Transformation and Precipitation Reactions by Metal Active Gas Pulsed Welded Joints from X2CrNiMoN22-5-3 Duplex Stainless Steels
Abstract
:1. Introduction
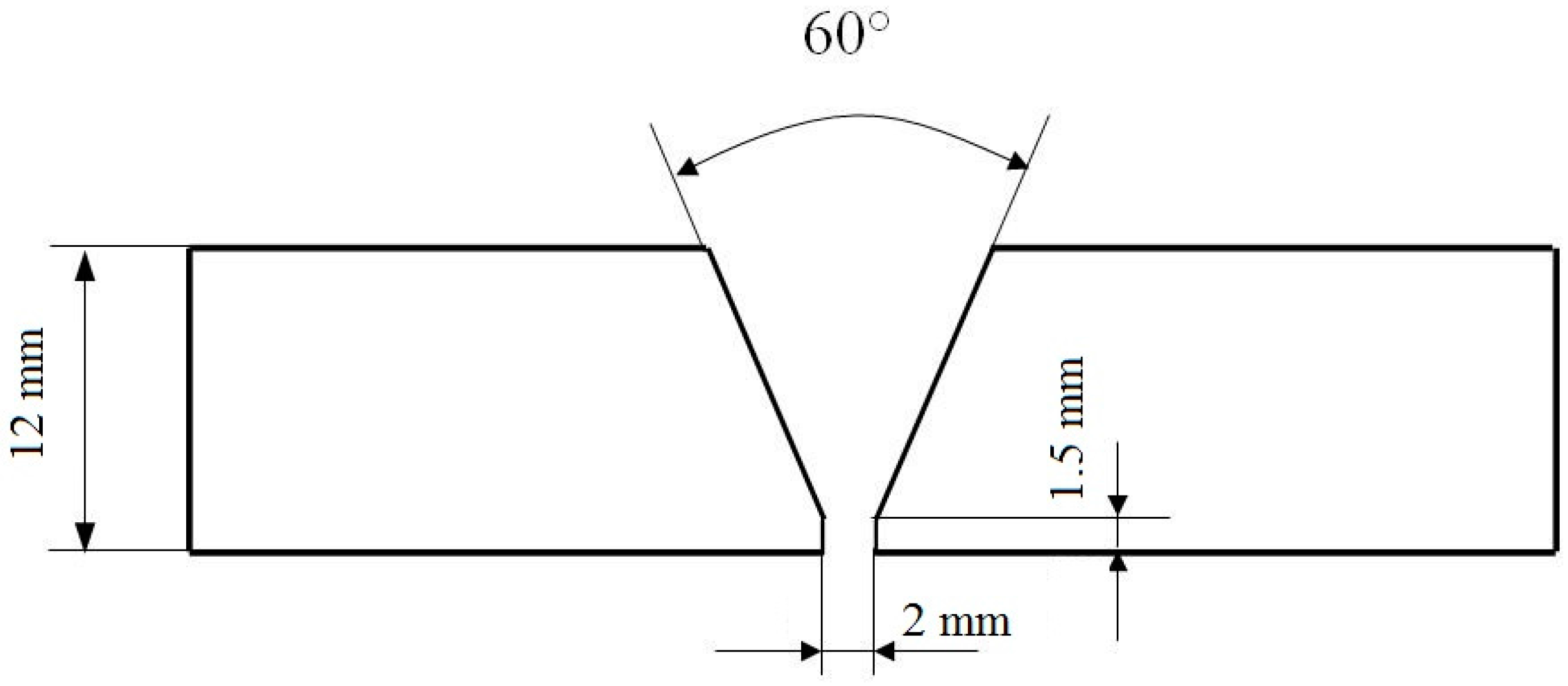
2. Experimental Procedure
- -
- defining of the welded joint: homogeneous;
- -
- base metal: Duplex stainless steel sheet: s = 12 mm;
- -
- type of the welded joint: butt penetrated joint;
- -
- weld thickness: 12 mm;
- -
- welding position: horizontal PA;
- -
- welding technique: pulsed MAG;
- -
- filler material: E 2209-16 wire (according to AWS A5.4); it has a Cr, Mo and N content similar to the base metal, but the more Ni (8%–10%);
- -
- diameter wire electrode: ds = 1.2 mm;
- -
- shielding gas: Cronigon 2 (97.5% Ar + 2.5% CO2), supplied by Linde Gas;
- -
- flow gas: 18 L/min;
- -
- welding direction: to the left;
- -
- inclination of the electrode wire: 85°.
3. Results and Discussion
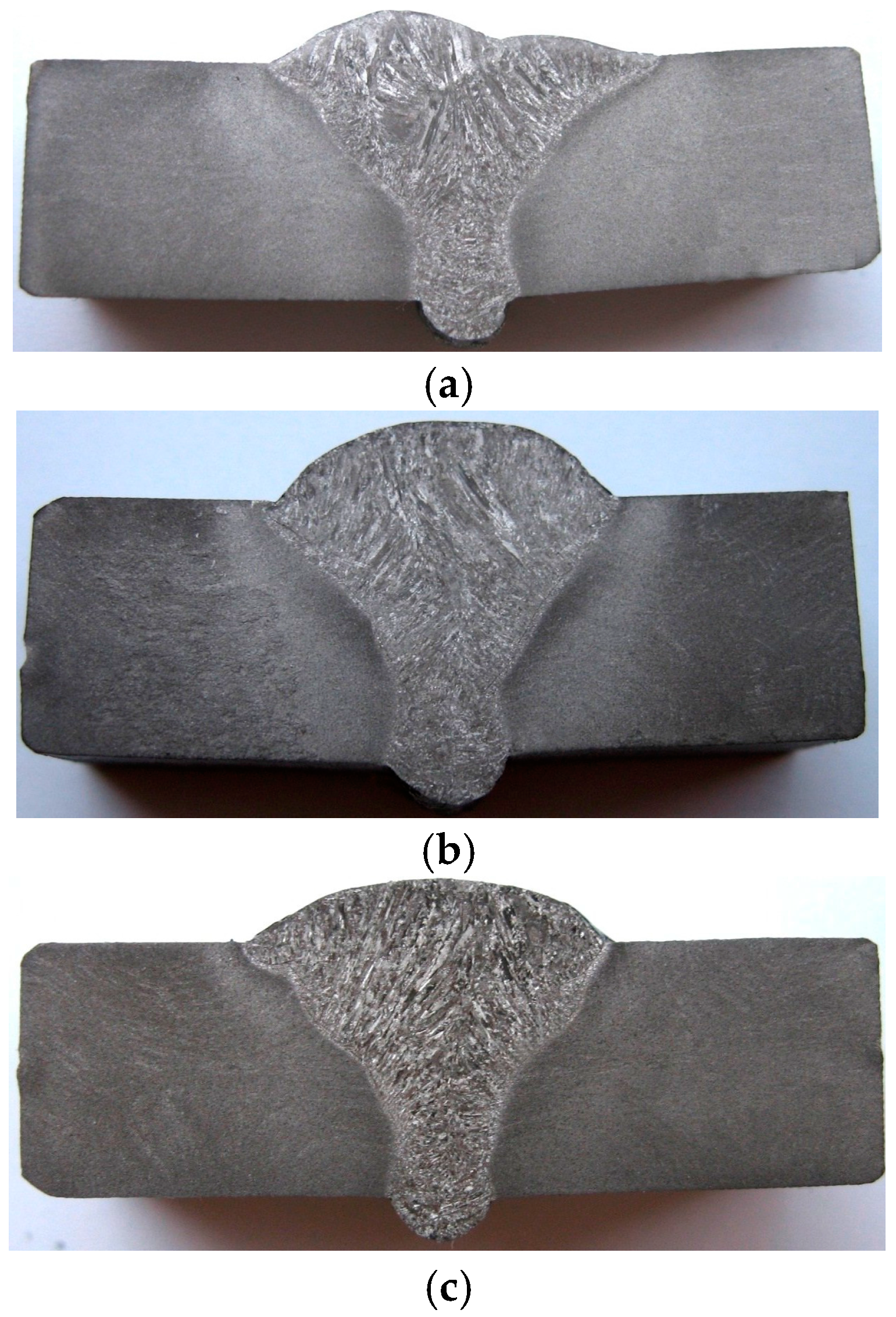
3.1. Macrographic Aspect of the Welded Joints
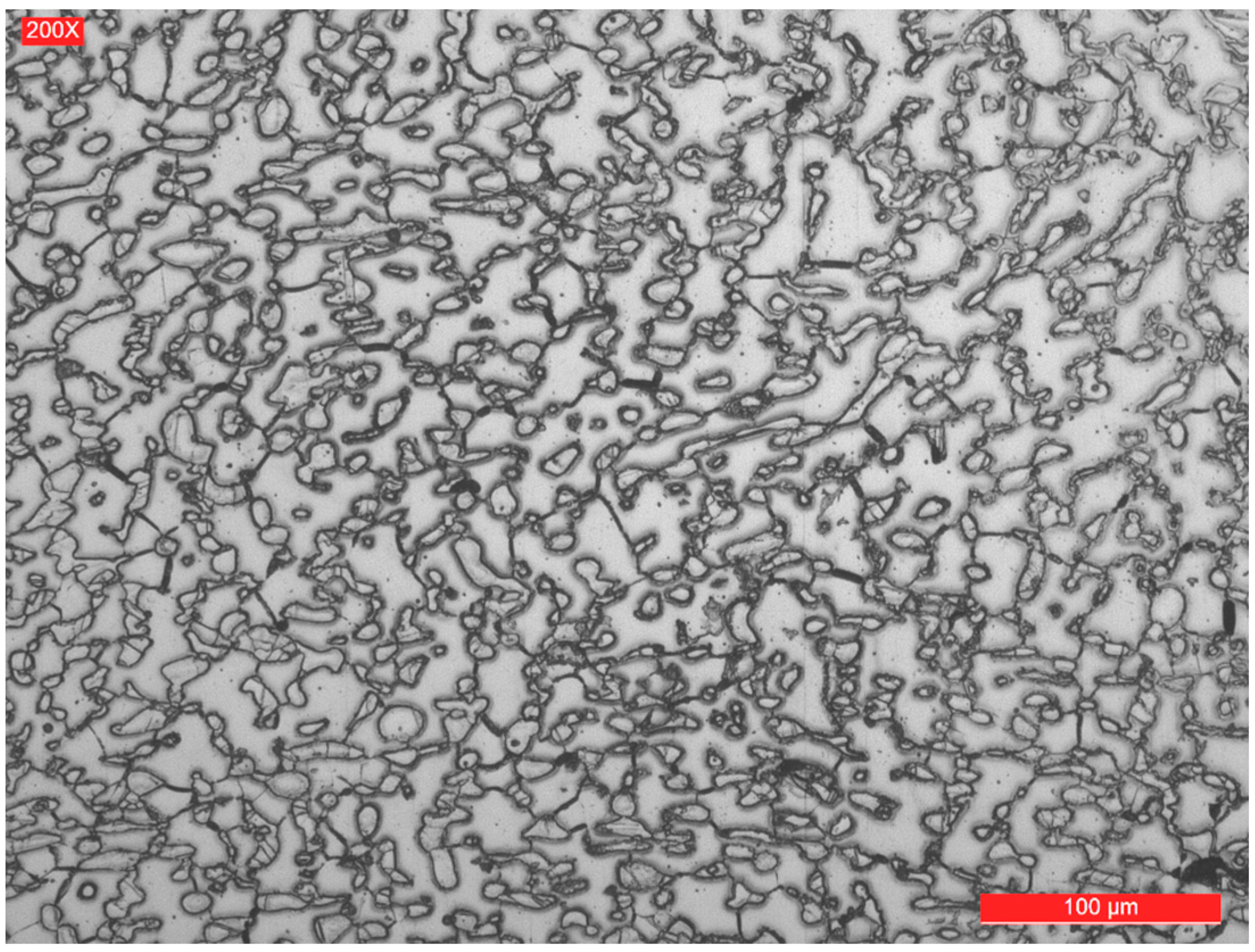
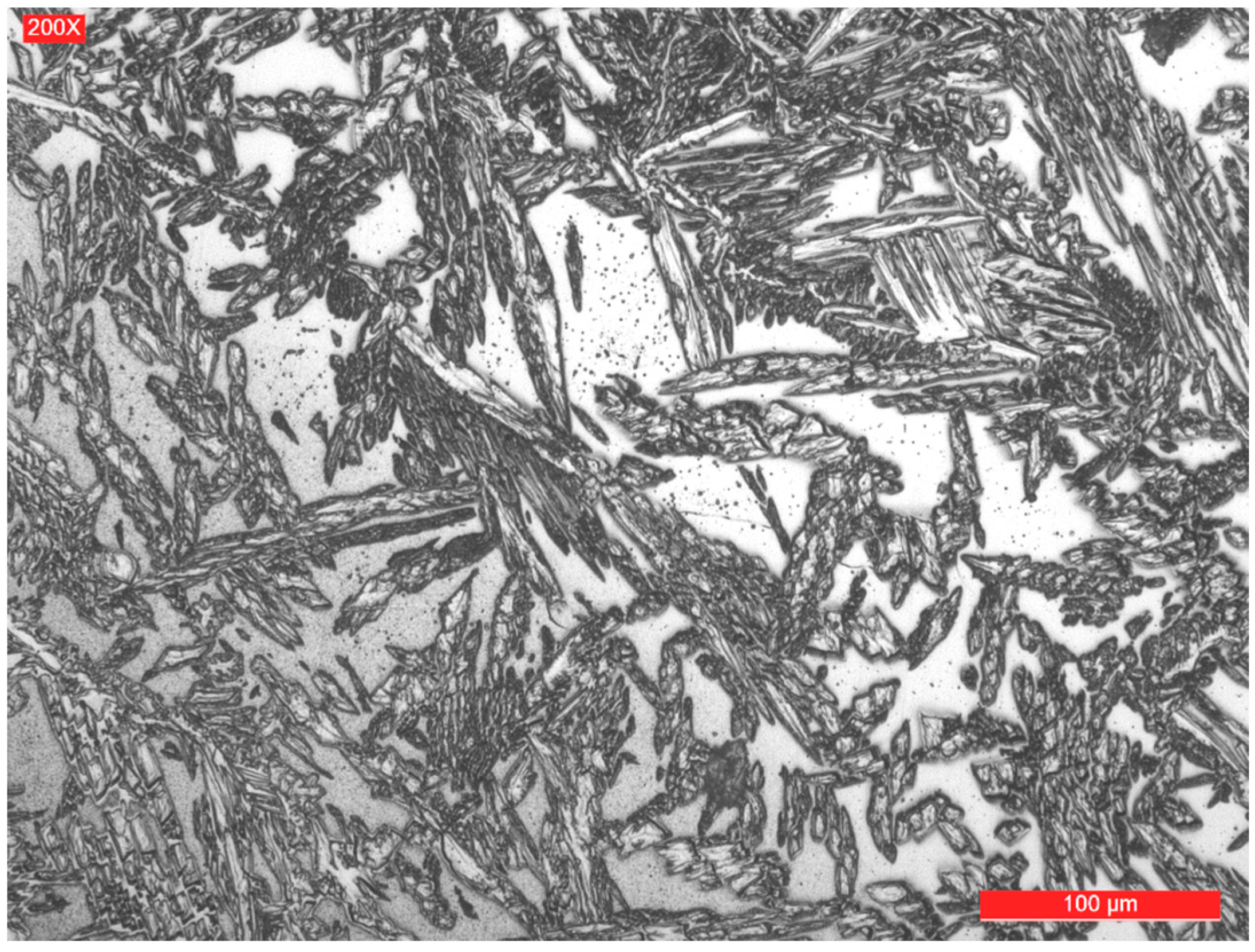
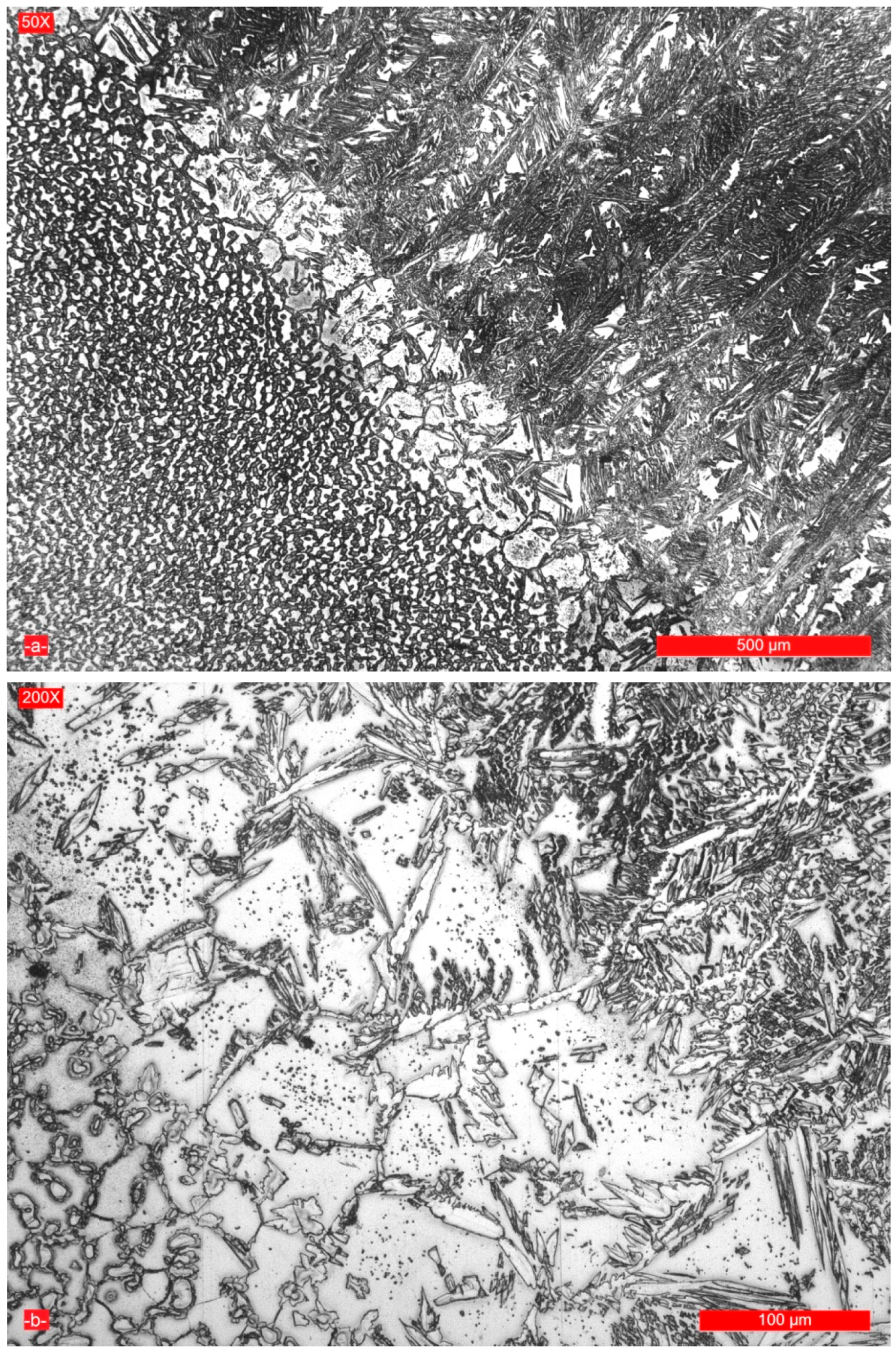
3.2. Microstructure of the Weld Seam and Joint Interface
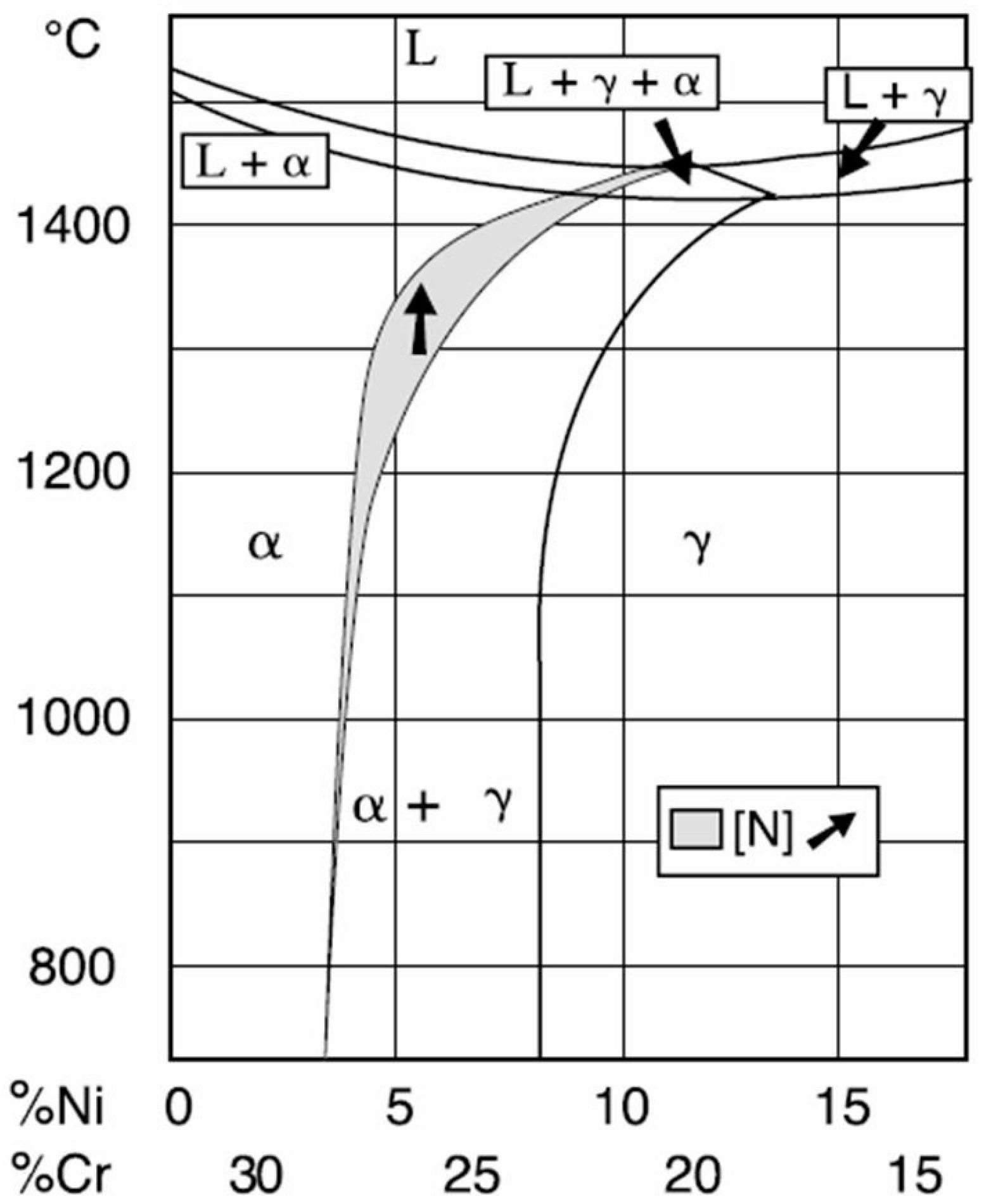
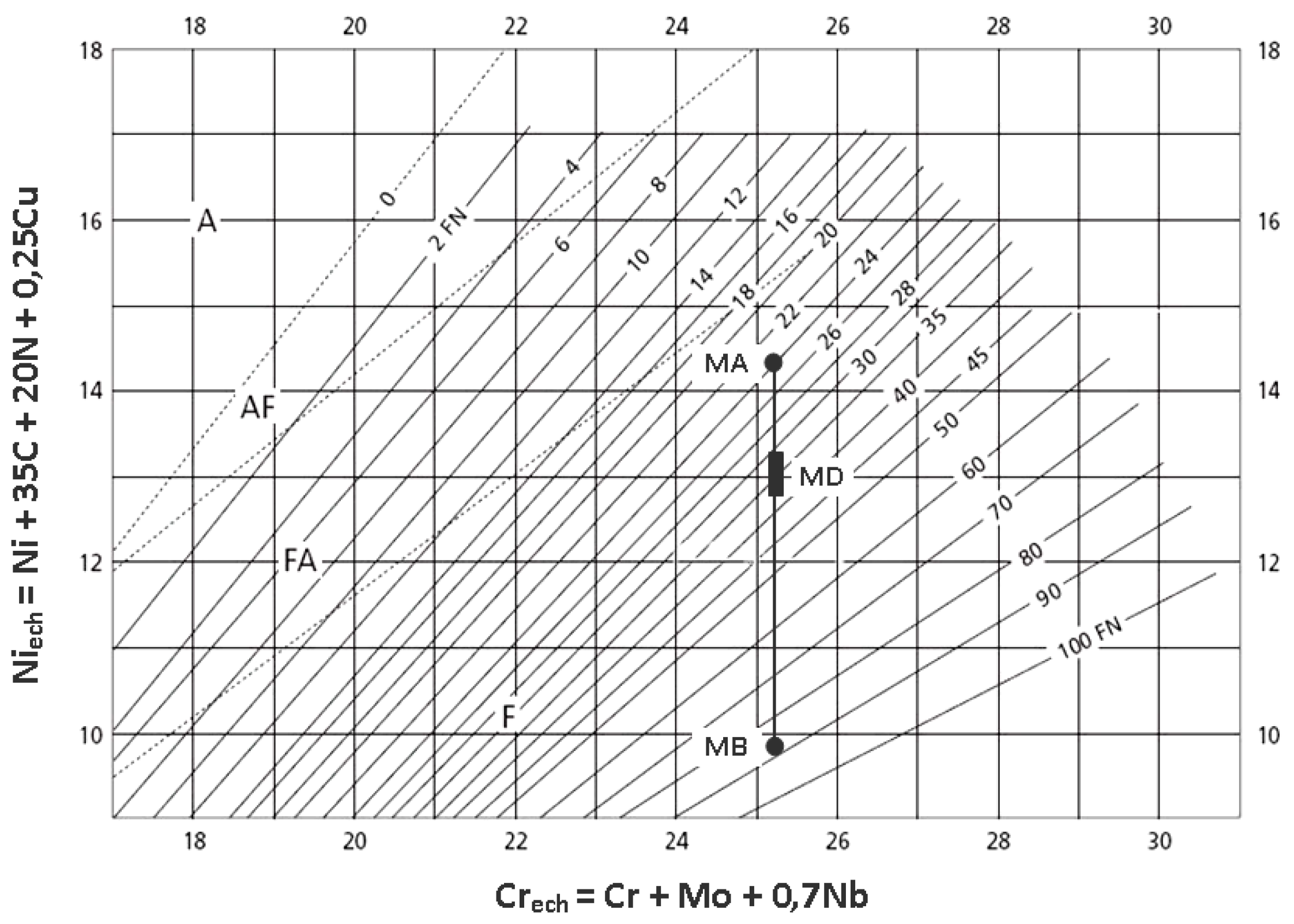
3.3. EDX Analysis
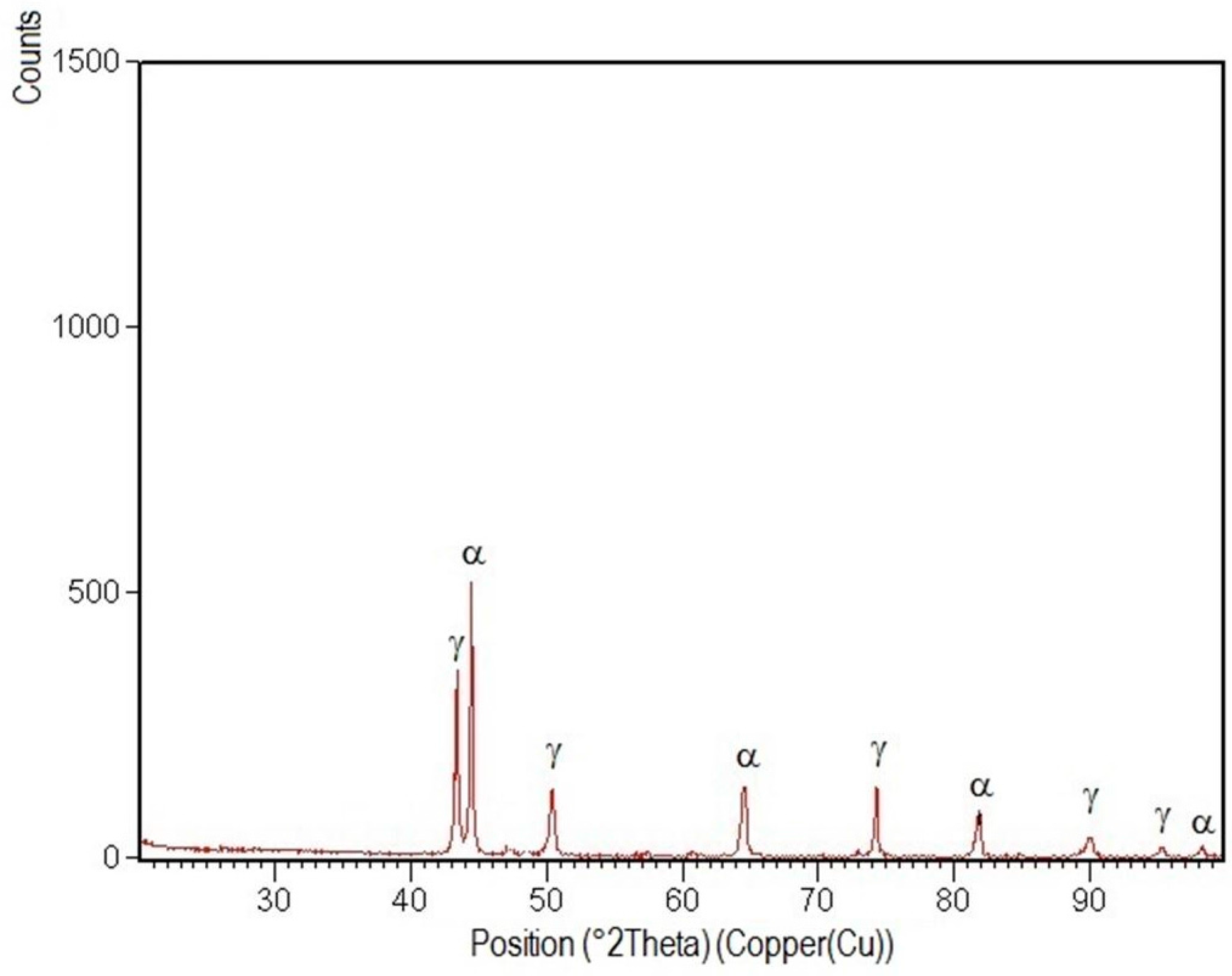
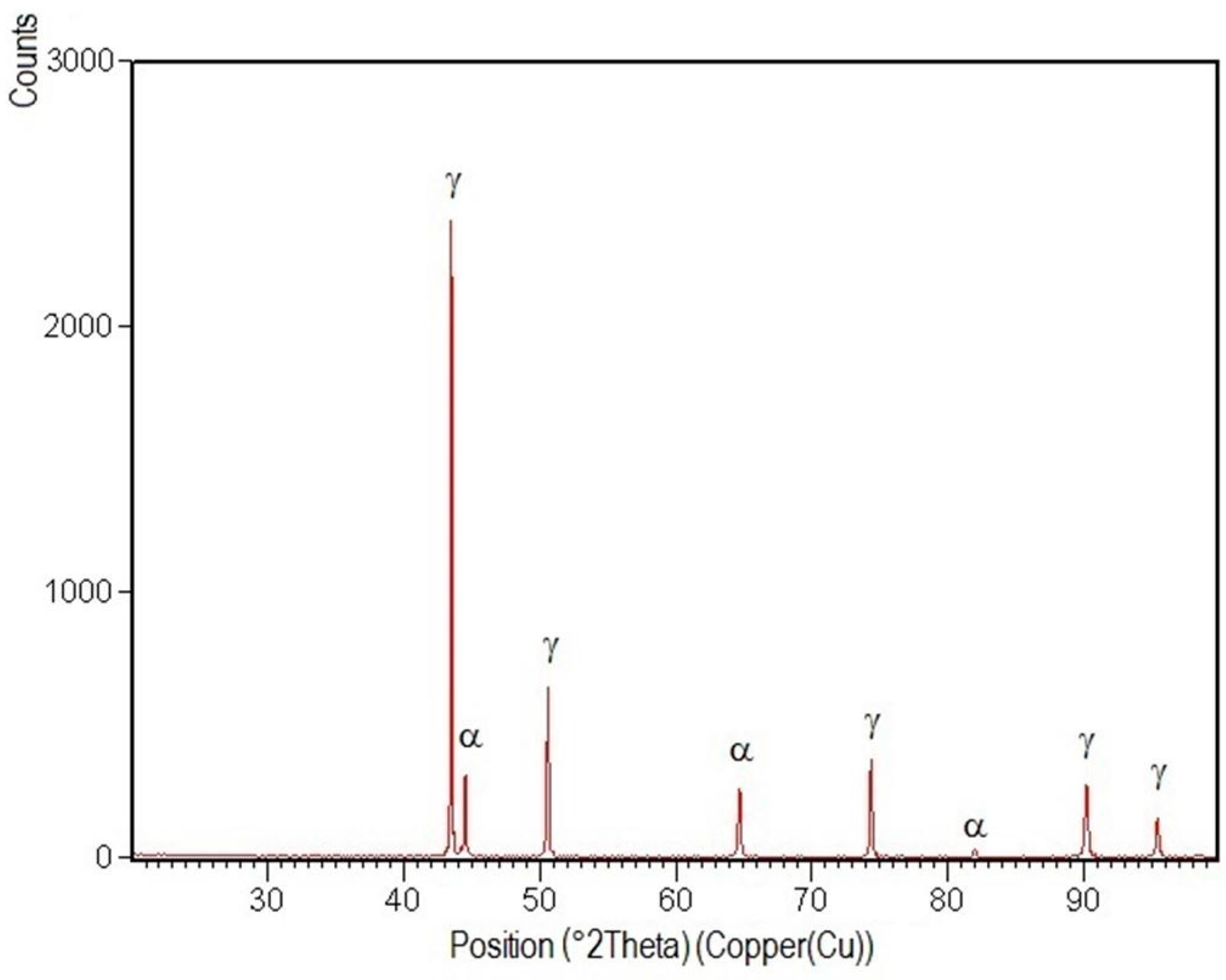
