Climate Response of Douglas Fir Reveals Recently Increased Sensitivity to Drought Stress in Central Europe
Abstract
:1. Introduction
2. Materials and Methods
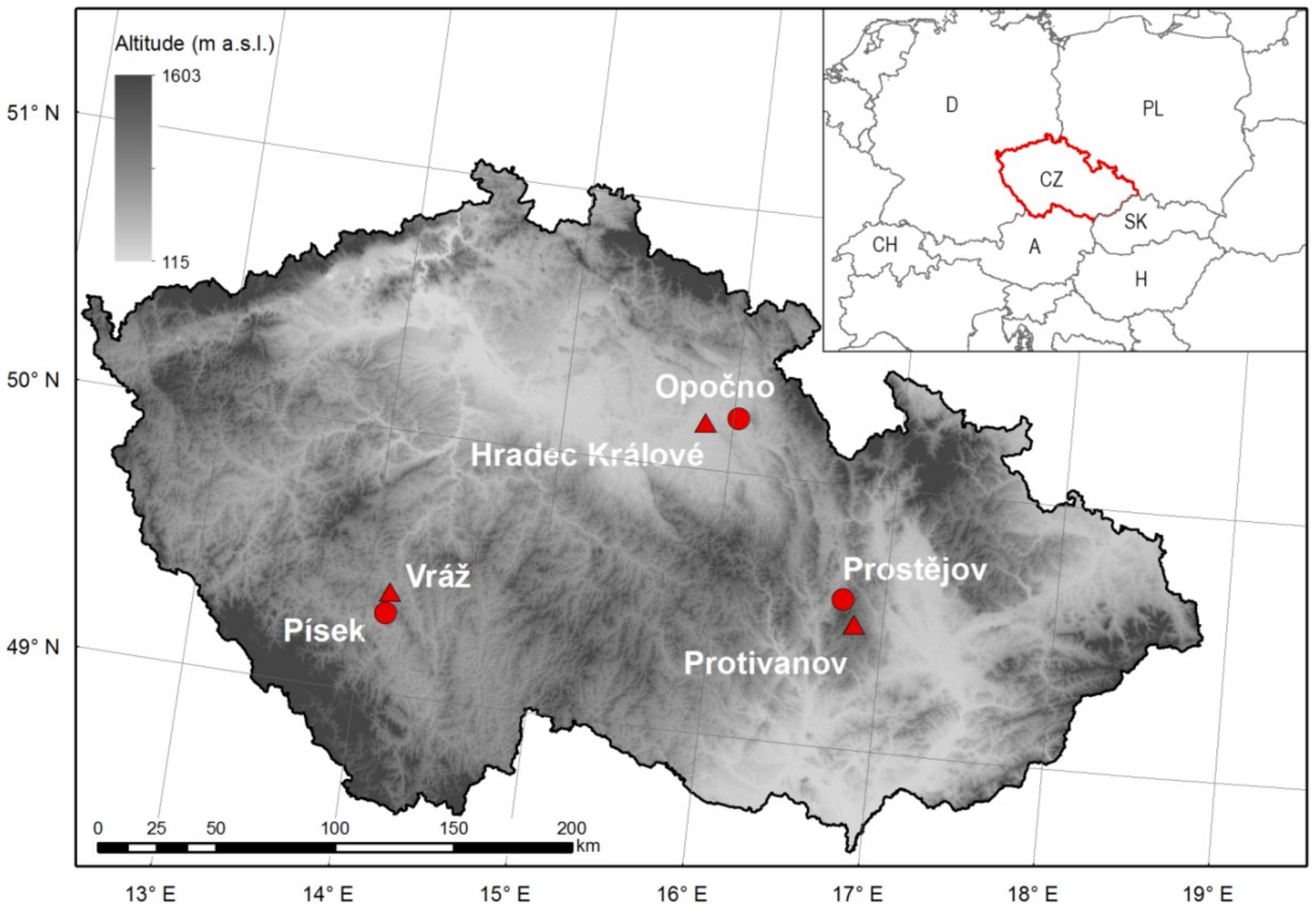
2.1. Study Sites and Data Collection
2.2. Data Processing and Chronology Development
2.3. Growth–Climate Relationship
3. Results
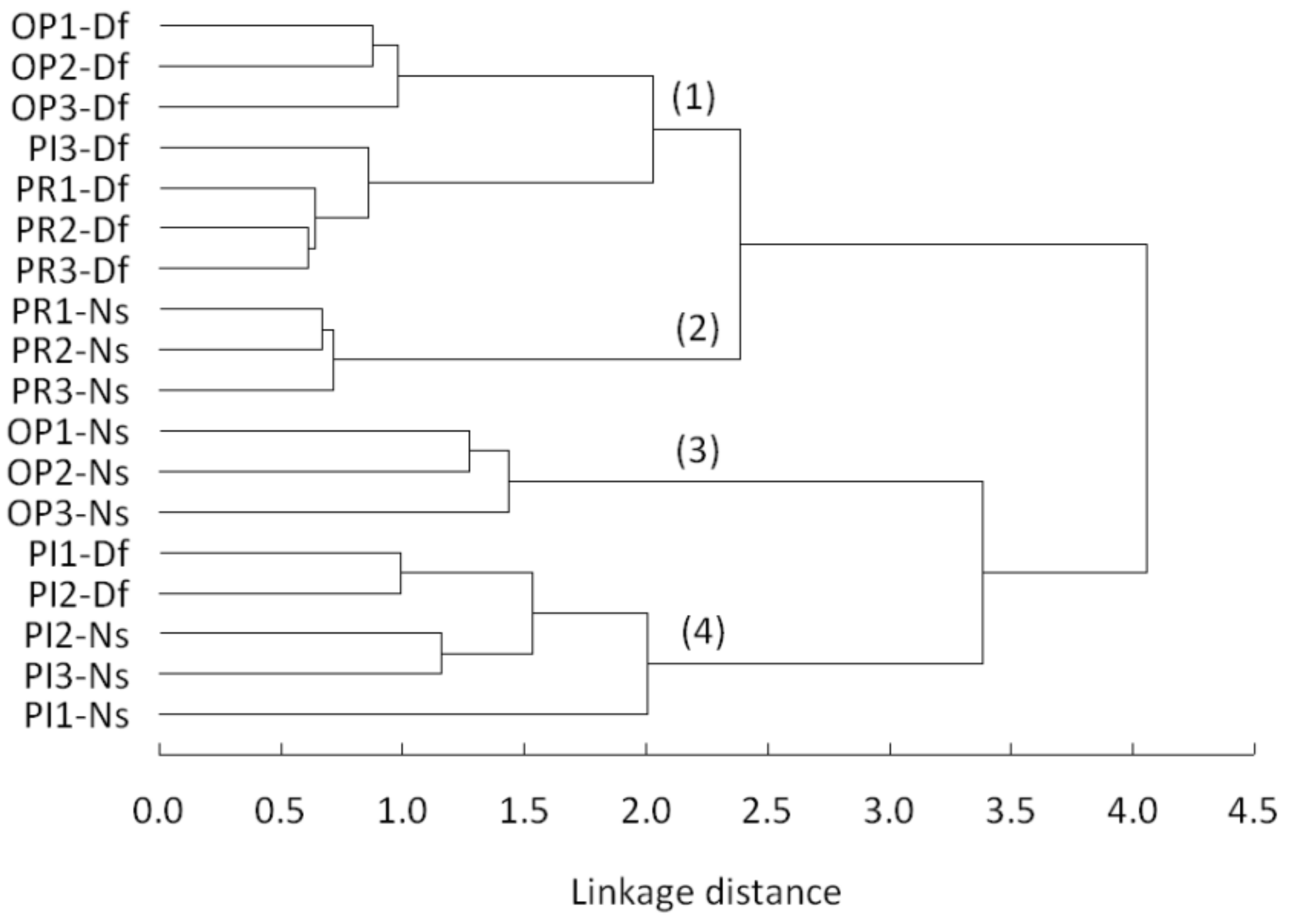
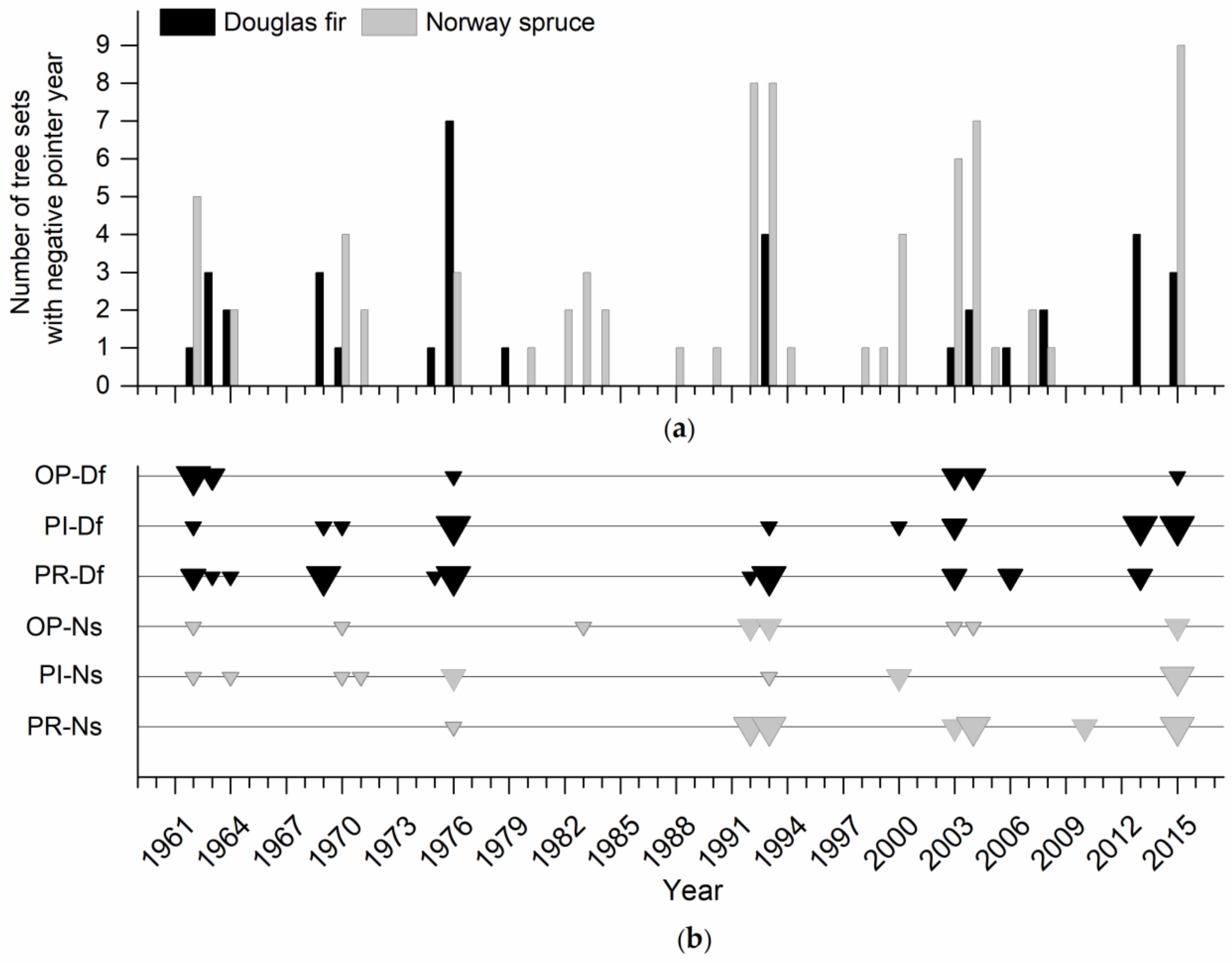
3.1. TRW Chronologies
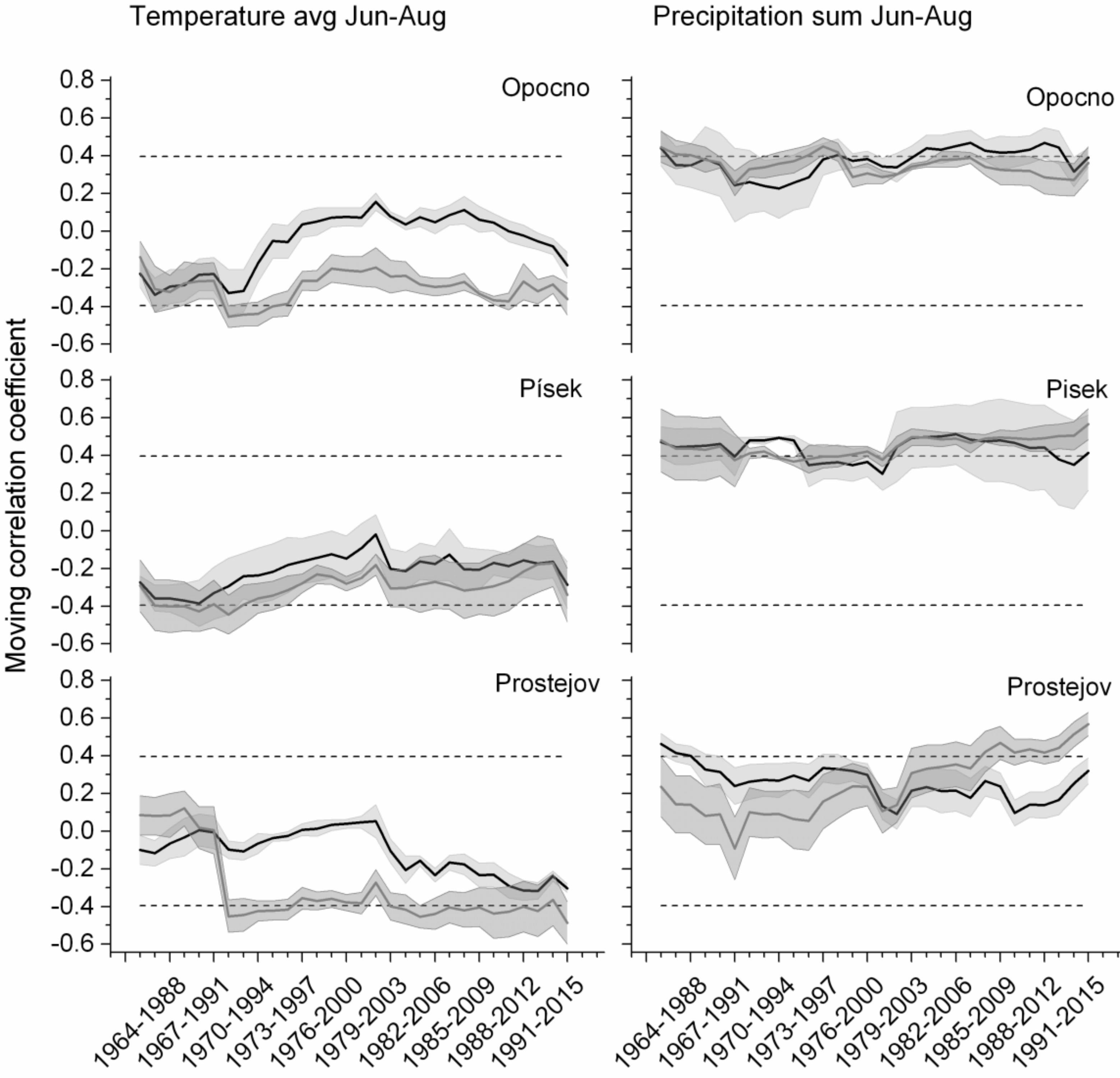
