Experimental Design
2.2. Sample Collection and Statistical Analyses
3. Results
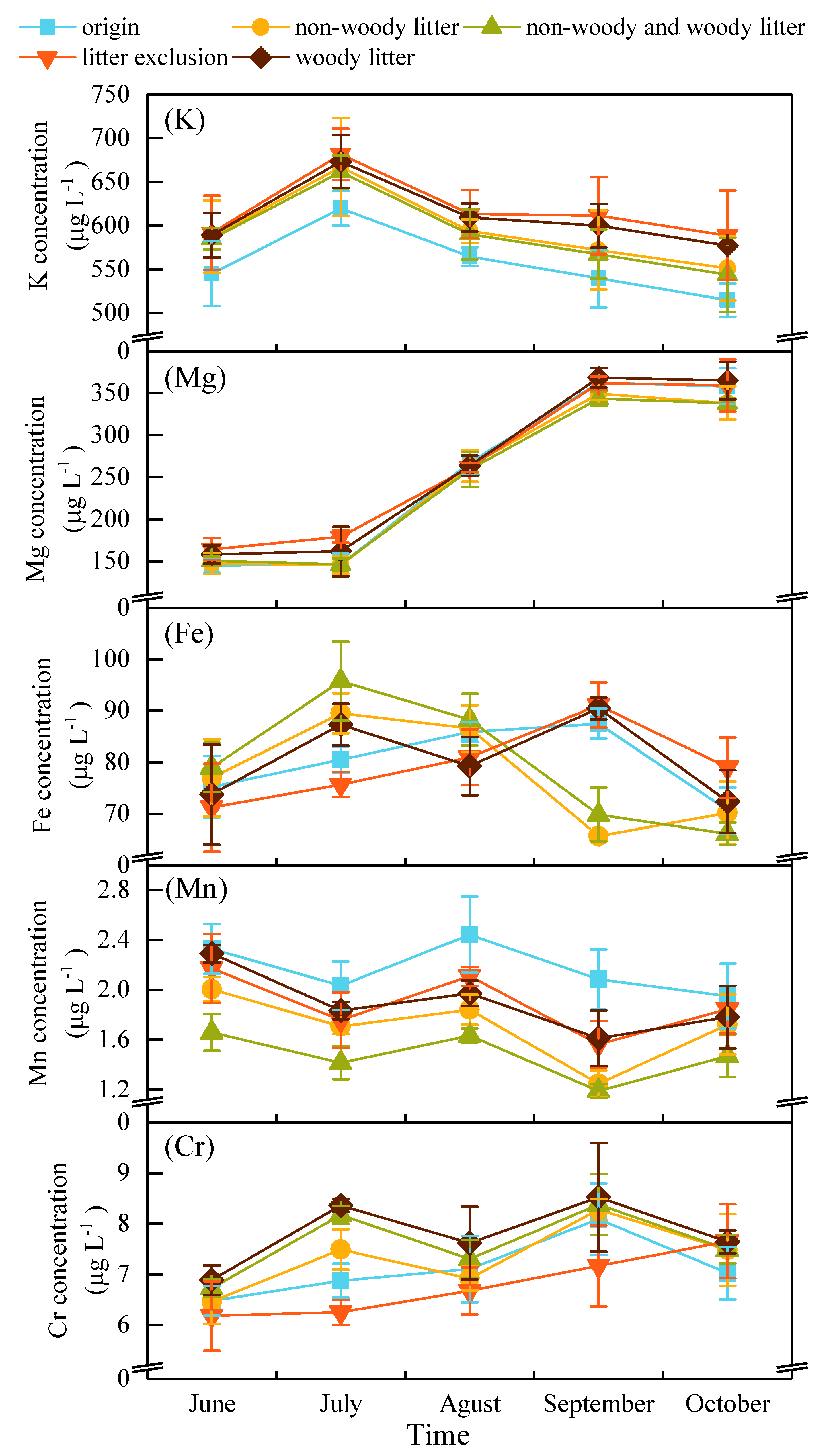
3.1. Dynamics of Metal Concentrations in Water
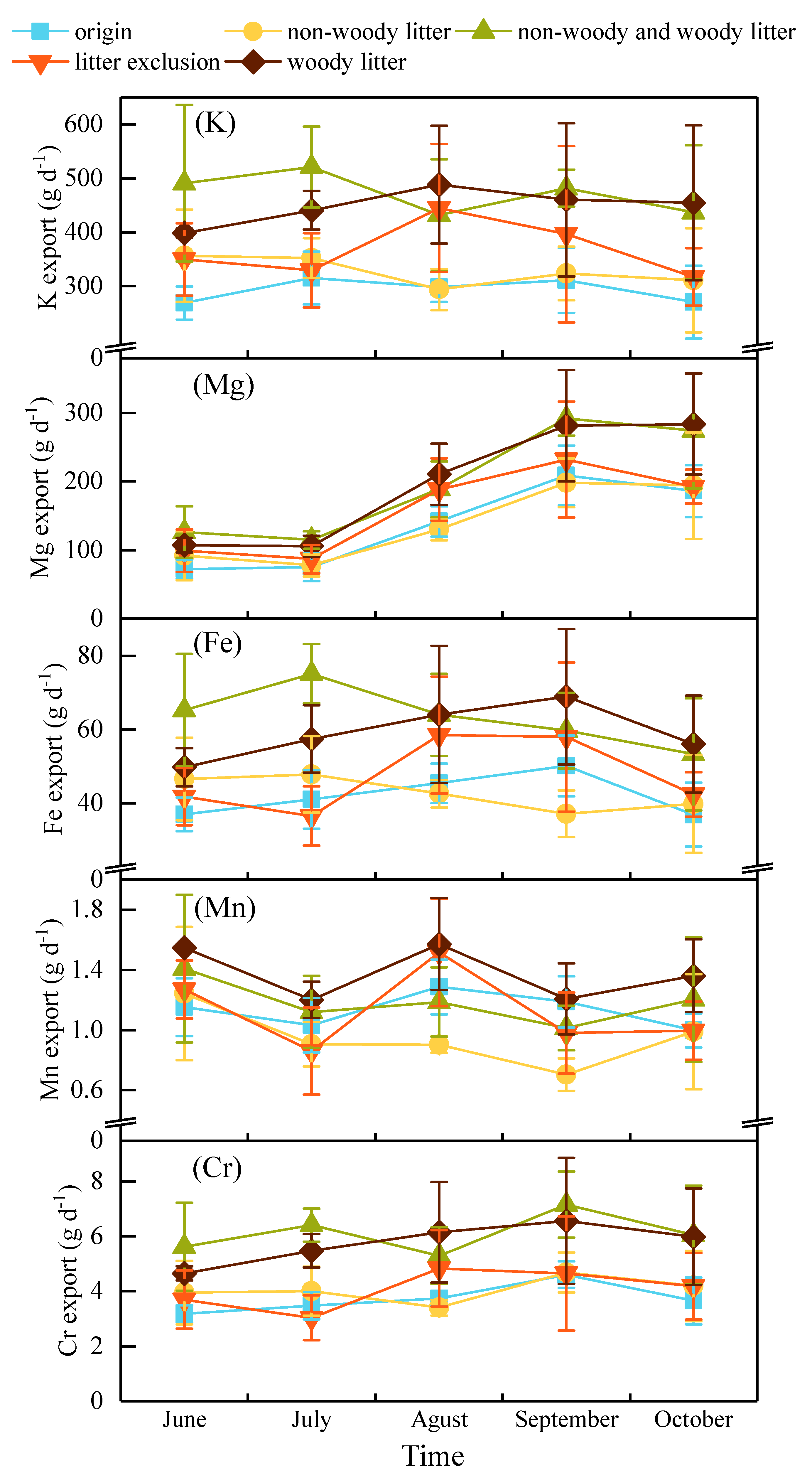
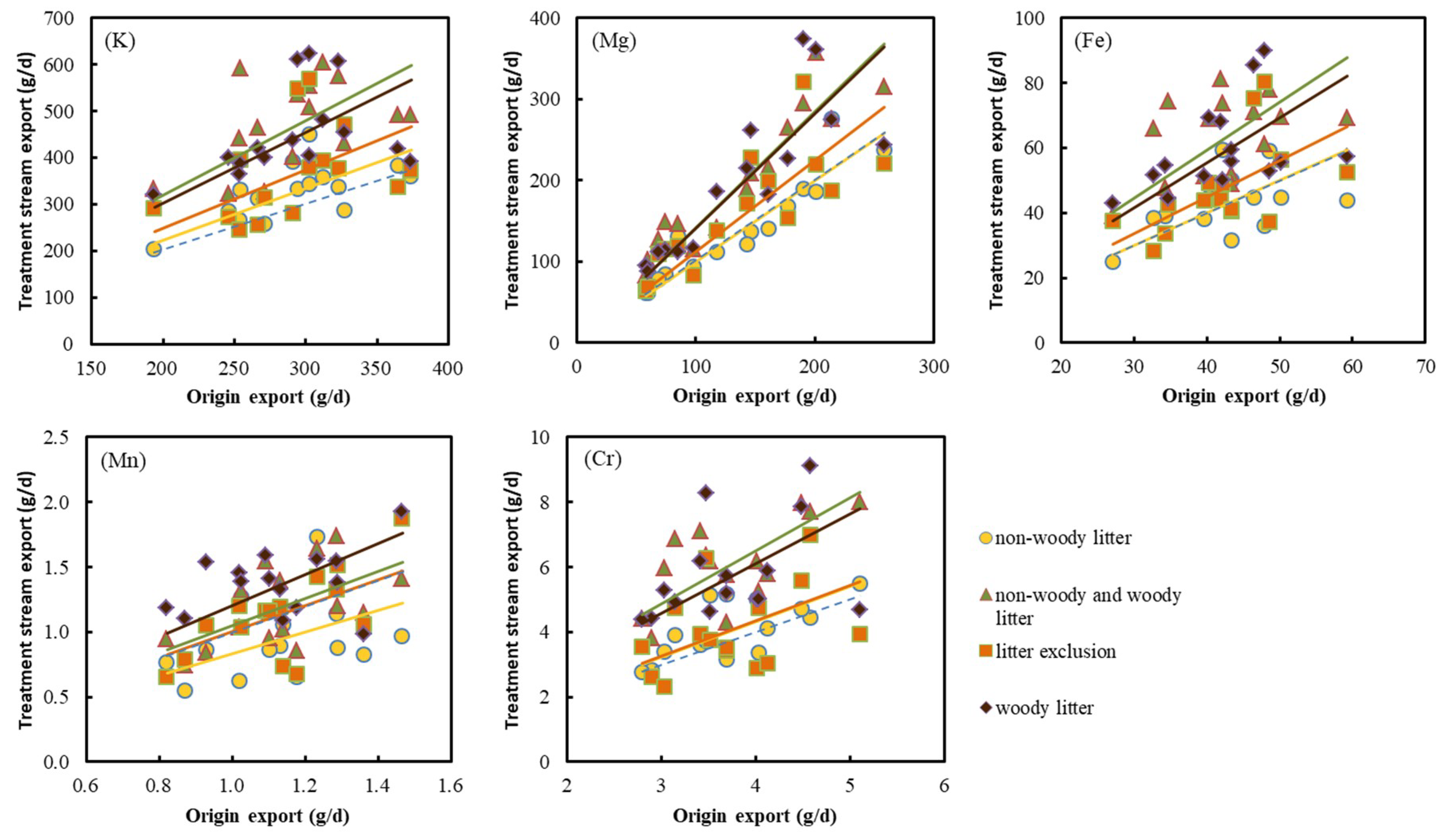
3.2. Dynamics of Metal Export in Water
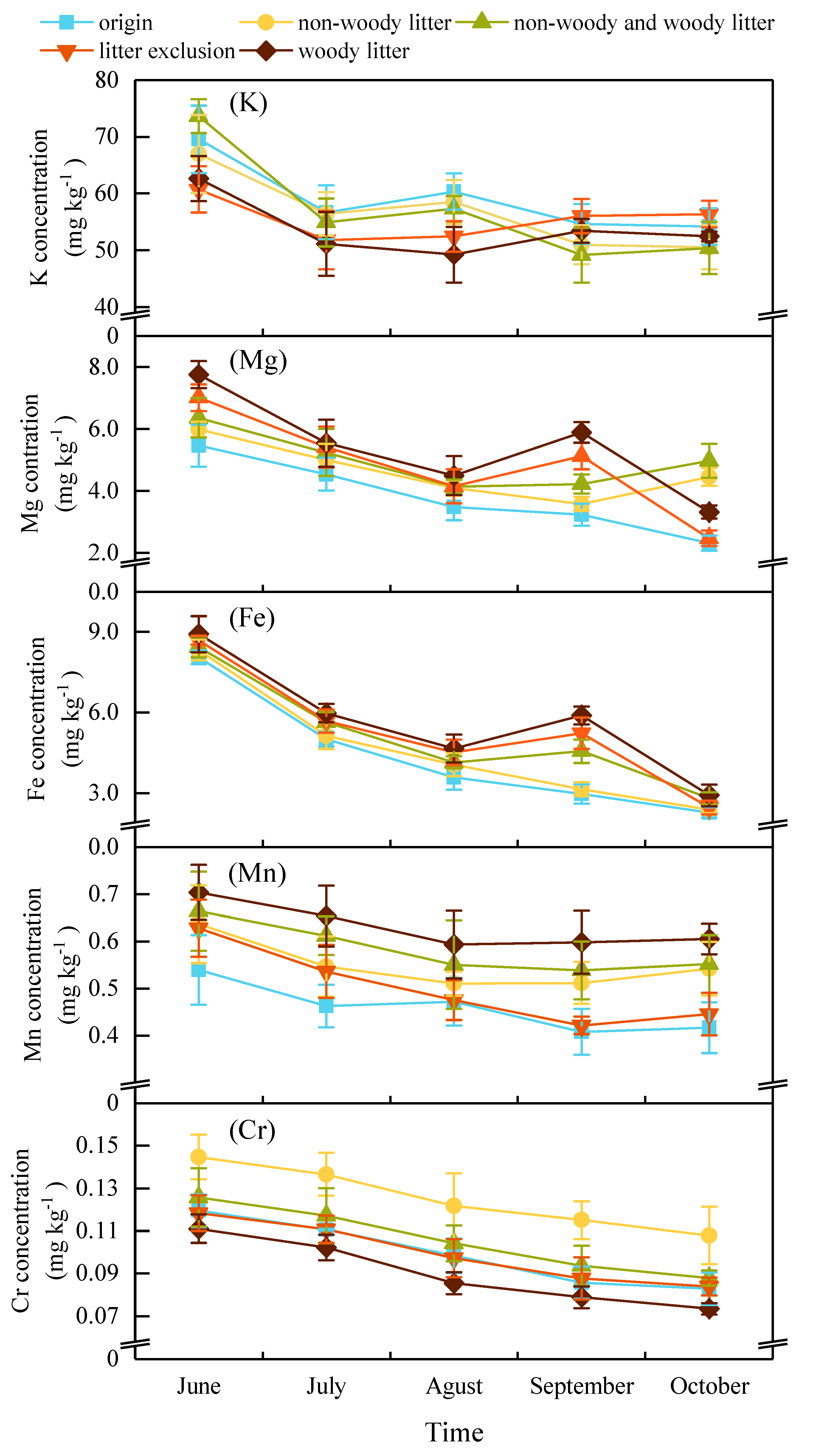
3.3. Dynamics of Metals Concentration in Sediment
3.4. Dynamics of Metals Storage in Sediment
4. Discussion
4.1. Concentration and Export of Metals in Water
4.2. Concentration and Storage of Metals in Sediment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Audry, S.; Schäfer, J.; Blanc, G.; Jouanneau, J. Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the lot river reservoirs (France). Environ. Pollut. 2004, 132, 413–426. [Google Scholar] [CrossRef]
- Taka, M.; Aalto, J.; Virkanen, J.; Luoto, M. The direct and indirect effects of watershed land use and soil type on stream water metal concentrations. Water Resour. Res. 2016, 52, 7711–7725. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The role of riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water. Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Daniels, R.B.; Gilliam, J.W. Sediment and chemical load reduction by grass and riparian filters. Soil Sci. Soc. Am. J. 1996, 60, 246–251. [Google Scholar] [CrossRef]
- Ohta, T.; Shin, K.C.; Saitoh, Y.; Nakano, T.; Hiura, T. The Effects of Differences in Vegetation on Calcium Dynamics in Headwater Streams. Ecosystems 2018, 21, 1390–1403. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Harmon, M.E.; Nadelhoffer, K.J.; Blair, J.M. Measuring decomposition, nutrient turnover, and stores in plant litter. In Standard Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 202–240. [Google Scholar]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 1997, 277, 102–104. [Google Scholar] [CrossRef]