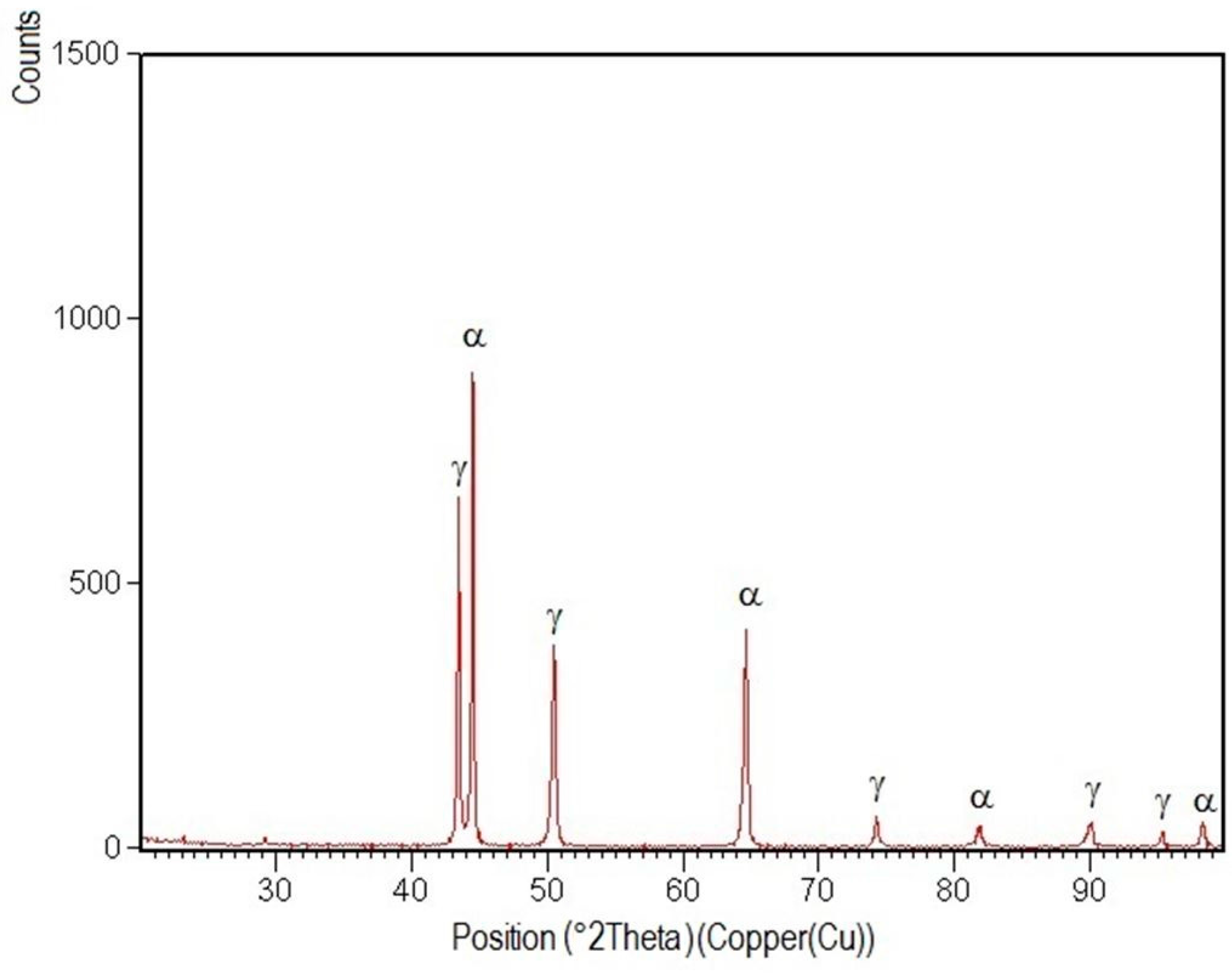
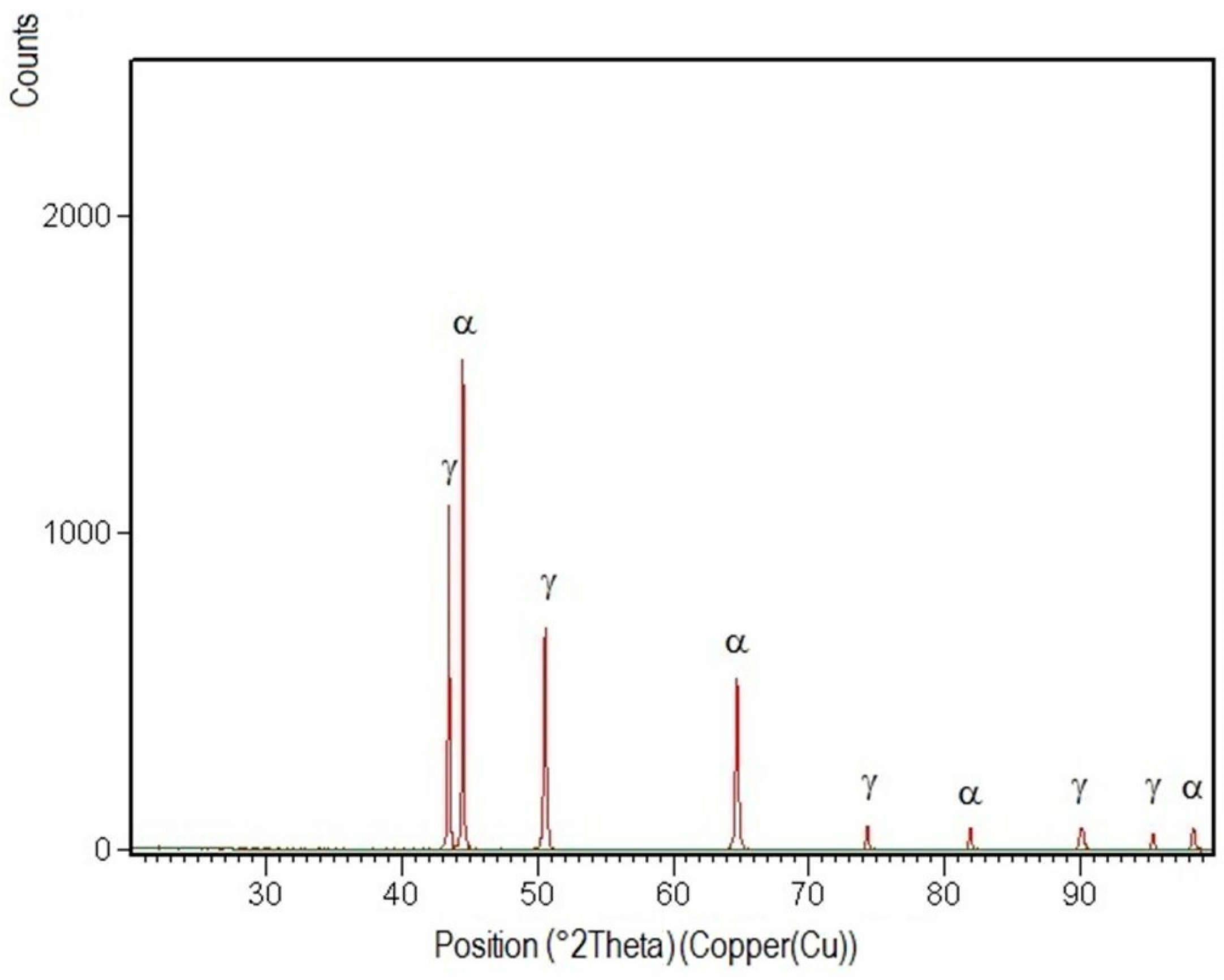
3.4. X-ray Diffraction Analysis
- -
- Structural phases detected in all areas of the welded joints are ferrite and austenite.
- -
- The high temperatures reached in the vicinity of the fusion line promote a predominantly ferrite microstructure in the HAZ zone (interference peaks are more pronounced), with a smaller proportion of austenite resulted from the solid state transformation, initiated in the welded joint cooling stage.
- -
- The specific global thermal cycles during successive welding passes, beside the higher Ni content of the selected filler material compared to the base metal, justify the data obtained regarding the quantitative report of the two main phases (A,F) present in the final microstructure following primary and secondary crystallization.
- -
- Both in the weld and HAZ zones, the intermetallic phases, which affect the mechanical properties of welded joints, were not highlighted.
4. Conclusions
- Variation of the linear energy within the range values of 10 kJ/cm and 20.7 kJ/cm lead to welded joints with a suitable macro-geometry, without metal continuity defects such as porosity, cracks or slag inclusions.
- The micrographic and X-ray diffraction analysis showed that for the welding conditions used, the base metal consists of approximately 51% ferrite and 49% austenite, and in the weld seam, the ferrite content decreases from the surface to the root layer.