3.2. Growth–Climate Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isaac-Renton, M.G.; Roberts, D.R.; Hamann, A.; Spiecker, H. Douglas-fir plantations in Europe: A retrospective test of assisted migration to address climate change. Glob. Chang. Biol. 2014, 20, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Wang, T.; Andre, K.; Konnert, M.; Lexer, M.J.; Matulla, C.; Schueler, S. Selecting Populations for non-analogous climate conditions using universal response functions: The case of Douglas-fir in Central Europe. PLoS ONE 2015, 10, e0136357. [Google Scholar] [CrossRef] [PubMed]
- Vinš, B.; Šika, A. Srovnání růstové reakce douglasky a smrku na stanovištní faktory v podmínkách ČSR [Comparison of the growth response of Douglas fir and Norway spruce to the site factors in the conditions of the Czech Republic]. Práce VÚLHM 1981, 58, 7–33. [Google Scholar]
- Podrázský, V.; Čermák, R.; Zahradník, D.; Kouba, J. Production of Douglas fir in the Czech Republic based on national forest inventory data. J. For. Sci. 2013, 59, 398–404. [Google Scholar] [CrossRef]
- Remeš, J.; Zeidler, A. Production potential and wood quality of Douglas fir from selected sites in the Czech Republic. Wood Res. 2014, 59, 509–520. [Google Scholar]
- Zeidler, A.; Borůvka, V.; Schönfelder, O. Comparison of wood quality of douglas fir and spruce from afforested agricultural land and permanent forest land in the Czech Republic. Forests 2018, 9, 13. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. (Eds.) Silvics of North America: Volume 1 Conifers; Agriculture Handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 527–540.