- Webster, J.R.; Tank, J.L.; Wallace, J.B.; Meyer, J.L.; Eggert, S.L.; Ehrman, T.P.; Ward, B.R.; Bennett, B.L.; Wagner, P.F.; McTammany, M.E. Effects of litter exclusion and wood removal on phosphorus and nitrogen retention in a forest stream. Verh. Int. Verein. Limnol. 2000, 27, 1337–1340. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, Y.; Zhang, C.; Huang, C.P.; Wu, F.Z. Chromium, cadmium, and lead dynamics during winter foliar litter decomposition in an alpine forest river. Arct. Antarct. Alp. Res. 2016, 48, 79–91. [Google Scholar] [CrossRef]
- Downing, J.A.; Cole, J.J.; Duarte, C.M.; Middelburg, J.J.; Melack, J.M.; Prairie, Y.T.; Kortelainen, P.; Striegl, R.G.; McDowell, W.H.; Tranvik, L.J. Global abundance and size distribution of streams and rivers. Inland Waters 2012, 2, 229–236. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Kobayashi, S.; Kagaya, T. Differences in patches of retention among leaves, woods and small litter particles in a headwater stream: The importance of particle morphology. Limnology 2008, 9, 47–55. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; Mckie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Yue, K.; García-Palacios, P.; Parsons, S.A.; Yang, W.Q.; Peng, Y.; Tan, B.; Huang, C.P.; Wu, F.Z. Assessing the temporal dynamics of aquatic and terrestrial litter decomposition in an alpine forest. Funct. Ecol. 2018, 32, 2464–2475. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xie, Y.Z.; Ren, Q.S.; Li, C.X. Leaf decomposition and nutrient release of three tree species in the hydro-fluctuation zone of the Three Gorges Dam Reservoir, China. Environ. Sci. Pollut. Res. 2018, 25, 23261–23275. [Google Scholar] [CrossRef]
- Rinella, D.J.; Booz, M.; Bogan, D.L.; Boggs, K.; Sturdy, M.; Rinella, M.J. Large woody litter and salmonid habitat in the Anchor River basin, Alaska, following an extensive spruce beetle (Dendroctonus rufipennis) outbreak. Northwest Sci. 2009, 83, 57–69. [Google Scholar] [CrossRef]
- Tank, J.L.; Rosi-Marshall, E.J.; Griffiths, N.A.; Entrekin, S.A.; Stephen, M.L. A review of allochthonous organic matter dynamics and metabolism in streams. J. N. Am. Benthol. Soc. 2010, 29, 118–146. [Google Scholar] [CrossRef]
- Gomi, T.; Sidle, R.C.; Bryant, M.D.; Woodsmith, R.D. The characteristics of woody litter and sediment distribution in headwater streams, southeastern Alaska. Can. J. For. Res. 2001, 31, 1386–1399. [Google Scholar] [CrossRef]