- The high cooling rates during primary and secondary crystallisation of the molten metal bath is associated with the segregation of the alloying elements and their redistributing between ferrite and austenite.
- As a result of the high temperatures reached in the vicinity of the fusion line, with a small thickness of approximately 120–160 μm, its microstructure became predominantly ferritic and by subsequent cooling its partial transformation in austenite occurred.
- The deposition of the root layer, with a linear energy of 6.9 kJ/cm, and of the filling layers, with values of 10 kJ/cm to 20 kJ/cm, prevents the cracking by liquation phenomenon of the weld and limits the fragile intermetallic phases’ precipitation in the welded joint area.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tavares, S.S.M.; Pardal, J.M.; Lima, L.D.; Bastos, I.N.; Nascimento, A.M.; Souza, J.A. Characterization of microstructure, chemical composition, corrosion resistance and toughness of a multipass weld joint of super duplex stainless steel UNS S32750. Mater. Charact. 2007, 58, 610–616. [Google Scholar] [CrossRef]
- Udayakumar, T.; Raja, K.; Tanksale, A.A.; Sathiya, P. Experimental investigation on mechanical and metallurgical properties of super duplex stainless steel joints using friction welding process. J. Manuf. Process. 2013, 15, 558–571. [Google Scholar] [CrossRef]
- Sarkari, K.M.; Mostafaei, M.A.; Pouraliakbar, H.; Kokabi, A.H. Study on microstructure and mechanical characteristics of low-carbon steel and ferritic stainless steel joints. Mater. Sci. Eng. A 2014, 608, 35–45. [Google Scholar] [CrossRef]
- Badji, R.; Bouabdallah, M.; Bacroix, B.; Kahloun, C.; Belkessa, B.; Maza, H. Phase transformation and mechanical behavior in annealed 2205 duplex stainless steel welds. Mater. Charact. 2008, 59, 447–453. [Google Scholar] [CrossRef]
- Luo, J.; Dong, Y.; Li, L.; Wang, X. Microstructure of 2205 duplex stainless steel joint in submerged arc welding by post weld heat treatment. J. Manuf. Process. 2014, 16, 144–148. [Google Scholar] [CrossRef]
- Chern, T.S.; Tseng, K.H.; Tsai, H.L. Study of the characteristics of duplex stainless steel activated tungsten inert gas welds. J. Mater. Des. 2011, 32, 255–263. [Google Scholar] [CrossRef]
- Ureňa, A.; Otero, E.; Utrilla, M.V.; Munez, C.J. Weldability of a 2205 duplex stainless steel using plasma arc welding. J. Mater. Process. Technol. 