- Brus, D.; Hengeveld, G.; Walvoort, D.; Goedhart, P.; Heidema, A.; Nabuurs, G.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2011, 131, 145–157. [Google Scholar] [CrossRef]
- Kubeček, J.; Štefančík, I.; Podrázský, V.; Longauer, R. Výsledky výzkumu douglasky tisolisté (Pseudotsuga menziesii/Mirb./Franco) v České republice a na Slovensku—Přehled [Results of the research of Douglas-fir in the Czech Republic and Slovakia—A review]. Lesnícky Časopis For. J. 2014, 60, 116–124. [Google Scholar] [CrossRef]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.J.; Nabuurs, G.J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Šrámek, V.; Novotný, R.; Fadrhonsová, V. Chřadnutí smrkových porostů a stav lesních půd v oblasti severní Moravy a Slezska (PLO 29 a 39) [Decay of Norway spruce stands and quality of forest soils in the region of Northern Moravia and Silesia]. Zprávy Lesnického Výzkumu 2015, 60, 147–153. [Google Scholar]
- Cienciala, E.; Tumajer, J.; Zatloukal, V.; Beranová, J.; Holá, Š.; Hůnová, I.; Russ, R. Recent spruce decline with biotic pathogen infestation as a result of interacting climate, deposition and soil variables. Eur. J. For. Res. 2017, 136, 307–317. [Google Scholar] [CrossRef]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species` drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Nadezhdina, N.; Urban, J.; Čermák, J.; Nadezhdin, V.; Kantor, P. Comparative study of long-term water uptake of Norway spruce and Douglas-fir in Moravian upland. J. Hydrol. Hydromech. 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Mauer, O.; Palátová, E. Root system development in Douglas fir (Pseudotsuga menziesii/Mirb./Franco) on fertile sites. J. For. Sci. 2012, 58, 400–409. [Google Scholar] [CrossRef]
- Lassoie, J.P.; Salo, D.J. Physiological response of large Douglas fir to natural and induced soil water deficits. Can. J. For. Res. 1981, 11, 139–144. [Google Scholar] [CrossRef]
- Pinol, J.; Sala, A. Ecological implications of xylem cavitation for several Pinaceae in the Pacific Northern USA. Funct. Ecol. 2000, 14, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Littell, J.S.; Peterson, D.L.; Tjoelker, M. Douglas-fir growth in mountain ecosystems: Water limits tree growth from stand to region. Ecol. Monogr. 2008, 78, 349–368. [Google Scholar] [CrossRef]
- Sergent, A.S.; Rozenberg, P.; Breda, N. Douglas-fir is vulnerable to exceptional and recurrent drought episodes and recovers less well on less fertile sites. Ann. For. Sci. 2014, 71, 697–708. [Google Scholar] [CrossRef]
- Trnka, M.; Balek, J.; Zahradníček, P.; Eitzinger, J.; Formayer, H.; Turňa, M.; Nejedlík, P.; Semerádová, D.; Hlavinka, R.; Brázdil, R. Drought trends over part of Central Europe between 1961 and 2014. Clim. Res. 2016, 70, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.; Coppola, E. European climate-change oscillation (ECO). Geophys. Res. Lett. 2007, 34, L21703. [Google Scholar] [CrossRef]
- Beran, F. Douglaska tisolistá v ČR—Biologie, genetika, provenienční výzkum [Douglas fir in the Czech Republic/biology, genetics, provenance studies]. In Pěstební Postupy Při Zavádění Douglasky do Porostních Směsí v Podmínkách ČR [Silvicultural Approaches for Introduction of Douglas-Fir into the Forest Mixed Stands in Conditions of the Czech Republic]; Slodičák, M., Novák, J., Eds.; Lesnická Práce: Kostelec nad Černými Lesy, Czech Republic, 2014; pp. 22–47. [Google Scholar]