- Viers, J.; Dupre, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, M.L.; Soto-Varela, F.; Taboada-Castro, M.M.; Taboada-Castro, M.T. Using hysteresis analysis to infer controls on sediment-associated and dissolved metals transport in a small humid temperate catchment. J. Hydrol. 2018. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, C.H.; Peng, Y.; Zhang, C.; Huang, C.P.; Tan, Y.; Wu, F.Z. Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Sci. Total Environ. 2016, 566, 279–287. [Google Scholar] [CrossRef]
- Fu, C.K.; Yang, W.Q.; Tan, B.; Xu, Z.F.; Zhang, Y.; Yang, J.P.; Ni, X.Y.; Wu, F.Z. Seasonal Dynamics of Litterfall in a Sub-Alpine Spruce-Fir Forest on the Eastern Tibetan Plateau: Allometric Scaling Relationships Based on One Year of Observations. Forests 2017, 8, 314. [Google Scholar] [CrossRef]
- Ni, X.Y.; Yang, W.Q.; Tan, B.; He, J.; Xu, L.Y.; Li, H.; Wu, F.Z. Accelerated foliar litter humification in forest gaps: Dual feedbacks of carbon sequestration during winter and the growing season in an alpine forest. Geoderma 2015, 241, 136–144. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Bärlocher, F.; Gessner, M.O. Part 1. Litter dynamics. Coarse benthic organic matter. In Methods to Study Litter Decomposition a Practical Guide; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Yue, K.; Yang, W.Q.; Peng, Y.; Zhang, C.; Huang, C.P.; Xu, Z.F.; Tan, B.; Wu, F.Z. Dynamics of multiple metallic elements during foliar litter decomposition in an alpine forest river. Ann. For. Sci. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Mutema, M.; Chaplot, V.; Jewitt, G.; Chivenge, P.; Blöschl, G. Annual water, sediment, nutrient, and organic carbon fluxes in river basins: A global meta-analysis as a function of scale. Water Resour. Res. 2016, 51, 8949–8972. [Google Scholar] [CrossRef]
- Song, C.L.; Wang, G.X.; Sun, X.Y.; Chang, R.Y.; Mao, T.X. Control factors and scale analysis of annual river water, sediments and carbon transport in China. Sci. Rep. 2016, 6, 25963. [Google Scholar] [CrossRef]