2007, 182, 624–631. [Google Scholar] [CrossRef]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; John Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sadeghian, M.; Shamanian, M.; Shafyei, A. Effect of heat input on microstructure and mechanical properties of dissimilar joints between super duplex stainless steel and high strength low alloy steel. J. Mater. Des. 2014, 60, 678–684. [Google Scholar] [CrossRef]
- Silva, E.D.; Costa de Albuquerque, V.H.; Leite, J.P.; Varela, A.C.G.; Moura, E.P.; Tavares, J.M.R.S. Phase transformations evaluation on a UNS S31803 duplex stainless steel based on nondestructive testing. Mater. Sci. Eng. A 2009, 516, 126–130. [Google Scholar] [CrossRef]
- Mitelea, I.; Roşu, R. Sudabilitatea Oţelurilor Inoxidabile; Editura Politehnica Timişoara: Timisoara, Romania, 2010; pp. 92–98. [Google Scholar]
- Santos, T.F.A.; Marinho, R.R.; Paes, M.T.P.; Ramirez, A.J. Microstructure evaluation of UNS S32205 duplex stainless steel friction stir welds. Rev. Esc. Minas. 2013, 66, 187–191. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D.J.; Zhao, G.J.; Wang, X.J.; Ran, H.Q. Relationship between microstructure of fusion zone and mechanical properties of 2205 duplex stainless steel joint in double-sided submerged arc welding. Rare Metal. Mater. Eng. 2011, 40, 369–374. [Google Scholar]
- Casalino, G.; Campanelli, S.L.; Domenico, L.A. Laser-arc hybrid welding of wrought to selective laser molten stainless steel. Int. J. Adv. Manuf. Technol. 2013, 68, 209–216. [Google Scholar] [CrossRef]