- Chytrý, M. Current vegetation of the Czech Republic. In Flora and Vegetation of the Czech Republic; Chytrý, M., Danihelka, J., Kaplan, Z., Pyšek, P., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 229–337. [Google Scholar]
- Viewegh, J.; Kusbach, A.; Mikeska, M. Czech forest ecosystem classification. J. For. Sci. 2003, 49, 74–82. [Google Scholar] [CrossRef]
- Knibbe, B. PAST 4—Personal Analysis Systém for Tree Ring Research, Version 4. Instruction Manual; SCIEM/Bernhard Knibbe: Vienna, Austria, 2004. [Google Scholar]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 39, 77–82. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Science; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1990. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizingforest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 198, 41, 45–53. [Google Scholar]
- Cook, E.R.; Krusic, P.J. ARSTAN v. 41d: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Tree-Ring Laboratory, Lamont-Doherty Earth Observatoryof Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984. 23, 201–213. [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Schweingruber, F.H.; Eckstein, D.; Serre-Bachet, F.; Bräker, O.U. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 1990, 8, 8–38. [Google Scholar]
- Jetschke, G.; van der Maaten, E.; van der Maaten-Theunissen, M. Towards the extremes: A critical analysis of pointer year detection methods. Dendrochronologia 2019, 53, 55–62. [Google Scholar] [CrossRef]
- Kaiser, H.F. On Cliff’s formula, the Kaiser–Guttman rule, and the number of factors. Percept. Motor Skills 1992, 74, 595–598. [Google Scholar] [CrossRef]
- Gugger, P.F.; Gonzalez-Rodriguez, A.; Rodriguez-Correa, H.; Sugita, S.; Cavender-Bares, J. Southward pleistocene migration of Douglas-fir into Mexico: Phylogeography, ecological niche modeling, and conservation of ‘rear edge’populations. New Phytol. 2011, 189, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Plíva, K. Trvale Udržitelné Obhospodařování Lesů Podle Souborů Lesních Typů [Sustainable Forest Management According to Forest Types]; Ústav pro hospodářskou úpravu lesů: Brandýs nad Labem, Czech Republic, 2000. [Google Scholar]
- Andrews, J.F. The weather and circulation of February 1956. Mon. Weather Rev. 1956, 84, 66–74. [Google Scholar] [CrossRef]
- Lebourgeois, F. Climatic signal in annual growth variation of silver fir (Abies alba Mill.) and spruce (Picea abies Karst.) from the French Permanent Plot Network (RENECOFOR). Ann. For. Sci. 2007, 64, 333–343. [Google Scholar] [CrossRef]
- Blinka, P. Klimatologické hodnocení sucha a suchých období na území ČR v letech 1876–2003 [Climatological assessment of drought and dry periods in the Czech Republic in 1876–2003]. In Proceedings of the “Extrémy počasí a podnebí” [Extremes of weather and climate], Brno, Czech Republic, 11 March 2004. [Google Scholar]
- Potop, V.; Türkott, L. Rizika výskytu pozdních jarních mrazů a prvních podzimních mrazů při pěstování cukrové řepy ve středních Čechách [The risks of late spring frosts and early autumn frosts in sugar beet growing in Central Bohemia]. Listy Cukrovarnické a Řepařské 2013, 129, 283–291. [Google Scholar]
- Kroupová, M. Dendroecological study of spruce growth in regions under long-term air pollution load. J. For. Sci. 2002, 48, 536–548. [Google Scholar]
- Brázdil, R.; Trnka, M.; Dobrovolný, P.; Chromá, K.; Hlavinka, P.; Žalud, Z. Variability of droughts in the Czech Republic, 1881–2006. Theor. Appl. Climatol. 2009, 97, 297–315. [Google Scholar] [CrossRef]
