Wood Protection through Plasma Powder Deposition—An Alternative Coating Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Process
2.2. Surface Characterization
2.3. Water Uptake and Release Tests
2.3.1. Absorption
2.3.2. Desorption
2.3.3. Liquid Water Uptake
3. Results and Discussion
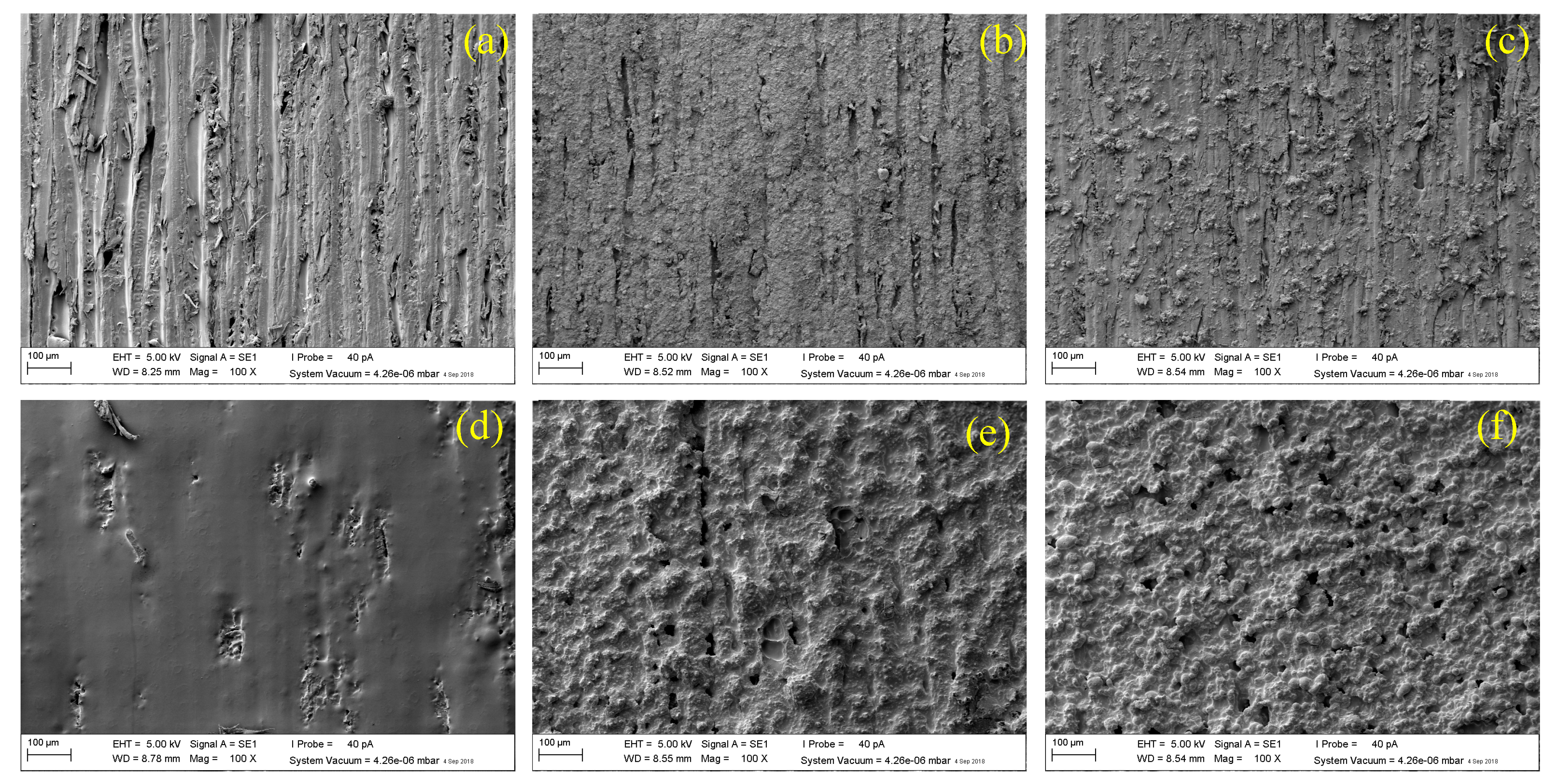
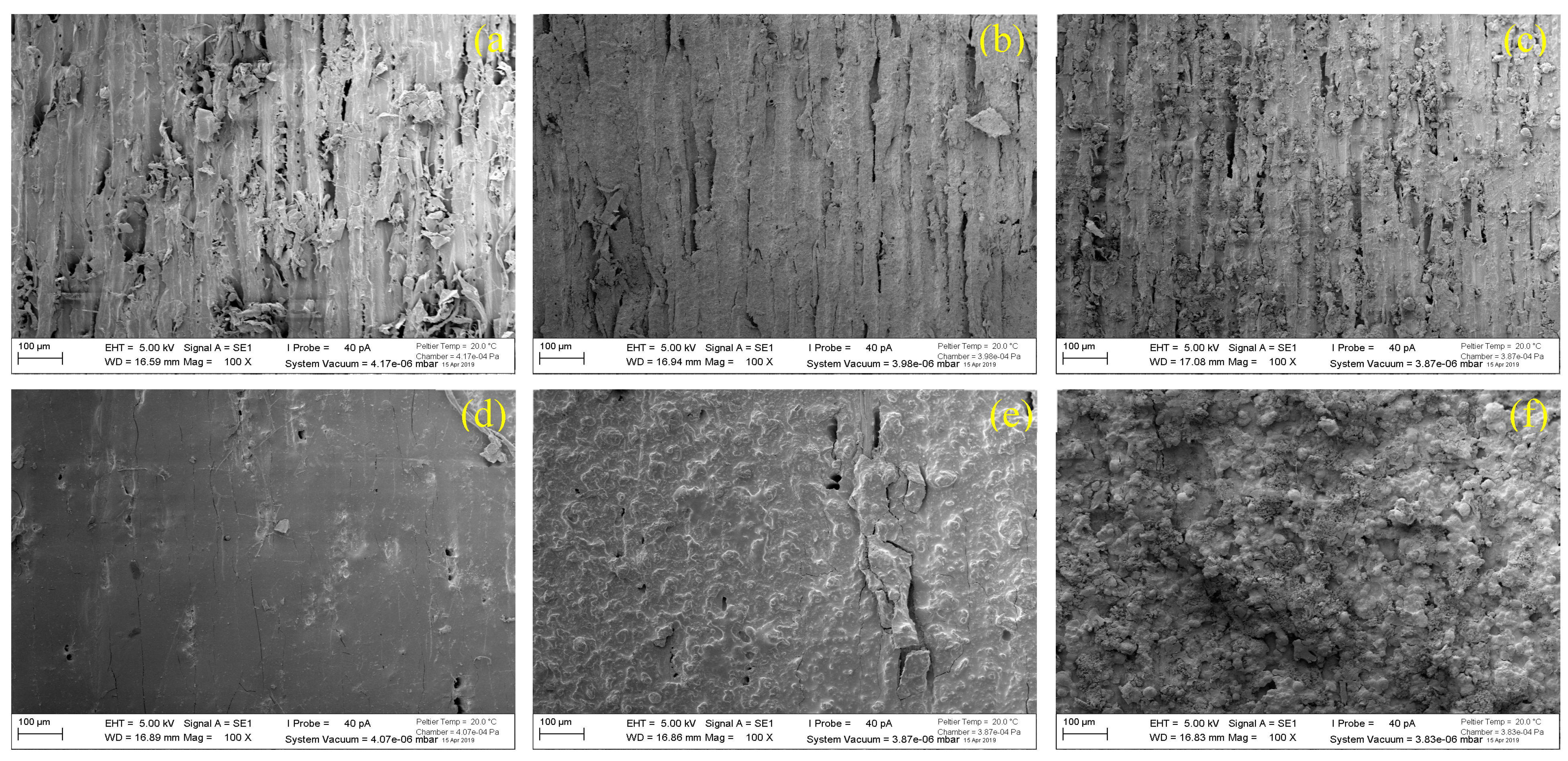
3.1. Average Thickness and Surface Morphology
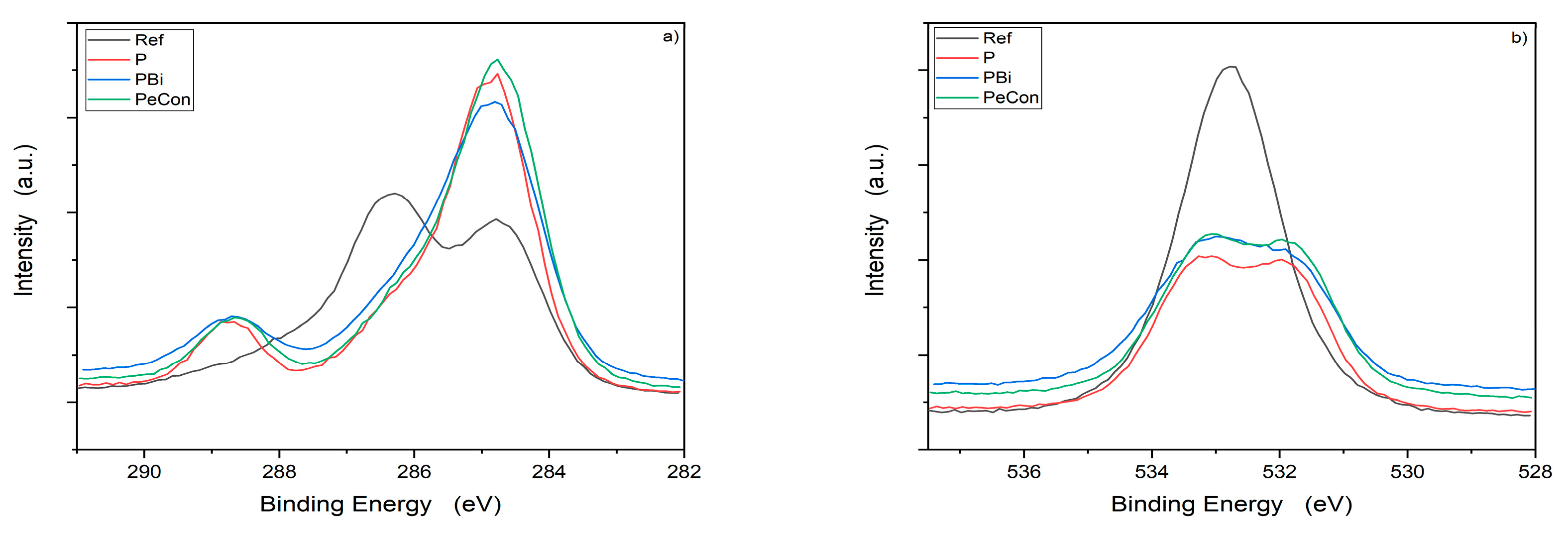
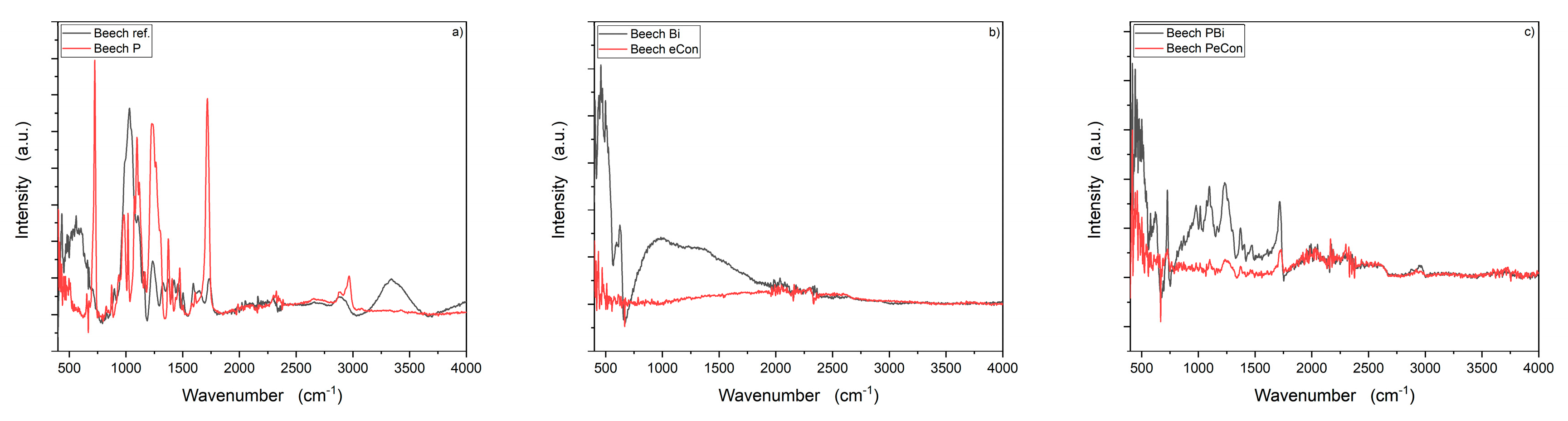
3.2. Chemical Surface Characterisation
- Wood reference and polyester layer: carbon (C 1s) and oxygen (O 1s),
- Bi coating and PBi: C 1s, O 1s and bismuth (Bi 4f)
- eCon and PeCon: C 1s, O 1s, aluminum (Al 2p) and silver (Ag 3d5).
