Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Study Design and Sampling Scheme
2.3. ECM Assessment
2.4. Identification of Mycorrhizal Fungi
2.5. Fine root and Tree Health Assessment
2.6. Physicochemical Analysis of the Soil
2.7. Data Analysis
3. Results
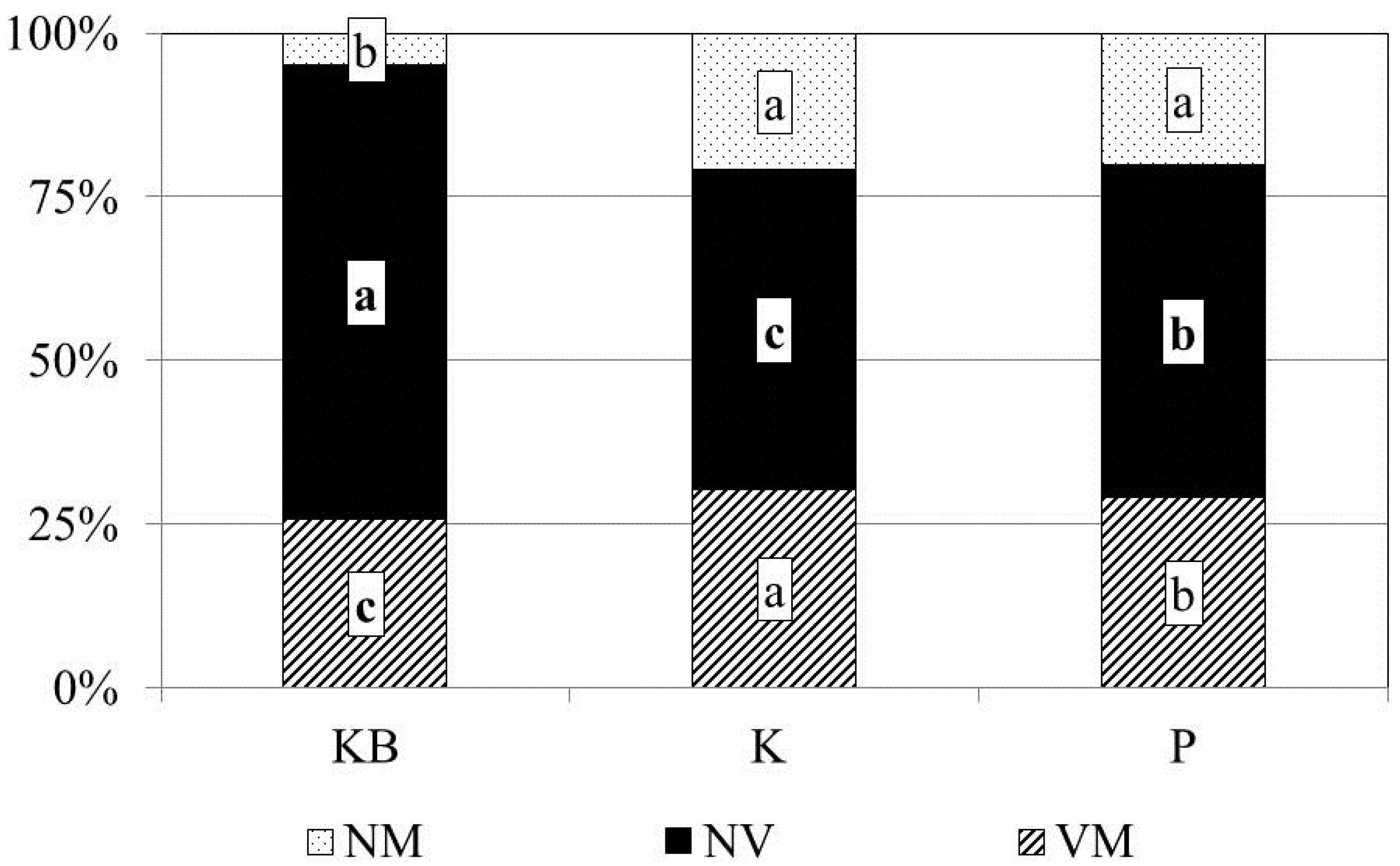
3.1. Mycorrhizal Colonisation
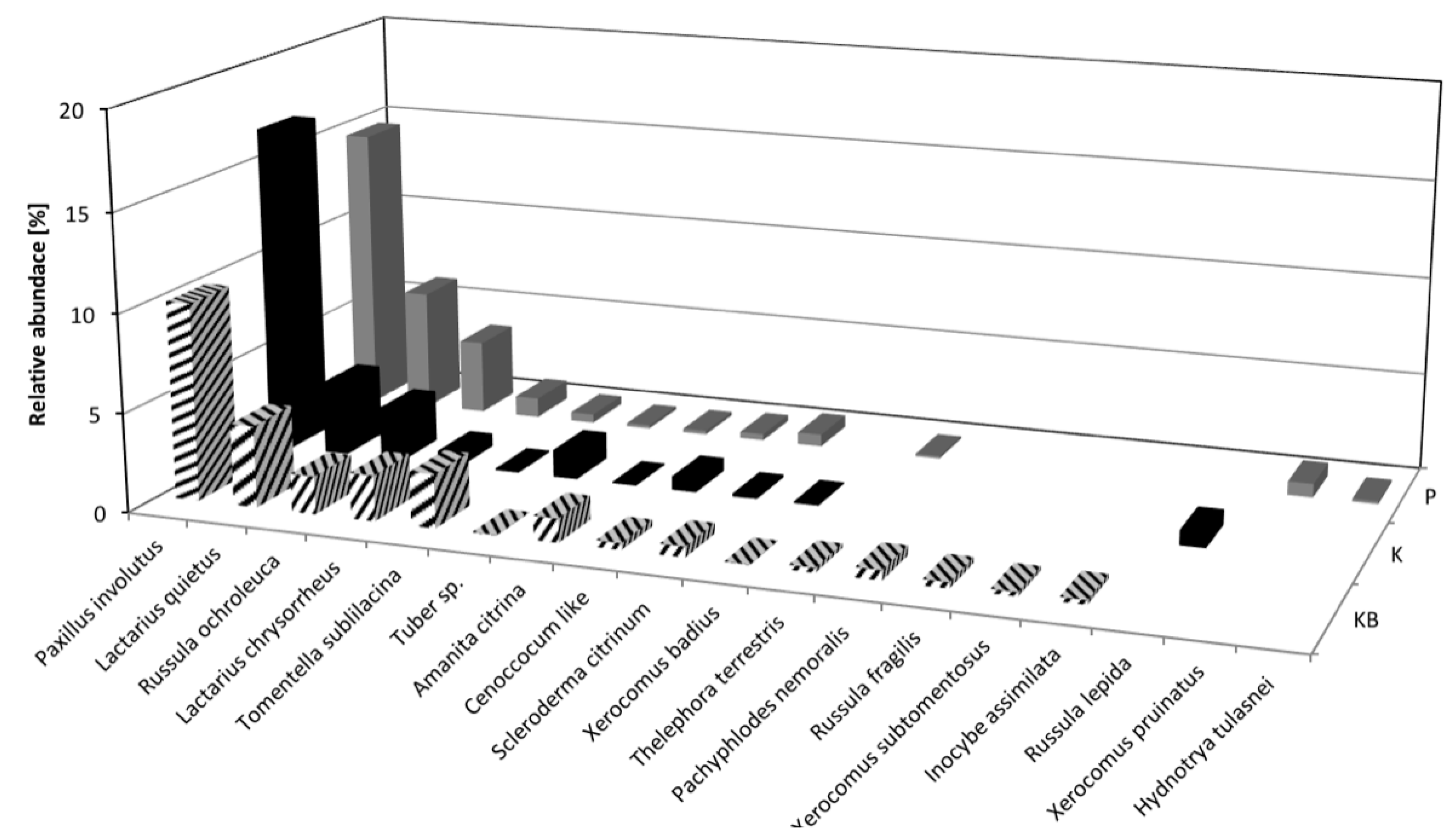
3.2. Ectomycorrhizal Fungal Communities
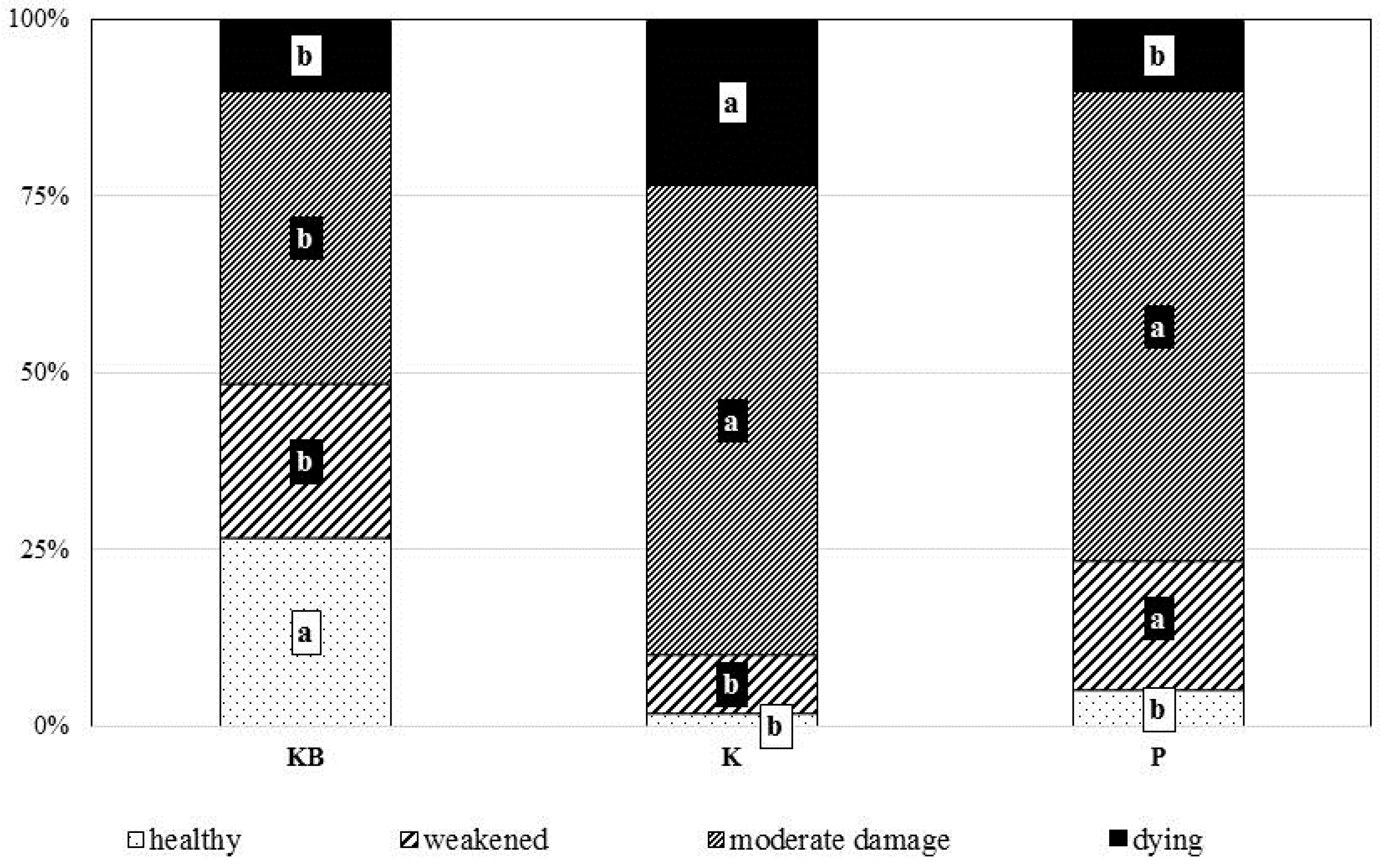
3.3. Crown Health Status and Biometrical Parameters of Root Systems
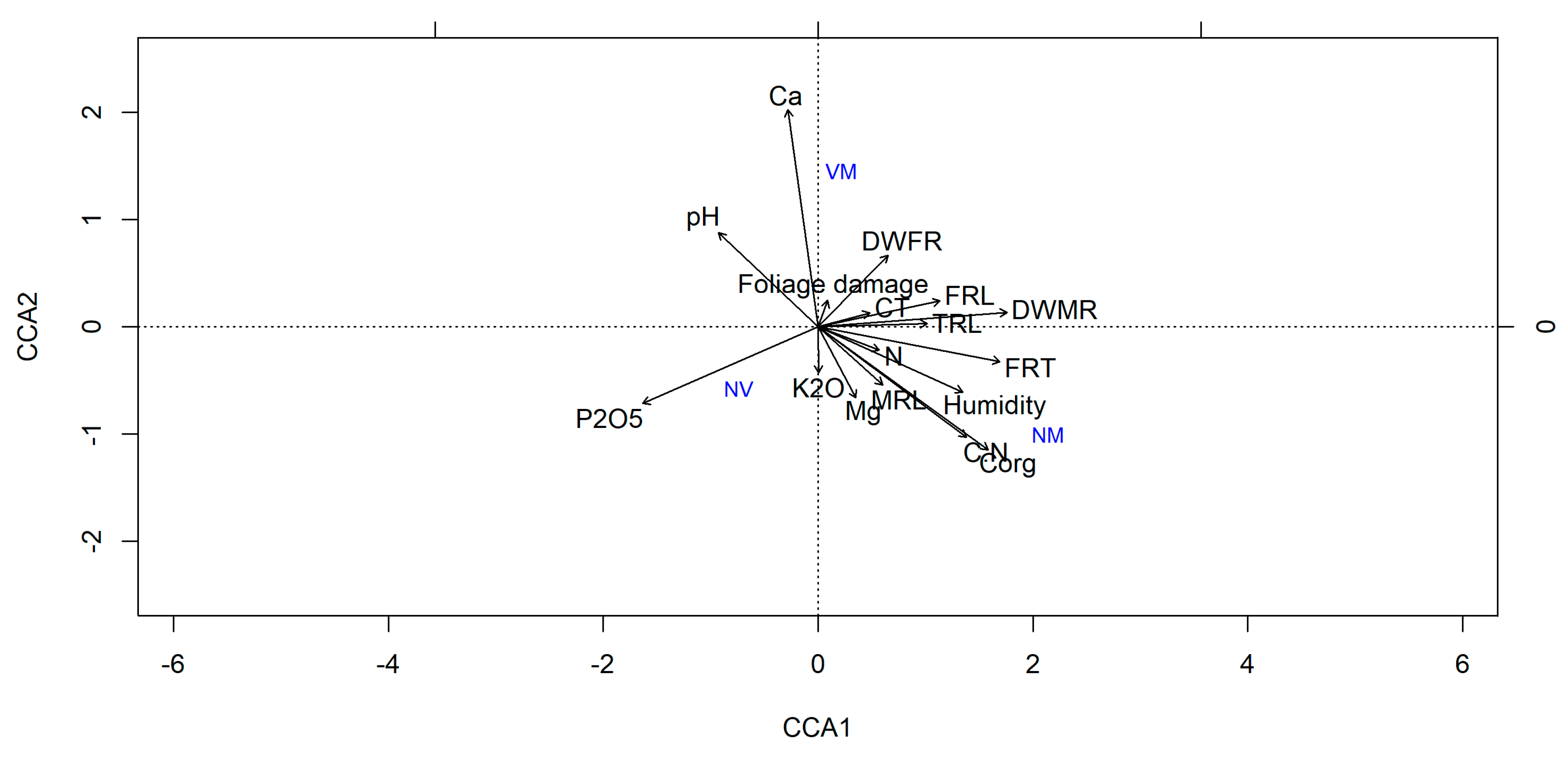
3.4. Soil Substrate Properties
4. Discussion
4.1. Degree of Mycorrhizal Colonisation
4.2. ECM Symbiosis and Fungal Community Composition
4.3. Ecological Importance of Exploration Types of Ectomycorrhizae
4.4. Tree Health Status and Ectomycorrhizal Colonization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zanetto, A.; Roussel, G.; Kremer, A. Geographic variation of inter-specific differentiation between Quercus robur L. and Quercus petrea (Matt) Liebl. For. Genet. 1994, 2, 111–123. [Google Scholar]
- Ducousso, A.; Bordacs, S. Pedunculate and sessile oaks (Quercus robur and Quercus petraea). In Technical Guidelines for Genetic Conservation and Use; EUFORGEN: Bonn, Germany, 2003; pp. 1–6. [Google Scholar]
- Bréda, N.; Cochard, H.; Dreyer, E.; Granier, A. Field comparison of transpiration, stomatal conductance and vulnerability to cavitation of Quercus petraea and Quercus robur under water stress. Ann. Sci. For. 1993, 50, 571–582. [Google Scholar] [CrossRef]
- Bourtsoukidis, E.; Kawaletz, H.; Radacki, D.; Schütz, S.; Hakola, H.; Hellén, H. Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees 2014, 28, 193–204. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration patterns of European oak species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in dependence of environment and neighborhood. PLoS ONE 2015, 12, e10. [Google Scholar] [CrossRef] [PubMed]
- Falck, R. Oak decline in Lödderitz forest district and in Westphalia. Z. Forst. Jagdwes. 1918, 50, 123–132. [Google Scholar]