- Palmer, T.A.; Elmer, J.W.; Babu, S.S.; Vitek, J.M. Observations of ferrite/austenite transformations in the heat affected zone of 2205 duplex stainless steel spot welds using time resolved X-ray diffraction. Mater. Sci. Eng. A 2004, 374, 307–321. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.Y.; Jiang, Y.M.; Sun, T.; Xu, J.L.; Li, J. Effect of thermal cycles on the corrosion and mechanical properties of UNSS31803 duplex stainless steel. Corros. Sci. 2009, 51, 2969–2975. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Łabanowski, J.; Fydrych, D. The effect of welding conditions on mechanical properties of superduplex stainless steel welded joints. Adv. Mater. Sci. 2014, 14, 14–22. [Google Scholar] [CrossRef]
- Sato, Y.S.; Nelson, T.W.; Sterling, C.J.; Steel, R.K.; Pettersson, C.O. Microstructure and mechanical properties of friction stir welded SAF 2507 super duplex stainless steel. Mater. Sci. Eng. A 2005, 397, 376–384. [Google Scholar] [CrossRef]
- Hilkes, J.; Bekkers, K. Welding Duplex Stainless Steel. Weld. J. 1995, 11, 51–54. [Google Scholar]
- Welding and Allied Processes—Welding Positions; ISO 6947/2011; ICS: Geneva, Switzerland, 2011.
- Casalino, G.; Mortello, M. Modeling and experimental analysis of fiber laser offset welding of Al-Ti butt joints. Int. J. Adv. Manuf. Technol. 2016, 83, 89–98. [Google Scholar] [CrossRef]