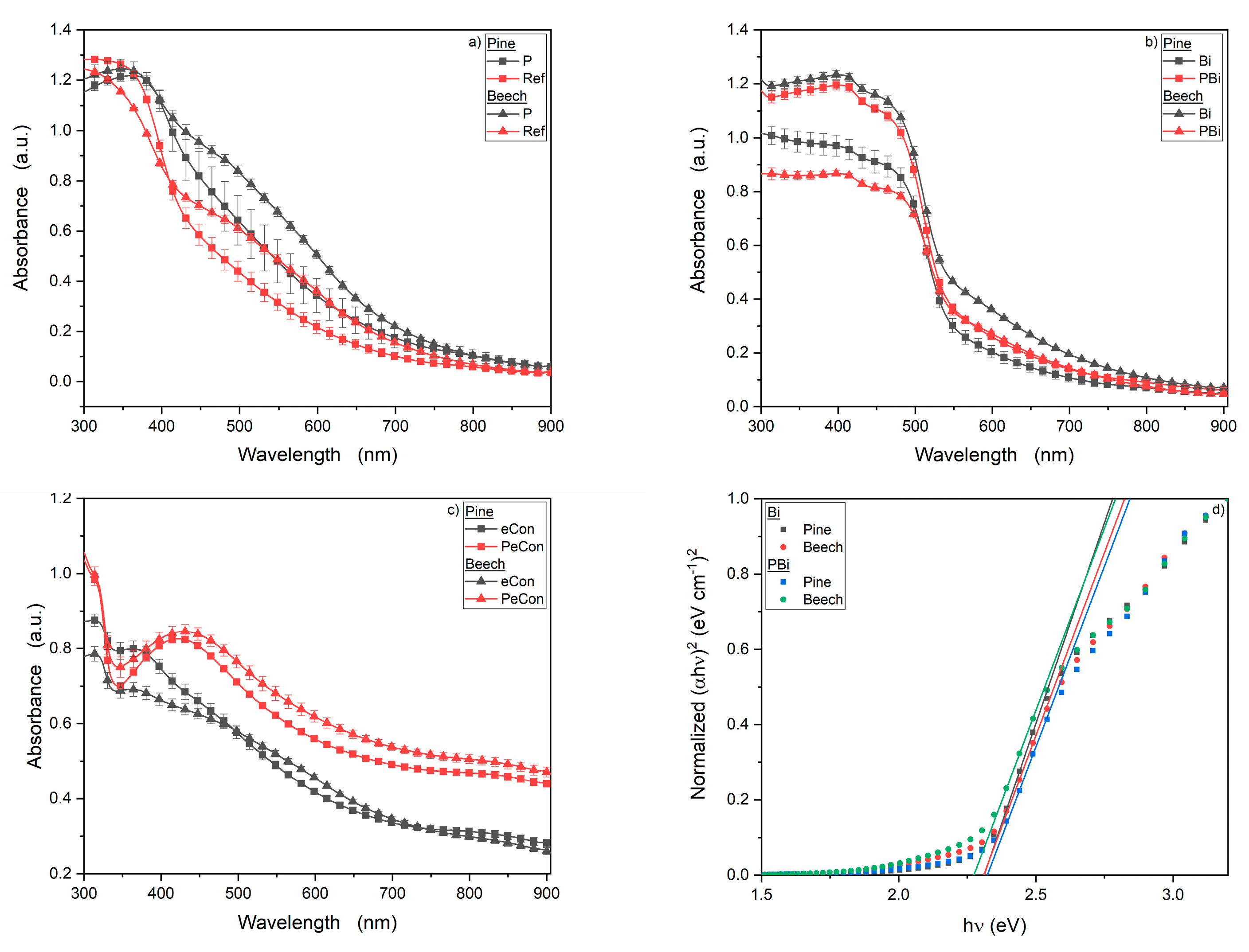
3.3. UV/Vis Spectroscopy
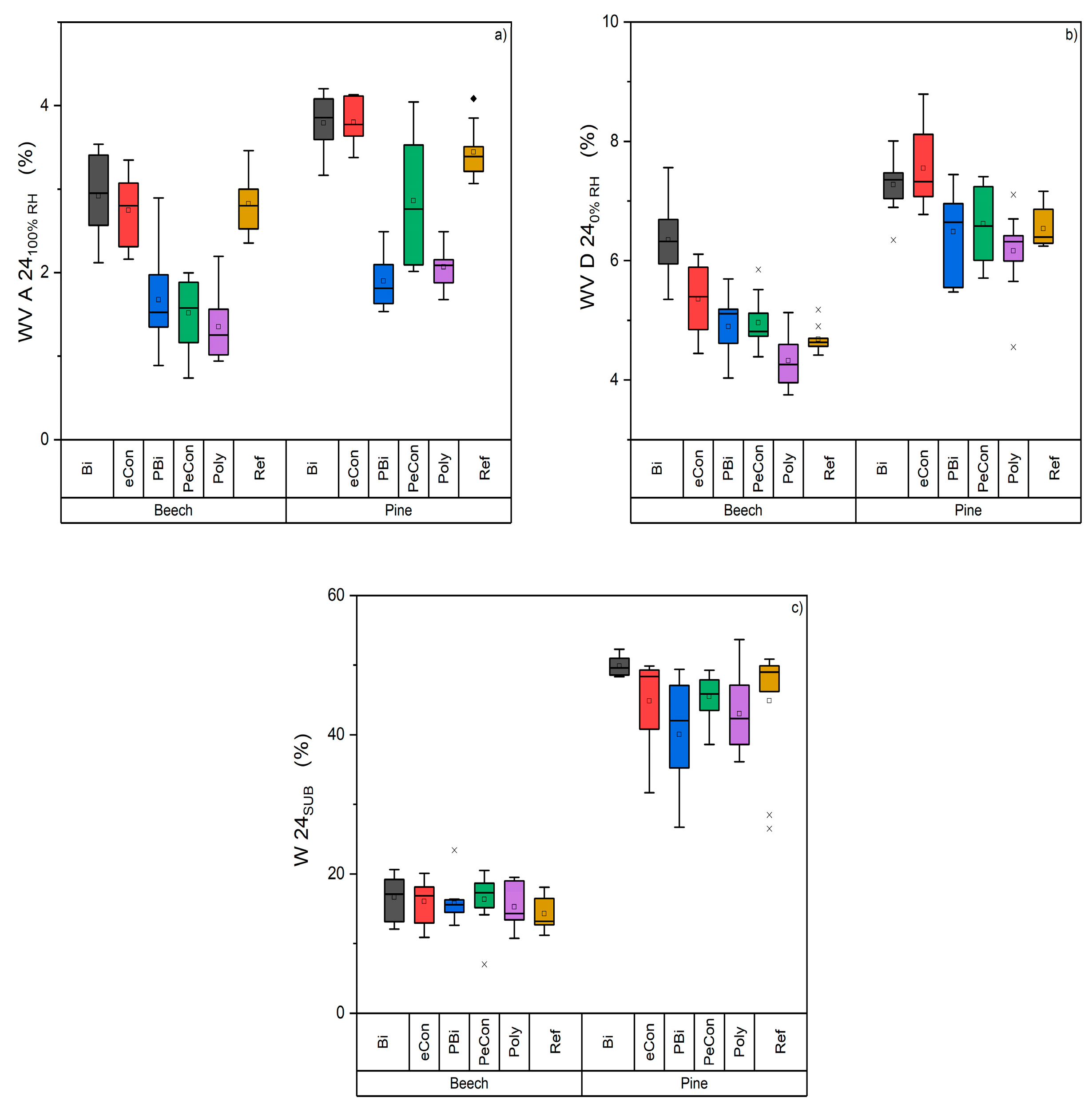
3.4. Water Uptake and Release Test
4. Conclusions
- In the CSs, the metal oxide particles are largely embedded in the polyester.
- The layers containing bismuth oxide show a band gap in the visible spectral range of light at 540 nm, which allows its use as a potential UV protection of wood.
- The eCon MOLs show no protection against UV radiation but could reflect infrared light (<15 µm).
- The CSs with eCon show plasmon resonance probably due to their structure, which also serves as UV protection for wood.
- The bismuth MOLs show good reflective properties in long-wavelength infrared.
- The polyester-containing layers show a lower absorption behavior than the metal oxide layers.
- The metal oxide layers have a higher desorption behavior than the polyester-containing layers.
- The good behavior of the layers against water vapor could not be shown in the submersion test.
- The combination of metal oxide and polyester in the one-layer system combines the positive properties of the polyester layer in absorption and the metal oxide layer in desorption. The layers created in this way protect the wood from water vapor and exhibit good desorption properties.