- Hansen, E.; Delatour, C. Phytophthora species in oak forests of north-east France. Ann. For. Sci. 1999, 56, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Tarasiuk, S.; Szczepkowski, A. The health status of endangered oak stands in Poland. Acta Sci. Pol. Silv. Colendar. Ratio Ind. Lignar. 2006, 5, 91–106. [Google Scholar]
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A description of the symptoms of Acute Oak Decline in Britain and a comparative review on causes of similar disorders on oak in Europe. Forestry 2014, 87, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Montecchio, L.; Causin, R.; Rossi, S.; Mutto Accordi, S. Changes in ectomycorrhizal diversity in a declining Quercus ilex coastal forest. Phytopathol. Mediterr. 2004, 43, 26–34. [Google Scholar]
- Power, S.A.; Ashmore, M.R. Nutrient relations and root mycorrhizal status of healthy and declining beech (Fagus sylvatica L) in southern Britain. Water Air Soil Pollut. 1996, 86, 317–333. [Google Scholar] [CrossRef]
- Kovacs, G.; Pausch, M.; Urban, A. Diversity of ectomycorrhizal morphotypes and oak decline. Phyton-Ann. Rei. Bot. A 2000, 40, 109–116. [Google Scholar]
- Pestana, M.; Santolamazza, S. Defoliation negatively affects plant growth and the ectomycorrhizal community of Pinus pinaster in Spain. Oecology 2011, 165, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, L.; Dal Maso, E.; Mutto Accordi, S.; Sella, L.; Montecchio, L. Detecting asymptomatic ink-diseased chestnut trees by the composition of the ectomycorrhizal community. For. Pathol. 2012, 42, 501–509. [Google Scholar] [CrossRef]
- Kernaghan, G.; Harper, K.A. Community structure of ectomycorrhizal fungi across an alpine/subalpine ecotone. Ecography 2001, 24, 181–188. [Google Scholar] [CrossRef]
- Erland, S.; Taylor, A.F.S. Diversity of ectomycorrhizal fungal communities in relation to the abiotic environment. In Mycorrhizal Ecology; Van der Heijden, M.G.A., Sanders, I., Eds.; Springer: Berlin, Germany, 2002; pp. 163–200. [Google Scholar]