- Ionita, M.; Tallaksen, L.M.; Kingston, D.G.; Stagge, J.H.; Laaha, G.; Van Lanen, H.A.J.; Scholz, P.; Chelcea, S.M.; Haslinger, K. The European 2015 drought from a climatological perspective. Hydrol. Earth Syst. Sci. 2017, 21, 1397–1419. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, G.A.; Adams, W.T.; Aitken, S.N. Quantitative genetics of spring and fall cold hardiness in seedlings from two Oregon populations of coastal Douglas-fir. For. Ecol. Manag. 2001, 149, 305–318. [Google Scholar] [CrossRef]
- Malmqvist, C.; Wallertz, K.; Johansson, U. Survival, early growth and impact of damage by late-spring frost and winter desiccation on Douglas-fir seedlings in southern Sweden. New For. 2018, 49, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Rais, A.; van de Kuilen, J.-W.G.; Pretzsch, H. 2014. Growth reaction patterns of tree height, diameter, and volume of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) under acute drought stress in Southern Germany. Eur. J. For. Res. 2014, 133, 1043–1056. [Google Scholar] [CrossRef]
- Fischer, S.; Neuwirth, B. Klimasensitivität der Douglasie in Eifel und Kellerwald [Climate sensitivity of Douglas-fir in Eifel and Kellerwald]. Allg. Forst Jagdztg. 2012, 183, 23–33. [Google Scholar]
- Hofman, J. Pěstování Douglasky [Plantation of Douglas fir], 1st ed.; Státní zemědělské nakladatelství: Praha, Czech Republic, 1964. [Google Scholar]
- Bansal, S.; St. Clair, J.B.; Harrington, C.A.; Gould, P.J. Impact of climate change on cold hardiness of Douglas-fir (Pseudotsuga menziesii): Environmental and genetic considerations. Glob. Chang. Biol. 2015, 21, 3814–3826. [Google Scholar] [CrossRef] [PubMed]
- Tumajer, J.; Altman, J.; Štěpánek, P.; Treml, V.; Doležal, J.; Cienciala, E. Increasing moisture limitation of Norway spruce in Central Europe revealed by forward modelling of tree growth in tree-ring network. Agric. For. Meteorol. 2017, 247, 56–64. [Google Scholar] [CrossRef]
- Pretel, J. Zpřesnění Dosavadních Odhadů Dopadů Klimatické Změny V Sektorech Vodního Hospodářství, Zemědělství A Lesnictví A Návrhy Adaptačních Opatření [Clarification of Existing Estimates of Impacts of Climate Change in the Water, Agriculture And Forestry Sectors and Proposals for Adaptation Measures], Technical Summary of Project Results 2007–2011. 2011. Available online: http://portal.chmi.cz/files/portal/docs/meteo/ok/klimazmena/files/vav_TECHNICKE_SHRNUTI_2011.pdf (accessed on 30 August 2018).
- Hlásny, T.; Mátyás, C.; Seidl, R.; Kulla, L.; Merganičová, K.; Trombik, J.; Dobor, L.; Barcza, Z.; Konôpka, B. Climate change increases the drought risk in Central European forests: What are the options for adaptation? Lesnícky Časopis For. J. 2014, 60, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Štěpánek, P.; Zahradníček, P.; Farda, A.; Skalák, P.; Trnka, M.; Meitner, J.; Rajdl, K. Projection of drought-inducing climate conditions in the Czech Republic according to Euro-CORDEX models. Clim. Res. 2016, 70, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Leites, L.P.; Robinson, A.P.; Rehfeldt, G.E.; Marshall, J.D.; Crookston, N.L. Height-growth response to climatic changes differs among populations of Douglas-fir: A novel analysis of historic data. Ecol. Appl. 2012, 22, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Eilmann, B.; de Vries, S.M.G.; den Ouden, J.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For. Ecol. Manag. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- Bansal, S.; Harrington, C.A. ; St. Clair, J.B. Tolerance to multiple climate stressors: A case study of Douglas-fir drought and cold hardiness. Ecol. Evol. 2016, 6, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmit, J. Mit der Douglasie in die Zukunft. Ökologische und ökonomische Bilanz: Genetik. Forst und Holz 2000, 55, 713–715. [Google Scholar]