- The combination of metal oxide and polyester in the one-layer system combines the protection properties of the single coatings against UV radiation and water vapor.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishimaru, Y.; Arai, K.; Mizutani, M.; Oshima, K.; Iida, I. Physical and mechanical properties of wood after moisture conditioning. J. Wood Sci. 2001, 47, 185–191. [Google Scholar] [CrossRef]
- Weichelt, F.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M.R.; Beyer, M. ZnO-Based UV Nanocomposites for Wood Coatings in Outdoor Applications. Macromol. Mater. Eng. 2009, 295, 130–136. [Google Scholar] [CrossRef]
- Gezici-Koç, Ö.; Erich, S.J.F.; Huinink, H.P.; van der Ven, L.G.J.; Adan, O.C.G. Bound and free water distribution in wood during water uptake and drying as measured by 1D magnetic resonance imaging. Cellulose 2017, 24, 535–553. [Google Scholar] [CrossRef]
- Pearnchob, N.; Bodmeier, R. Dry polymer powder coating and comparison with conventional liquid-based coatings for Eudragit® RS, ethylcellulose and shellac. Eur. J. Pharm. Biopharm. 2003, 56, 363–369. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Rerich, R.; Vovk, M.; Dahle, S.; Militz, H.; Ohms, G.; Viöl, W. Morphologic and Chemical Properties of PMMA/ATH Layers with Enhanced Abrasion Resistance Realised by Cold Plasma Spraying at Atmospheric Pressure. Adv. Condens. Matter Phys. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wallenhorst, L.M.; Dahle, S.; Vovk, M.; Wurlitzer, L.; Loewenthal, L.; Mainusch, N.; Gerhard, C.; Viöl, W. Characterisation of PMMA/ATH Layers Realised by Means of Atmospheric Pressure Plasma Powder Deposition. Adv. Condens. Matter Phys. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Köhler, R.; Sauerbier, P.; Militz, H.; Viöl, W. Atmospheric Pressure Plasma Coating of Wood and MDF with Polyester Powder. Coatings 2017, 7, 171. [Google Scholar] [CrossRef]
- Sakakibara, A.; Samo, Y. Chemistry of Lignin. In Wood and Cellulosic Chemistry, 2nd ed.; Hon, D.N.-S., Shiraishi, N., Eds.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Kiguchi, M.; Evans, P.D.; Ekstedt, J.; Williams, R.S.; Kataoka, Y. Improvement of the durability of clear coatings by grafting of UV-absorbers on to wood. Surf. Coat. Int. Part B Coat. Trans. 2001, 84, 263–270. [Google Scholar] [CrossRef]
- Aloui, F.; Ahajji, A.; Irmouli, Y.; George, B.; Charrier, B.; Merlin, A. Inorganic UV absorbers for the photostabilisation of wood-clearcoating systems: Comparison with organic UV absorbers. Appl. Surf. Sci. 2007, 253, 3737–3745. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. Alloy Formation of Gold−Silver Nanoparticles and the Dependence of the Plasmon Absorption on Their Composition. J. Phys. Chem. B 1999, 103, 3529–3533. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood–the state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet. Coatings 2019, 9, 441. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Harada, T.; Matsumura, M. Synthesis, characterization and photocatalytic performance of visible light induced bismuth oxide nanoparticle. J. Alloys Compd. 2015, 648, 641–650. [Google Scholar] [CrossRef]
- Blaber, M.G.; Arnold, M.D.; Harris, N.; Ford, M.J.; Cortie, M.B. Plasmon absorption in nanospheres: A comparison of sodium, potassium, aluminum, silver and gold. Phys. B Condens. Matter 2007, 394, 184–187. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Gurău, L.; Gellerich, A.; Militz, H.; Ohms, G.; Viöl, W. UV-blocking properties of Zn/ZnO coatings on wood deposited by cold plasma spraying at atmospheric pressure. Appl. Surf. Sci. 2018, 434, 1183–1192. [Google Scholar] [CrossRef]
- Wallenhorst, L.M.; Loewenthal, L.; Avramidis, G.; Gerhard, C.; Militz, H.; Ohms, G.; Viöl, W. Topographic, optical and chemical properties of zinc particle coatings deposited by means of atmospheric pressure plasma. Appl. Surf. Sci. 2017, 410, 485–493. [Google Scholar] [CrossRef]
- Gascón-Garrido, P.; Mainusch, N.; Militz, H.; Viöl, W.; Mai, C. Effects of copper-plasma deposition on weathering properties of wood surfaces. Appl. Surf. Sci. 2016, 366, 112–119. [Google Scholar] [CrossRef]