- Jones, M.D.; Durall, D.M.; Cairney, J.W.G. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 2003, 157, 399–422. [Google Scholar] [CrossRef] [Green Version]
- Richard, F.; Moreau, P.; Selosse, M.; Gardes, M. Diversity and fruiting patterns of ectomycorrhizal and saprobic fungi in an old-growth Mediterranean forest dominated by Quercus ilex L. Can. J. Bot. 2004, 82, 1711–1729. [Google Scholar] [CrossRef]
- Kernaghan, G. Mycorrhizal diversity: Cause and effect. Pedobiologia 2005, 49, 511–520. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Dickie, I. Does host plant richness explain diversity of ectomycorrhizal fungi? Re-evaluation of Gao et al. (2013) data sets reveals sampling effects. Mol. Ecol. 2014, 23, 992–995. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.; Douma, J.; Akhmetzhanova, A.; van Bodegom, P.; Cornwell, W.; Moens, E.; Treseder, K.; Tibbett, M.; Wang, Y.; Cornelissen, J. Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob. Ecol. Biogeogr. 2015, 24, 371–382. [Google Scholar] [CrossRef]
- Kuikka, K.; Härmä, E.; Markkola, A.; Rautio, P.; Roitto, M.; Saikkonen, K.; Ahonen-Jonnarth, U.; Finlay, R.; Tuomi, J. Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 2003, 84, 2051–2061. [Google Scholar] [CrossRef]
- Saravesi, K.; Markkola, A.M.; Rautio, P.; Roitto, M.; Tuomi, J. Defoliation causes parallel temporal responses in a host tree and its fungal symbionts. Oecology 2008, 156, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Corcobado, T.; Vivas, M.; Moreno, G.; Solla, A. Ectomycorrhizal symbiosis in declining and non-declining Quercus ilex trees infected with or free of Phytophthora cinnamomi. For. Ecol. Manag. 2014, 324, 72–80. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Boratyński, A.; Boratyńska, K.; Filipiak, M. Morfologia, systematyka i geograficzne rozmieszczenie. Systematyka i rozmieszczenie [Morphology, systematics and geographical distribution. Systematics and distribution]. In Nasze Drzewa Leśne. Monografie Popularnonaukowe [Our Forest Trees. Popular Science Monographs]; Polska Akademia Nauk, Instytut Dendrologii, Poznań: Kórnik, Poland, 2006; Volume 11. [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae, 1st ed.; Einhorn-Verlag: Munich, Germany, 1987–2008. [Google Scholar]