- Richardson, J.S.; Danehy, R.J. A synthesis of the ecology of headwater streams and their riparian zones in temperate forests. For. Sci. 2007, 53, 131–147. [Google Scholar] [CrossRef]
- Huser, B.; Fölster, J.; Köhler, S. Lead, zinc, and chromium concentrations in acidic headwater streams in Sweden explained by chemical, climatic, and land-use variations. Biogeosciences 2012, 9, 4323–4335. [Google Scholar] [CrossRef]
- Landre, A.L.; Watmough, S.A.; Dillon, P.J. The effects of dissolved organic carbon, acidity and seasonality on metal geochemistry within a forested catchment on the Precambrian Shield, central Ontario, Canada. Biogeochemistry 2009, 93, 271–289. [Google Scholar] [CrossRef]
- Elosegi, A.; Díez, J.; Pozo, J. Contribution of dead wood to the carbon flux in forested streams. Earth Surf. Proc. Landf. 2007, 32, 1219–1228. [Google Scholar] [CrossRef]
- Wallace, J.B.; Webster, J.R.; Eggert, S.L.; Meyer, J.L.; Siler, E.R. Large woody litter in a headwater stream: Long-term legacies of forest disturbance. Int. Rev. Hydrobiol. 2001, 86, 501–513. [Google Scholar] [CrossRef]
- Young, R.G.; Matthaei, C.D.; Townsend, C.R. Organic matter breakdown and ecosystem metabolism: Functional indicators for assessing river ecosystem health. J. N. Am. Benthol. Soc. 2008, 27, 605–625. [Google Scholar] [CrossRef]
- Foucreau, N.; Puijalon, S.; Hervant, F.; Piscart, C. Effect of leaf litter characteristics on leaf conditioning and on consumption by Gammarus pulex. Freshw. Biol. 2013, 58, 1672–1681. [Google Scholar] [CrossRef]
- Merten, E.C.; Vaz, P.G.; Decker-Fritz, J.A.; Finlay, J.C.; Stefan, H.G. Relative importance of breakage and decay as processes depleting large wood from streams. Geomorphology 2013, 190, 40–47. [Google Scholar] [CrossRef]
- Quinn, J.M.; Phillips, N.R.; Parkyn, S.M. Factors influencing retention of coarse particulate organic matter in streams. Earth Surf. Proc. Landf. 2010, 32, 1186–1203. [Google Scholar] [CrossRef]
- Osei, N.A.; Gurnell, A.M.; Harvey, G.L. The role of large wood in retaining fine sediment, organic matter and plant propagules in a small, single-thread forest river. Geomorphology 2015, 235, 77–87. [Google Scholar] [CrossRef]