- Nejad, M.; Shafaghi, R.; Pershin, L.; Mostaghimi, J.; Cooper, P. Thermal Spray Coating: A New Way of Protecting Wood. BioResources 2016, 12. [Google Scholar] [CrossRef] [Green Version]
- Gascón-Garrido, P.; Thévenon, M.F.; Mainusch, N.; Militz, H.; Viöl, W.; Mai, C. Siloxane-treated and copper-plasma-coated wood: Resistance to the blue stain fungus Aureobasidium pullulans and the termite Reticulitermes flavipes. Int. Biodeterior. Biodegrad. 2017, 120, 84–90. [Google Scholar] [CrossRef]
- Beier, O.; Pfuch, A.; Horn, K.; Weisser, J.; Schnabelrauch, M.; Schimanski, A. Low Temperature Deposition of Antibacterially Active Silicon Oxide Layers Containing Silver Nanoparticles, Prepared by Atmospheric Pressure Plasma Chemical Vapor Deposition. Plasma Process. Polym. 2013, 10, 77–87. [Google Scholar] [CrossRef]
- Gerullis, S.; Pfuch, A.; Spange, S.; Kettner, F.; Plaschkies, K.; Küzün, B.; Kosmachev, P.V.; Volokitin, G.G.; Grünler, B. Thin antimicrobial silver, copper or zinc containing SiOx films on wood polymer composites (WPC) applied by atmospheric pressure plasma chemical vapour deposition (APCVD) and sol–gel technology. Eur. J. Wood Prod. 2018, 76, 229–241. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; Sung, L.-P. Wood surface modification by in-situ sol-gel deposition of hybrid inorganic–organic thin films. J. Coat. Technol. Res. 2007, 4, 483–490. [Google Scholar] [CrossRef]
- Köhler, R.; Siebert, D.; Kochanneck, L.; Ohms, G.; Viöl, W. Bismuth Oxide Faceted Structures as a Photocatalyst Produced Using an Atmospheric Pressure Plasma Jet. Catalysts 2019, 9, 533. [Google Scholar] [CrossRef]
- Meyer-Veltrup, L.; Brischke, C.; Alfredsen, G.; Humar, M.; Flæte, P.-O.; Isaksson, T.; Brelid, P.L.; Westin, M.; Jermer, J. The combined effect of wetting ability and durability on outdoor performance of wood: Development and verification of a new prediction approach. Wood Sci. Technol. 2017, 51, 615–637. [Google Scholar] [CrossRef]
- Meng, L.; Xu, W.; Zhang, Q.; Yang, T.; Shi, S. Study of nanostructural bismuth oxide films prepared by radio frequency reactive magnetron sputtering. Appl. Surf. Sci. 2019, 472, 165–171. [Google Scholar] [CrossRef]
- Chang, B.; Liu, Q.; Chen, N.; Yang, Y. A Flower-like Bismuth Oxide as an Efficient, Durable and Selective Electrocatalyst for Artificial N 2 Fixation in Ambient Condition. ChemCatChem 2019, 11, 1884–1888. [Google Scholar] [CrossRef]
- Kowalska, E.; Wei, Z.; Karabiyik, B.; Herissan, A.; Janczarek, M.; Endo, M.; Markowska-Szczupak, A.; Remita, H.; Ohtani, B. Silver-modified titania with enhanced photocatalytic and antimicrobial properties under UV and visible light irradiation. Catal. Today 2015, 252, 136–142. [Google Scholar] [CrossRef]
- Maiti, N.; Thomas, S.; Debnath, A.; Kapoor, S. Raman and XPS study on the interaction of taurine with silver nanoparticles. RSC Adv. 2016, 6, 56406–56411. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Ivanova, T.; Homola, T.; Bryukvin, A.; Cameron, D. Catalytic Performance of Ag2O and Ag Doped CeO2 Prepared by Atomic Layer Deposition for Diesel Soot Oxidation. Coatings 2018, 8, 237. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Chen, D.-H. Microwave-assisted green synthesis of Ag/reduced graphene oxide nanocomposite as a surface-enhanced Raman scattering substrate with high uniformity. Nanoscale Res. Lett. 2014, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.B.; Powell, M.J.; Parkin, I.P.; Carmalt, C.J. Aluminum /gallium, indium/gallium, and aluminum /indium co-doped ZnO thin films deposited via aerosol assisted CVD. J. Mater. Chem. C 2018, 6, 588–597. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Astuti, Y.; Fauziyah, A.; Nurhayati, S.; Wulansari, A.D.; Andianingrum, R.; Hakim, A.R.; Bhaduri, G. Synthesis of α-Bismuth oxide using solution combustion method and its photocatalytic properties. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 12006. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Köhler, R.; Ohms, G.; Militz, H.; Viöl, W. Atmospheric Pressure Plasma Coating of Bismuth Oxide Circular Droplets. Coatings 2018, 8, 312. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xiong, T.; Jiang, G.; Zhang, Y.; Wu, Z.; Dong, F. Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible-light photocatalysis. J. Catal. 2017, 352, 102–112. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Yang, J.; Chen, Z.; Zhang, W.; Zhou, L.; Liu, S. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Appl. Catal. A Gen. 2006, 308, 105–110. [Google Scholar] [CrossRef]