- Agerer, R.; Rambold, G. DEEMY-an Information System for Characterization and Determination of Ectomycorrhizae. 2004–2015. Available online: http://www.deemy.de (accessed on 12 November 2018).
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Thermofisher.com. Available online: https://www.thermofisher.com (accessed on 15 November 2018).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Ncbi.nlm.nih.gov. Available online: http://www.ncbi.nlm.nih.gov (accessed on 15 November 2018).
- Roloff, A. Kronenentwicklung und Vitalitätsbeurteilung Ausgewählter Baumarten der Gemäßigten Breiten; Schriften aus der Forstlichen Fakultät der Universität Göttingen und der Niedersächsischen Forstlichen Versuchsanstalt: Frankfurt am Main, Germany, 1989. [Google Scholar]
- Dmyterko, E. Methods of assessing damage in oak stands. Sylwan 1998, 10, 29–38. [Google Scholar]
- Thien, S.J. A flow diagram for teaching texture by feel analysis. J. Agron. Educ. 1979, 8, 54–55. [Google Scholar]
- Anderson, D.W.; Saggar, S.; Bettany, J.R.; Stewart, J.W.B. Particle size fractionation and their use in studies of Soil Organic Matter: I. The Nature and Distribution of Forms of Carbon, Nitrogen, and Sulfur. Soil Sci. Soc. Am. J. 1981, 48, 298–301. [Google Scholar] [CrossRef]
- Schlichting, E.; Blume, H.P.; Stahr, K. Bodenkundliches Praktikum; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995. [Google Scholar]
- R Core Team. R A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R-project.org/ (accessed on 15 November 2018).
- Oksanen, J.; Blanche, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B. Vegan: Community Ecology Package. R Package Version 2.4-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 15 November 2018).