- Turowski, J.M.; Hilton, R.G.; Sparkes, R. Decadal carbon discharge by a mountain stream is dominated by coarse organic matter. Geology 2016, 44, 27–30. [Google Scholar] [CrossRef]
- Ruiz-Villanueva, V.; Piégay, H.; Gaertner, V.; Perret, F.; Stoffel, M. Wood density and moisture sorption and its influence on large wood mobility in rivers. Catena 2016, 140, 182–194. [Google Scholar] [CrossRef]
- Wohl, E.; Scott, D.N. Wood and sediment storage and dynamics in river corridors. Earth Surf. Proc. Landf. 2017, 42, 5–23. [Google Scholar] [CrossRef]
- Cornut, J.; Elger, A.; Greugny, A.; Bonnet, M.; Chauvet, E. Coarse particulate organic matter in the interstitial zone of three French headwater streams. Ann. Limnol.-Int. J. Limnol. 2012, 48, 303–313. [Google Scholar] [CrossRef]
- Leithold, E.L.; Blair, N.E.; Perkey, D.W. Geomorphologic controls on the age of particulate organic carbon from small mountainous and upland rivers. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Eggert, S.L.; Wallace, J.B.; Meyer, J.L.; Webster, J.R. Storage and export of organic matter in a headwater stream: Responses to long-term detrital manipulations. Ecosphere 2012, 3, 1–25. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Tan, B.; Peng, Y.; Huang, C.P.; Xu, Z.F.; Ni, X.Y.; Yang, Y.; Zhou, W.; Zhang, L.; et al. Immobilization of heavy metals during aquatic and terrestrial litter decomposition in an alpine forest. Chemosphere 2019, 216, 419–427. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J.; Hill, W.R. Multiple scales of temporal variability in ecosystem metabolism rates: Results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 2007, 10, 558–606. [Google Scholar] [CrossRef]
- Tockner, K.; Pusch, M.; Borchardt, D.; Lorang, M.S. Multiple stressors in coupled river-floodplain ecosystems. Freshw. Boil. 2010, 55, 135–151. [Google Scholar] [CrossRef]


| Streams | Temperature (°C) | Dissolved Oxygen (mg/L) | Conductivity (μs/cm) | pH | Illumination (lx) | Discharge (L/s) |
|---|---|---|---|---|---|---|