| Site Characteristics | Raw Measurements | Residual Chronology | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Tree Set (Site 1, Tree Species 2) | Altitude (m a.s.l.) | Forest Type 3 | Site Index 4 | No of Trees/Series | Mean/Max Segment Length (year) | Mean TRW 5 (mm) | Proportion of Missing Rings (%) | Series Intercorrel. vs. Mean | Mean rbar | Mean Sensitivity | 1st Order Autocorrel. | Mean Sensitivity | EPS 6 |
| Opočno | OP1-Df | 350 | 3I | 34 | 20/40 | 46/55 | 3.66 | 0 | 0.577 | 0.492 | 0.216 | 0.636 | 0.164 | 0.96 |
| OP1-Ns | 350 | 3I | 26 | 19/38 | 70/77 | 2.25 | 0 | 0.711 | 0.577 | 0.308 | 0.688 | 0.270 | 0.96 | |
| OP2-Df | 350 | 3K | 30 | 20/40 | 64/78 | 3.43 | 0 | 0.641 | 0.359 | 0.222 | 0.669 | 0.196 | 0.97 | |
| OP2-Ns | 350 | 3K | 28 | 20/40 | 77/83 | 2.13 | 0 | 0.662 | 0.542 | 0.251 | 0.720 | 0.210 | 0.97 | |
| OP3-Df | 260 | 1M | 28 | 17/34 | 110/119 | 2.27 | 0.16 | 0.732 | 0.445 | 0.284 | 0.692 | 0.265 | 0.96 | |
| OP3-Ns | 260 | 1M | 24 | 20/40 | 119/128 | 1.44 | 0 | 0.729 | 0.510 | 0.326 | 0.658 | 0.296 | 0.98 | |
| Písek | PI1-Df | 479 | 3K | 34 | 20/40 | 70/81 | 2.24 | 0.04 | 0.629 | 0.544 | 0.303 | 0.627 | 0.226 | 0.96 |
| PI1-Ns | 479 | 3K | 26 | 18/36 | 84/103 | 2.04 | 0.15 | 0.714 | 0.386 | 0.399 | 0.536 | 0.339 | 0.97 | |
| PI2-Df | 410 | 3S | 36 | 19/38 | 62/69 | 2.93 | 0 | 0.702 | 0.548 | 0.290 | 0.656 | 0.208 | 0.96 | |
| PI2-Ns | 410 | 3S | 28 | 20/40 | 64/68 | 2.59 | 0 | 0.711 | 0.581 | 0.348 | 0.692 | 0.311 | 0.97 | |
| PI3-Df | 525 | 4B | 34 | 20/40 | 73/80 | 3.16 | 0 | 0.459 | 0.329 | 0.259 | 0.664 | 0.174 | 0.91 | |
| PI3-Ns | 525 | 4B | 30 | 19/38 | 64/77 | 2.83 | 0.08 | 0.559 | 0.306 | 0.298 | 0.646 | 0.263 | 0.92 | |
| Prostějov | PR1-Df | 600 | 5K | 34 | 20/40 | 76/79 | 2.53 | 0.23 | 0.619 | 0.489 | 0.240 | 0.710 | 0.193 | 0.95 |
| PR1-Ns | 600 | 5K | 30 | 20/40 | 78/83 | 2.22 | 0 | 0.698 | 0.510 | 0.217 | 0.716 | 0.183 | 0.97 | |
| PR2-Df | 560 | 5B | 32 | 20/40 | 95/108 | 2.09 | 0.03 | 0.710 | 0.646 | 0.230 | 0.752 | 0.206 | 0.97 | |
| PR2-Ns | 560 | 5B | 28 | 20/40 | 91/98 | 1.84 | 0 | 0.729 | 0.504 | 0.221 | 0.660 | 0.201 | 0.97 | |
| PR3-Df | 540 | 5K | 36 | 19/38 | 78/83 | 2.43 | 0 | 0.616 | 0.570 | 0.264 | 0.711 | 0.199 | 0.95 | |
| PR3-Ns | 540 | 5K | 28 | 20/40 | 83/90 | 1.99 | 0.15 | 0.704 | 0.701 | 0.240 | 0.784 | 0.210 | 0.97 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejpustková, M.; Čihák, T. Climate Response of Douglas Fir Reveals Recently Increased Sensitivity to Drought Stress in Central Europe. Forests 2019, 10, 97. https://doi.org/10.3390/f10020097
Vejpustková M, Čihák T. Climate Response of Douglas Fir Reveals Recently Increased Sensitivity to Drought Stress in Central Europe. Forests. 2019; 10(2):97. https://doi.org/10.3390/f10020097
Chicago/Turabian StyleVejpustková, Monika, and Tomáš Čihák. 2019. "Climate Response of Douglas Fir Reveals Recently Increased Sensitivity to Drought Stress in Central Europe" Forests 10, no. 2: 97. https://doi.org/10.3390/f10020097