- Courty, P.E.; Breda, N.; Garbaye, J. Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol. Biochem. 2007, 39, 1655–1663. [Google Scholar] [CrossRef]
- Jakucs, E.; Eros-Honti, Z. Morphological-anatomical characterization and identification of Tomentella ectomycorrhizas. Mycorrhiza 2008, 18, 277–278. [Google Scholar] [CrossRef]
- Eyre, C.; Muftah, W.; Hiscox, J.; Hunt, J.; Kille, P.; Boddy, L.; Rogers, H.J. Microarray analysis of differential gene expression elicited in Trametes versicolor during interspecific mycelial interactions. Fungal Biol. 2010, 114, 646–660. [Google Scholar] [CrossRef]
- Mac Nally, R.C. Ecological Versatility and Community Ecology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Pena, R.; Lang, C.; Naumann, A.; Polle, A. Ectomycorrhizal identification in environmental samples of tree roots by Fourier-transform infrared (FTIR) spectroscopy. Front. Plant Sci. 2014, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ainsworth, M. Antrodia ramentacea on Salix in s.e. England. Field Mycol. 2001, 2, 46–49. [Google Scholar] [CrossRef]
- Yurkov, A.; Wehde, T.; Kahl, T.; Begerow, D. Aboveground Deadwood Deposition Supports Development of Soil Yeasts. Diversity 2012, 4, 453–474. [Google Scholar] [CrossRef] [Green Version]
- Chlebicki, A. Fungi on higher plants of the upper limit of alpine zone in Tian Shan. Mycotaxon 2010, 110, 451–454. [Google Scholar] [CrossRef]
- Itoo, Z.A.; Reshi, Z.A.; Andrabi, K.I. Characterization and identification of Russula firmula and Russula postiana from Himalayan moist temperate forests of Kashmir. Afr. J. Biotechnol. 2013, 12, 3643–3647. [Google Scholar]
- Folman, L.B.; Klein Gunnewiek, P.J.A.; Boddy, L.; de Boer, W. Impact of white-rot fungi on numbers and community composition of bacteria colonizing beech wood from forest soil. FEMS Microbiol. Ecol. 2008, 63, 181–191. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, D.J.; Spatafora, J.W. Systematics and Evolution; Springer Science and Business Media: Berlin, Germany, 2013. [Google Scholar]
- Murat, C.; Vizzini, A.; Bonfante, P.; Mello, A. Morphological and molecular typing of the below-ground fungal community in a natural Tuber magnatum truffle-ground. FEMS Microbiol. Lett. 2005, 245, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.; Groenewald, J.Z.; Cecília, R.; Oliveira, H.; Crous, P.W. Cite as Cylindrocarpon root rot: Multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef]
- Bushley, KE.; Raja, R.; Jaiswal, P.; Cumbie, J.S.; Nonogaki, M.; Boyd, A.E.; Owensby, C.A.; Knaus, B.J.; Elser, J.; Miller, D.; et al. The Genome of Tolypocladium inflatum: Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster. PLoS Genet. 2013, 9, e1003496. [Google Scholar] [CrossRef]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants an Identification Handbook; Richmond Publishing: Puebla, Mexico, 1997. [Google Scholar]
- Healy, R.; Hobart, C.; Tocci, G.E.; Bóna, L.; Merényi, Z.; Paz Conde, A.; Smith, M.E. Fun with the discomycetes: Revisiting collections of Korf’s anamorphic Pezizales and Thaxter’s New England truffles leads to a connection between forms and the description of two new truffle species: Pachyphlodes pfisteri and P. nemoralis. Ascomycete 2015, 7, 357–366. [Google Scholar]
- Suz, L.M.; Barsoum, N.; Benham, S.; Dietrich, H.P.; Fetzer, K.D.; Fischer, R.; García, P.; Gehrman, J.; Kristöfel, F.; Manninger, M.; et al. Environmental drivers of ectomycorrhizal communities in Europe’s temperate oak forests. Mol. Ecol. 2014, 23, 5628–5644. [Google Scholar] [CrossRef]
- Fodor, E.; Timofte, A.; Geambau, T. Mycorrhizal status of several Quercus species in Romania (Quercus cerris, Q. frainetto, Q. robur) and the optimization perspective of growth conditions for in vitro propagated plants transplanted in the field. Ann. For. Res. 2011, 54, 57–71. [Google Scholar]
- Bakker, M.R.; Garbaye, J.; Nys, C. Effect of liming on the ectomycorrhizal status of oak. For. Ecol. Manag. 2000, 126, 121–131. [Google Scholar] [CrossRef]
- Børja, I.; Nilsen, P. Long term effect of liming and fertilization on ectomicorrhizal colonization and tree growth in old scots pine (Pinus sylvestris L.) stand. Plant Soil 2009, 314, 109–119. [Google Scholar] [CrossRef]
- Nowotny, I.; Dähne, J.; Klingelhöfer, D.; Rothe, G.M. Effect of artificial soil acidification and liming on growth and nutrient status of mycorrhizal roots of Norway spruce (Picea abies [L.] Karst.). Plant Soil 1998, 199, 29–40. [Google Scholar] [CrossRef]
- Marx, D.H.; Zak, B. Effect of pH on mycorrhizal formation of slash pine in aseptic culture. For. Sci. 1965, 11, 66–75. [Google Scholar]