| origin | 6.87 ± 1.20 a | 7.60 ± 0.23 a | 23.62 ± 5.13 a | 6.42 ± 0.22 a | 12,256 ± 11,061 a | 6.09 ± 0.95 c |
| non-woody litter | 6.87 ± 1.20 a | 7.60 ± 0.23 a | 23.62 ± 5.13 a | 6.42 ± 0.22 a | 13,422 ± 14,124 a | 6.43 ± 1.46 c |
| non-woody and woody litter | 6.71 ± 0.65 a | 7.62 ± 0.27 a | 23.85 ± 4.07 a | 6.54 ± 0.21 a | 7981 ± 6294 b | 9.28 ± 1.79 a |
| litter exclusion | 6.65 ± 0.64 a | 7.51 ± 0.24 a | 24.87 ± 6.37 a | 6.44 ± 0.21 a | 12,405 ± 14,637 a | 6.91 ± 1.83 bc |
| woody litter | 6.71 ± 0.68 a | 7.57 ± 0.28 a | 24.69 ± 3.81 a | 6.40 ± 0.28 a | 8077 ± 5343 b | 8.53 ± 1.81 ab |
| Factor | df | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Time | 4 | 26.743 | <0.001 | 717.856 | <0.001 | 18.730 | <0.001 | 25.690 | <0.001 | 27.040 | <0.001 |
| Input | 4 | 5.042 | <0.05 | 3.186 | 0.062 | 0.740 | 0.586 | 21.203 | <0.001 | 3.778 | <0.05 |
| Time × Input | 16 | 0.194 | 1.000 | 0.829 | 0.647 | 6.582 | <0.001 | 1.194 | 0.314 | 2.268 | <0.05 |
| origin | 556.67 b | 255.84 a | 79.96 a | 2.17 a | 7.11 ab | ||||||
| non-woody litter | 594.20 ab | 248.72 a | 77.77 a | 1.70 bc | 7.33 ab | ||||||
| non-woody and woody litter | 589.41 ab | 247.57 a | 79.80 a | 1.47 c | 7.62 ab | ||||||
| litter exclusion | 617.30 a | 265.26 a | 79.58 a | 1.89 b | 6.78 b | ||||||
| woody litter | 609.62 a | 263.37 a | 80.63 a | 1.90 b | 7.81 a | ||||||
| Factor | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Export | Concentration | Export | Concentration | Export | Concentration | Export | Concentration | Export | |
| Temperature | 0.297 ** | 0.183 | −0.028 | 0.092 | 0.426 ** | 0.291 * | −0.054 | 0.041 | 0.136 | 0.159 |
| Dissolved Oxygen | 0.355 ** | 0.400 ** | −0.483 ** | −0.260 * | 0.054 | 0.281 * | −0.065 | 0.237 * | 0.034 | 0.258 * |
| Conductivity | 0.187 | 0.139 | −0.223 | −0.150 | 0.252 * | 0.208 | 0.188 | 0.226 | −0.199 | 0.016 |
| pH | −0.036 | −0.102 | −0.307 ** | −0.229 * | −0.113 | −0.128 | 0.078 | −0.021 | −0.112 | −0.079 |
| Illumination | −0.104 | −0.264 * | 0.174 | 0.011 | 0.144 | −0.147 | 0.173 | −0.135 | −0.150 | −0.257 * |