- Jiang, M.-M.; Chen, H.-Y.; Li, B.-H.; Liu, K.-W.; Shan, C.-X.; Shen, D.-Z. Hybrid quadrupolar resonances stimulated at short wavelengths using coupled plasmonic silver nanoparticle aggregation. J. Mater. Chem. C 2014, 2, 56–63. [Google Scholar] [CrossRef]
- Pawar, O.; Deshpande, N.; Dagade, S.; Waghmode, S.; Nigam Joshi, P. Green synthesis of silver nanoparticles from purple acid phosphatase apoenzyme isolated from a new source Limonia acidissima. J. Exp. Nanosci. 2016, 11, 28–37. [Google Scholar] [CrossRef]
- Klantsataya, E.; François, A.; Ebendorff-Heidepriem, H.; Sciacca, B.; Zuber, A.; Monro, T.M. Effect of surface roughness on metal enhanced fluorescence in planar substrates and optical fibers. Opt. Mater. Express 2016, 6, 2128. [Google Scholar] [CrossRef] [Green Version]
- Feng, A.L.; You, M.L.; Tian, L.; Singamaneni, S.; Liu, M.; Duan, Z.; Lu, T.J.; Xu, F.; Lin, M. Distance-dependent plasmon-enhanced fluorescence of upconversion nanoparticles using polyelectrolyte multilayers as tunable spacers. Sci. Rep. 2015, 5, 7779. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Rifat, A.A.; Mahdiraji, G.A.; Chow, D.M.; Shee, Y.G.; Ahmed, R.; Adikan, F.R.M. Photonic crystal fiber-based surface plasmon resonance sensor with selective analyte channels and graphene-silver deposited core. Sensors (Basel) 2015, 15, 11499–11510. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Choi, H.; Kim, J.Y.; Lee, T.-W. Silver-Based Nanoparticles for Surface Plasmon Resonance in Organic Optoelectronics. Part. Part. Syst. Charact. 2015, 32, 164–175. [Google Scholar] [CrossRef]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Annealed silver-island films for applications in metal-enhanced fluorescence: Interpretation in terms of radiating plasmons. J. Fluoresc. 2005, 15, 643–654. [Google Scholar] [CrossRef]


| Powder | Plasma Power (%) | Process Gas Flow Rate (L/min) | Substrate Scan Speed (mm/s) | Powder Feed Rate (m³/h) | Powder Feed Speed (mm/h) |
|---|---|---|---|---|---|
| Polyester | 100 | 60 | 40 | 4.3 | 150 |
| Metal oxide/metal | 100 | 60 | 40 | 2.2 | 100 |
| Sample | C 1s | O 1s | Al 2p | Ag 3d5 | Bi 4f | F 1s |
|---|---|---|---|---|---|---|
| Beech reference | 68.14 | 31.86 | – | – | – | – |
| Pine reference | 71.22 | 28.78 | – | – | – | – |
| Beech P | 77.81 | 21.93 | – | – | – | 0.26 |
| Pine P | 77.98 | 21.7 | – | – | – | 0.32 |
| Beech Bi | 50.58 | 32.76 | – | – | 16.66 | – |
| Pine Bi | 63.63 | 24.75 | – | – | 11.62 | – |
| Beech PBi | 77.18 | 22.39 | – | – | 0.18 | 0.25 |
| Pine PBi | 77.3 | 22.29 | – | – | 0.15 | 0.26 |
| Beech eCon | 58.07 | 34.92 | 3.76 | 3.25 | – | – |
| Pine eCon | 58.24 | 32.82 | 5.09 | 3.85 | – | – |
| Beech PeCon | 77.13 | 22.17 | 0 | 0.21 | – | 0.49 |
| Pine PeCon | 77.05 | 22.51 | 0 | 0.22 | – | 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, R.; Sauerbier, P.; Ohms, G.; Viöl, W.; Militz, H. Wood Protection through Plasma Powder Deposition—An Alternative Coating Process. Forests 2019, 10, 898. https://doi.org/10.3390/f10100898
Köhler R, Sauerbier P, Ohms G, Viöl W, Militz H. Wood Protection through Plasma Powder Deposition—An Alternative Coating Process. Forests. 2019; 10(10):898. https://doi.org/10.3390/f10100898
Chicago/Turabian StyleKöhler, Robert, Philipp Sauerbier, Gisela Ohms, Wolfgang Viöl, and Holger Militz. 2019. "Wood Protection through Plasma Powder Deposition—An Alternative Coating Process" Forests 10, no. 10: 898. https://doi.org/10.3390/f10100898