- Erland, S.; Söderström, B. Effect of liming on ectomycorrhizal fungi infecting Pinus sylvestris L. 1. Mycorrhizal infection in limed humus in the laboratory and isolation of fungi from mycorrhizal roots. New Phytol. 1991, 115, 675–682. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef]
- Iwanski, M.; Rudawska, M.; Leski, T. Mycorrhizal associationns of nursery grown Scots pine (Pinus sylvestris L.) seedlings in Poland. Ann. For. Sci. 2006, 639, 715–724. [Google Scholar] [CrossRef]
- Bougher, N.L.; Grove, T.S.; Malajczuk, N. Growth and phosphorus acquisition of karri (Eucalyptus diversicolor F. Muell.) seedlings inoculated with ectomycorrhizal fungi in relation to phosphorus supply. New Phytol. 1990, 114, 77–85. [Google Scholar] [CrossRef]
- Ibáñez, B.; Gómez-Aparicio, L.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Marañón, T. Impact of tree decline on spatial patterns of seedling-mycorrhiza interactions: Implications for regeneration dynamics in Mediterranean forests. For. Ecol. Manag. 2015, 353, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Giltrap, N.J.; Lewis, D.H. Inhibition of growth of Ectomycorrhizal Fungi in culture by phosphate. New Phytol. 1981, 87, 669–675. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Baier, R.; Ettl, R.; Hahn, C.; Göttlein, A. Early development and nutrition of Norway spruce (Picea abies (L.) Karst.) seedlings on different seedbeds in the Bavarian limestone Alps—A bioassay. Ann. For. Sci. 2006, 63, 339–348. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Hendrick, R.L.; Fogel, R. The demography of fine roots in response to patches of water and nitrogen. New Phytol. 1993, 125, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Kjøller, R.; Nilsson, L.-O.; Hansen, K.; Schmidt, I.K.; Vesterdal, L.; Gundersen, P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol. 2012, 194, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Arnebrant, K. Nitrogen amendments reduce the growth of extramatrical ectomycorrhizal mycelium. Mycorrhiza 1994, 5, 7–15. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Wallander, H. Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol. 2003, 158, 409–416. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R. The effect of excess nitrogen and of insect defoliation on the frost hardiness of bark tissue of adult oaks. Ann. For. Sci. 1996, 53, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Richard, F.; Roy, M.; Shahin, O.; Sthultz, C.; Duchemin, M.; Joffre, R.; Selosse, M. Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: Seasonal dynamics and response to drought in the surface organic horizon. Ann. For. Sci. 2011, 68, 57–68. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Harrington, T.J. The macrofungal diversity and community of Atlantic oak (Quercus petraea and Q. robur) forests in Ireland. Anales del Jardín Botánico de Madrid 2012, 69, 107–117. [Google Scholar] [CrossRef]
- Trocha, L.K.; Kałucka, I.; Stasińska, M.; Nowak, W.; Dabert, M.; Leski, T.; Rudawska, M.; Oleksyn, J. Ectomycorrhizal fungal communities of native and non-native Pinus and Quercus species in a common garden of 35-year-old trees. Mycorrhiza 2012, 22, 121–134. [Google Scholar] [CrossRef]
- Keizer, P.J.; Arnolds, E. Succession of ectomycorrhizal fungi in roadside verges planted with common oak (Quercus robur L.) in Drenthe, The Netherlands. Mycorrhiza 1994, 4, 147–159. [Google Scholar] [CrossRef]
- Finlay, R.D.; Söderström, B. Mycorrhiza and carbon flow to the soil. In Mycorrhizal Functioning an Integrative Plant-Fungal Process; Allen, M.J., Ed.; Chapman and Hall: New York, NY, USA, 1992; pp. 134–160. [Google Scholar]
- Mosca, E.; Montecchio, L.; Sella, L.; Garbaye, J. Short-term effect of removing tree competition on the ectomycorrhizal status of a declining pedunculate oak forest (Quercus robur L.). For. Ecol. Manag. 2007, 244, 129–140. [Google Scholar] [CrossRef]
- Hagerman, S.H.; Sakakibara, S.M.; Durall, D.M. The potential for woody understory plants to provide refuge for ectomycorrhizal inoculum at an interior Douglas-fir forest after clear-cut logging. Can. J. For. Res. 2001, 31, 711–721. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M.; Aučina, A. The ectomycorrhizal status of European larch (Larix decidua Mill.) seedlings from bareroot forest nurseries. For. Ecol. Manag. 2008, 256, 2136–2144. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Stasinska, M. Species and functional diversity of ectomycorrhizal fungal communities on Scots pine (Pinus sylvestris L.) trees on three different sites. Ann. For. Sci. 2011, 68, 5–15. [Google Scholar] [CrossRef]