| Factor | df | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Time | 4 | 1.439 | 0.239 | 90.525 | <0.001 | 5.602 | <0.05 | 14.116 | <0.001 | 8.592 | <0.001 |
| Input | 4 | 3.098 | 0.067 | 2.070 | 0.160 | 3.024 | 0.071 | 1.646 | 0.238 | 3.284 | 0.058 |
| Time × Input | 16 | 1.516 | 0.142 | 1.229 | 0.324 | 3.189 | <0.05 | 2.077 | <0.05 | 1.520 | 0.140 |
| origin | 292.28 a | 136.82 a | 42.13 a | 1.13 a | 3.74 a | ||||||
| non-woody litter | 327.04 a | 138.41 a | 42.81 a | 0.95 a | 4.05 a | ||||||
| non-woody and woody litter | 472.06 a | 199.19 a | 63.44 a | 1.19 a | 6.11 a | ||||||
| litter exclusion | 367.12 a | 159.86 a | 47.46 a | 1.12 a | 4.08 a | ||||||
| woody litter | 448.14 a | 197.68 a | 59.23 a | 1.38 a | 5.77 a | ||||||
| K | Mg | Fe | Mn | Cr | |
|---|---|---|---|---|---|
| origin | 1.00 c | 1.00 b | 1.00 b | 1.00 b | 1.00 b |
| non-woody litter | 1.12b c | 1.04 b | 1.03 b | 0.84 b | 1.09 b |
| non-woody and woody litter | 1.63 a | 1.50 a | 1.53a | 1.05 a | 1.65 a |
| litter exclusion | 1.27 b | 1.21 b | 1.13 b | 0.99 b | 1.10 b |
| woody litter | 1.55 a | 1.47 a | 1.43 a | 1.24 a | 1.56 a |
| Factor | df | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Time | 4 | 45.982 | <0.001 | 133.525 | <0.001 | 487.003 | <0.001 | 34.831 | <0.001 | 110.740 | <0.001 |
| Input | 4 | 1.541 | 0.264 | 10.342 | <0.001 | 27.993 | <0.001 | 5.577 | <0.05 | 9.888 | <0.01 |
| Time × Input | 16 | 3.334 | <0.001 | 9.657 | <0.001 | 3.968 | <0.001 | 0.993 | 0.483 | 0.118 | 1.000 |
| origin | 59.08 a | 3.80 b | 4.37 c | 0.46 b | 0.10 b | ||||||
| non-woody litter | 56.71 a | 4.62 ab | 4.61 c | 0.55 ab | 0.13 a | ||||||
| non-woody and woody litter | 57.10 a | 4.98 a | 5.11 b | 0.58 ab | 0.11 b | ||||||
| litter exclusion | 55.49 a | 4.83 a | 5.31 ab | 0.50 ab | 0.10 b | ||||||
| woody litter | 53.78 a | 5.40 a | 5.67 a | 0.63 a | 0.90 b | ||||||
| Factor | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content | Storage | Content | Storage | Content | Storage | Content | Storage | Content | Storage | |
| Temperature | −0.153 | 0.410 ** | −0.079 | 0.399 ** | 0.035 | 0.363 ** | −0.157 | 0.472 ** | −0.022 | 0.498 ** |