- Rousseau, J.V.D.; Sylvia, D.M.; Fox, A.J. Contribution of ectomycorrhizal to the potential nutrient-absorbing surface of pine. New Phytol. 1994, 128, 639–644. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Shahin, O.; Martin-St, P.N.; Rambal, S.; Joffre, R.; Richard, F. Ectomycorrhizal fungal diversity in Quercus ilex Mediterranean woodlands: Variation among sites and over soil depth profiles in hyphal exploration types, species richness and community composition. Symbiosis 2013, 61, 1–12. [Google Scholar] [CrossRef]
- Moeller, H.V.; Peay, K.G.; Fukami, T. Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol. Ecol. 2013, 87, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Van Schöll, L.; Kuyper, T.W.; Smits, M.M.; Landeweert, R.; Hoffland, E.; Van Breemen, N. Rock-eating mycorrhizas: Their role in plant nutrition and biogeochemical cycles. Plant Soil 2008, 303, 35–47. [Google Scholar] [CrossRef]
- Laiho, O. Paxillus involutus as a mycorrhizal symbiont of forest trees. Acta For. Fenn. 1970, 106, 1–65. [Google Scholar] [CrossRef]
- Conjeaud, C.; Scheromm, P.; Mousain, D. Effects of phosphorus and ectomycorrhiza on maritime pine seedlings (Pinus pinaster). New Phytol. 1996, 133, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.R. Fine-root parameters as indicators of sustainability of forest ecosystems. For. Ecol. Manag. 1999, 122, 7–16. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Blaschke, H.; Osswald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef] [Green Version]
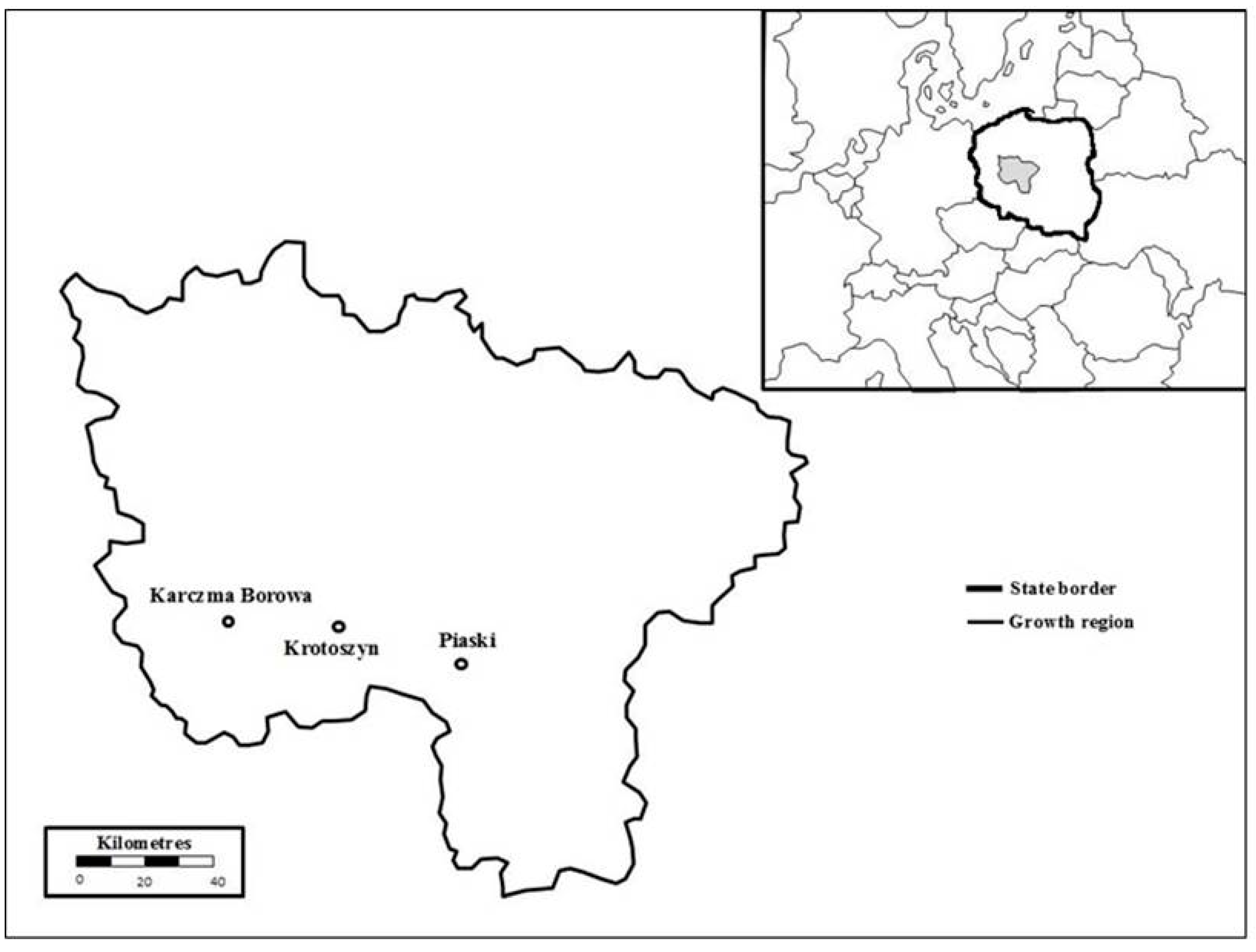



| Forest Inspectorate | Karczma Borowa | Krotoszyn | Piaski |
|---|---|---|---|
| Geographic coordinates | 51°50′51″ N, 16°37′34″ E | 51°42′11″ N, 17°33′52″ E | 51°49′36.4″ N 17°06′46.6″ E |
| Elevation (m) | 77 m.a.s.l | 127 m.a.s.l | 58 m.a.s.l |
| Area size (ha) | 5 | 5 | 0.05 |
| Average annual temperature (°C) | 8.3 | 8.0 | 8.5 |
| Average annual precipitation (mm) | 550 | 450 | 550 |
| Length of the growing season (days) | 226 | 210 | 220 |
| Bedrock | glacial clay | glacial tills | moraine clay |
| Soil type | luvisols | brown soil | brown rendzinas |
| Age of stands (years) | 120 | 120 | 60 |
| Forest stand | Quercus robur (60%), Corylus avellana (10%), Carpinus betulus (30%), sparse natural regeneration | Quercus robur (70%), Corylus avellana (10%), Carpinus betulus (20%) | Quercus robur (70%), Picea abies (30%) |
| Plant community | Galio silvatici-Carpinetum | Potentillo albae-Quercetum Libb | Tilio Carpinetum |
| Past history and status of stands | timber forests planted in previously oak stands | ||
| Ground water status | depended on precipitation | ||
| Identification | BLAST Top-Hit | Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Closest Match | NCBI | Identity [%] | Putative Ecology | References * | KB [n = 60] | K [n = 60] | P [n = 60] | |||||
| Freq. | Abun. | Freq. | Abun. | Freq. | Abun. | |||||||
| Basidiomycota | ||||||||||||
| Paxillus involutus | Paxillus involutus | KT334655 | 99 | ECM | [28] | 60.0 | 8.7 | 73.3 | 16.3 | 80.0 | 12.8 | |
| Russula ochroleuca | Russula ochroleuca | KT334671 | 100 | ECM | [28] | 15.0 | 2.9 | 35.0 | 4.6 | 25.0 | 5.1 | |
| Lactarius quietus | Lactarius quietus | KT334684 | 99 | ECM | [41] | 16.7 | 5.1 | 16.7 | 4.9 | 26.7 | 5.7 | |
| Tomentella sublilacina | Tomentella sublilacina | KT334657 | 100 | ECM | [42] | 11.7 | 3.0 | 5.0 | 0.1 | 5.0 | 1.4 | |
| Lactarius chrysorrheus | Lactarius chrysorrheus | KT334686 | 100 | ECM | [28] | 8.3 | 2.5 | 5.0 | 1.2 | 5.0 | 1.1 | |
| Scleroderma citrinum | Scleroderma citrinum | KT334757 | 99 | ECM | [28] | 5.0 | 0.4 | 3.3 | 0.2 | 6.7 | 1.6 | |
| Amanita citrina | Amanita citrina | KT334692 | 99 | ECM | [28] | 3.3 | 0.8 | 1.7 | 0.1 | 1.7 | 0.2 | |
| Thelephora terrestris | Thelephora terrestris | KT334743 | 99 | ECM | [28] | 1.7 | 0.2 | - | - | 5.0 | 0.1 | |
| Trametes versicolor | Trametes versicolor | KT334660 | 99 | Sapr | [43] | - | - | 3.3 | 0.6 | 1.7 | 0.1 | |
| Russula lepida | Russula lepida | KT334672 | 99 | ECM | [44] | - | - | 3.3 | 0.3 | - | - | |
| Xerocomus pruinatus | Xerocomus pruinatus | KT334702 | 100 | ECM | [45] | - | - | - | - | 5.0 | 0.5 | |
| Xerocomus subtomentosus | Xerocomus subtomentosus | KT334687 | 100 | ECM | [28] | 3.3 | 0.4 | - | - | - | - | |
| Xerocomus badius | Xerocomus badius | KT334738 | 100 | ECM | [28] | 1.7 | 0.02 | 1.7 | 0.1 | - | - | |
| Sporidiobolus metaroseus | Sporidiobolus metaroseus | KT334689 | 100 | Endoph | [46] | - | - | 1.7 | 0.5 | - | - | |
| Antrodia ramentacea | Antrodia ramentacea | KT334658 | 100 | Sapr | [47] | - | - | - | - | 1.7 | 0.6 | |
| Cryptococcus terricola | Cryptococcus terricola | HE863714 | 99 | Sapr | [48] | - | - | 1.7 | 0.3 | - | - | |
| Lagarobasidium detriticum | Lagarobasidium detriticum | KT334691 | 99 | Sapr | [49] | - | - | - | - | 1.7 | 0.5 | |
| Russula fragilis | Russula fragilis | KT334670 | 99 | ECM | [50] | 1.7 | 0.3 | - | - | - | - | |
| Inocybe assimilata | Inocybe assimilata | FN393147 | 99 | ECM | [28] | 1.7 | 0.2 | - | - | - | - | |
| Hypholoma fasciculare | Hypholoma fasciculare | KT334676 | 99 | Sapr | [51] | - | - | 1.7 | 0.1 | - | - | |
| Ascomycota | ||||||||||||
| Unidentified ascomycete | Uncultured ascomycete (Chaetothyriales. cf. Capronia) | KT334693 | 98 | Endoph | [52] | 3.3 | 0.1 | 3.3 | 0.1 | 3.3 | 0.1 | |
| Tuber sp. | Tuber sp. | KT334690 | 99 | ECM | [53] | 1.7 | 0.04 | 5.0 | 1.4 | 1.7 | 0.2 | |
| Cenoccocum-like | ECM | [28] | 3.3 | 0.8 | 3.3 | 0.9 | 1.7 | 0.1 | ||||
| Ilyonectria radicicola | Ilyonectria radicicola | KT334654 | 99 | Pl path | [54] | 3.3 | 1.3 | 1.7 | 0.7 | - | - | |
| Elaphocordyceps subsessilis/Tolypocladium inflatum | Elaphocordyceps subsessilis/Tolypocladium inflatum | JX488469/AB255606 | 99 | So fung/An par | [55] | - | - | 3.3 | 0.4 | - | - | |
| Cephalotheca sp. | Cephalotheca sulfurea | EU689260 | 82 | Sapr | [56] | 1.7 | 0.6 | - | - | - | - | |
| Pachyphlodes nemoralis | Pachyphlodes nemoralis | FJ013079 | 100 | ECM | [57] | 1.7 | 0.2 | - | - | - | - | |
| Hydnotrya tulasnei | Hydnotrya tulasnei | KT334735 | 99 | ECM | [58] | - | - | - | - | 1.7 | 0.2 | |
| Mycorrhizal fungal species richness [n] | 15 | 11 | 12 | |||||||||
| Degree of mycorrhization [%] | 25.6 | 30.2 | 29.1 | |||||||||
| Estimated species richness | ||||||||||||
| Chao1 | 2.50 | 2.36 | 2.45 | |||||||||
| Diversity | ||||||||||||
| Shannon–Wiener (H’) | 0.69 a | 0.57 c | 0.65 b | |||||||||
| Simpson 1/D | 0.43 a | 0.35 b | 0.41 a | |||||||||
| Parameters | Site | |||||
|---|---|---|---|---|---|---|
| KB | K | P | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| FRT = fine root tips (n) | 1635.7 | ±871.25 | 4291.5 | ±2531.70 | 3164.8 | ±1874.07 |
| MRL = mother root length (cm) | 140.0 | ±68.49 | 244.3 | ±103.50 | 188.7 | ±140.76 |
| Total root length (cm) | 1048.7 | ±526.71 | 2036.0 | ±899.40 | 1432.7 | ±772.52 |
| FRL = fine root length (cm) | 888.1 | ±464.34 | 1785.3 | ±806.92 | 1238.5 | ±648.51 |
| Dry weight of fine roots | 2.7 | ±1.71 | 6.3 | ±3.07 | 3.5 | ±1.67 |
| Dry weight of mother roots | 3.8 | ±1.31 | 7.0 | ±3.09 | 6.3 | ±2.22 |
| Crown transparency (%) | 32.3 | ±20.52 | 41.1 | ±14.09 | 38.8 | ±16.51 |
| Foliage damage | 1.3 | ±0.99 | 1.8 | ±0.648 | 1.6 | ±0.69 |
| Syn. | 1.1 | ±0.78 | 1.5 | ±0.51 | 1.4 | ±0.57 |
| Study Site | ||||||
|---|---|---|---|---|---|---|
| KB | K | P | ||||
| Physical and chemical characteristics | Mean | SE | Mean | SE | Mean | SE |
| mean pH KCl | 3.64 | ±0.25 | 3.40 | ±0.18 | 3.17 | ±0.16 |
| C-org. (mg/g) | 16.94 | ±6.5 | 34.02 | ±12.74 | 65.39 | ±32.41 |
| Total Kjeldahl Nitrogen = N-org. + NH4-N (mg/g) | 0.65 | ±0.26 | 1.44 | ±0.62 | 2.25 | ±1.11 |
| C:N ratio | 29.7 | ±17.2 | 26.9 | ±13.3 | 32.8 | ±17.1 |
| Ca (mg/L) | 106.92 | ±19.98 | 128.62 | ±35.81 | 99.90 | ±21.31 |
| P2O5 (mg/100g) | 4.06 | ±2.74 | 2.07 | ±0.85 | 4.09 | ±3.35 |
| K2O (mg/100g) | 2.77 | ±1.06 | 5.23 | ±2.48 | 7.14 | ±5.58 |
| Mg (mg/100g) | 1.79 | ±0.65 | 3.25 | ±1.23 | 3.85 | ±1.71 |
| Relative soil humidity (%) | 16.88 | ±4.40 | 23.28 | ±6.85 | 25.79 | ±8.51 |
| Mineral soil particle size (fraction in %) | ||||||
| sand (2–0.05 mm) | 83.16 | ±8.99 | 79.02 | ±3.89 | 79.09 | ±3.85 |
| coarse silt (0.05–0.02 mm) | 11.60 | ±5.20 | 13.27 | ±1.99 | 14.39 | ±2.40 |
| fine silt (0.02–0.002 mm) | 4.72 | ±3.31 | 6.74 | ±2.10 | 5.89 | ±1.74 |
| clay (<0.002 mm) | 0.54 | ±0.68 | 1.00 | ±0.47 | 0.61 | ±0.38 |
| fraction contained in % <0.02 mm | 5.27 | ±3.97 | 7.74 | ±2.50 | 6.34 | ±2.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Nowakowska, J.A.; Oszako, T.; Urban, A.; Hilszczańska, D. Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland. Forests 2019, 10, 30. https://doi.org/10.3390/f10010030
Bzdyk RM, Olchowik J, Studnicki M, Nowakowska JA, Oszako T, Urban A, Hilszczańska D. Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland. Forests. 2019; 10(1):30. https://doi.org/10.3390/f10010030
Chicago/Turabian StyleBzdyk, Roman Mariusz, Jacek Olchowik, Marcin Studnicki, Justyna Anna Nowakowska, Tomasz Oszako, Alexander Urban, and Dorota Hilszczańska. 2019. "Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland" Forests 10, no. 1: 30. https://doi.org/10.3390/f10010030