| Dissolved Oxygen | 0.207 | 0.161 | 0.233 ** | 0.255 * | 0.274 * | 0.289 * | −0.018 | 0.082 | 0.195 | 0.192 |
| Conductivity | 0.350 ** | 0.286 * | 0.089 | 0.235 * | 0.276 * | 0.354 ** | 0.095 | 0.226 | 0.197 | 0.273 * |
| pH | −0.025 | −0.256 * | 0.110 | −0.189 | 0.072 | −0.150 | 0.228 * | −0.186 | 0.275 * | −0.128 |
| Illumination | −0.049 | 0.398 ** | −0.192 | 0.284 * | −0.017 | 0.334 ** | −0.138 | 0.414 ** | 0.009 | 0.440 ** |
| Factor | df | K | Mg | Fe | Mn | Cr | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Time | 4 | 10.331 | <0.001 | 11.465 | <0.001 | 20.406 | <0.001 | 12.174 | <0.001 | 16.291 | <0.001 |
| Input | 4 | 0.099 | 0.980 | 0.236 | 0.912 | 0.184 | 0.942 | 0.309 | 0.866 | 0.561 | 0.697 |
| Time × Input | 16 | 0.785 | 0.692 | 1.503 | 0.219 | 1.046 | 0.434 | 0.235 | 0.999 | 0.400 | 0.975 |
| origin | 4385.02 a | 278.54 a | 306.11 a | 33.12 a | 7.22 a | ||||||
| non-woody litter | 3981.85 a | 316.76 a | 310.69 a | 38.00 a | 8.63 a | ||||||
| non-woody and woody litter | 3945.64 a | 339.82 a | 344.26 a | 39.80 a | 7.24 a | ||||||
| litter exclusion | 3944.64 a | 329.85 a | 367.09 a | 34.39 a | 6.97 a | ||||||
| woody litter | 3624.27 a | 353.65 a | 375.75 a | 41.70 a | 6.06 a | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Wu, F.; Ni, X.; Tan, B.; Zhang, L.; Xu, Z.; Hu, J.; Yue, K. Woody Litter Increases Headwater Stream Metal Export Ratio in an Alpine Forest. Forests 2019, 10, 379. https://doi.org/10.3390/f10050379
Liang Z, Wu F, Ni X, Tan B, Zhang L, Xu Z, Hu J, Yue K. Woody Litter Increases Headwater Stream Metal Export Ratio in an Alpine Forest. Forests. 2019; 10(5):379. https://doi.org/10.3390/f10050379
Chicago/Turabian StyleLiang, Ziyi, Fuzhong Wu, Xiangyin Ni, Bo Tan, Li Zhang, Zhenfeng Xu, Junyi Hu, and Kai Yue. 2019. "Woody Litter Increases Headwater Stream Metal Export Ratio in an Alpine Forest" Forests 10, no. 5: 379. https://doi.org/10.3390/f10050379
APA StyleLiang, Z., Wu, F., Ni, X., Tan, B., Zhang, L., Xu, Z., Hu, J., & Yue, K. (2019). Woody Litter Increases Headwater Stream Metal Export Ratio in an Alpine Forest. Forests, 10(5), 379. https://doi.org/10.3390/f10050379