- Urlan, S.; Mitelea, I.; Utu, I.D.; Burca, M. Microstructure of the pulsed MIG/MAG welded joints from Duplex stainless steel X2CrNiMoN 22-5-3. In Proceedings of the 24th International Conference on Metallurgy and Materials, Brno, Czech Republic, 3–5 June 2015; pp. 898–904.
- Lippold, J.C.; Kotecki, D.J. Weldability of stainless steels. In Welding Metallurgy; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 230–253. [Google Scholar]
- Askeland, D.R.; Wright, W.J. Essentials of Materials Science and Engineering, 3rd ed.; Global Engineering: Stamford, CT, USA, 2014; pp. 210–216. [Google Scholar]
- Jiang, Y.; Tan, H.; Wang, Z.; Hong, J.; Jiang, L.; Li, J. Influence of Creq/Nieq on pitting corrosion resistance and mechanical properties of UNS S32304 duplex stainless steel welded joints. Corros. Sci. 2013, 70, 252–259. [Google Scholar] [CrossRef]





| C, % | Si, % | Mn,% | P, % | S, % | Cr, % | Ni, % | Mo, % | N, % |
|---|---|---|---|---|---|---|---|---|
| 0.021 | 0.79 | 0.82 | 0.019 | 0.021 | 22.34 | 5.61 | 3.1 | 0.14 |
| Phase | Cr, wt % | Ni, wt % | Mo, wt % | N, wt % |
|---|---|---|---|---|
| Ferrite/Ferrite | 21.61–23.83 | 5.40–6.22 | 2.62–3.35 | <0.052 |
| Austenite/Austenite | 19.92–21.5 | 6.31–8.70 | 2.51–2.98 | 0.31–0.58 |
| Welded Joint Zone | Phase | Cr, wt % | Ni, wt % | Mo, wt % |
|---|---|---|---|---|
| Root weld | F/F A/A | 23.62 23.14 | 7.16 7.85 | 2.98 2.67 |
| Heat affected zone (ZIT) | F/F A/A | 21.88 20.76 | 5.17 5.83 | 3.39 2.87 |
| Weld-median zone | F/F A/A | 24.84 22.60 | 6.18 8.55 | 3.68 2.47 |
| Weld-outer zone | F/F A/A | 23.71 23.42 | 7.20 7.72 | 2.73 2.54 |
| Base metal | F/F A/A | 23.18 19.47 | 4.08 7.16 | 3.29 2.43 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utu, I.-D.; Mitelea, I.; Urlan, S.D.; Crăciunescu, C.M. Transformation and Precipitation Reactions by Metal Active Gas Pulsed Welded Joints from X2CrNiMoN22-5-3 Duplex Stainless Steels. Materials 2016, 9, 606. https://doi.org/10.3390/ma9070606
Utu I-D, Mitelea I, Urlan SD, Crăciunescu CM. Transformation and Precipitation Reactions by Metal Active Gas Pulsed Welded Joints from X2CrNiMoN22-5-3 Duplex Stainless Steels. Materials. 2016; 9(7):606. https://doi.org/10.3390/ma9070606
Chicago/Turabian StyleUtu, Ion-Dragos, Ion Mitelea, Sorin Dumitru Urlan, and Corneliu Marius Crăciunescu. 2016. "Transformation and Precipitation Reactions by Metal Active Gas Pulsed Welded Joints from X2CrNiMoN22-5-3 Duplex Stainless Steels" Materials 9, no. 7: 606. https://doi.org/10.3390/ma9070606
APA StyleUtu, I.-D., Mitelea, I., Urlan, S. D., & Crăciunescu, C. M. (2016). Transformation and Precipitation Reactions by Metal Active Gas Pulsed Welded Joints from X2CrNiMoN22-5-3 Duplex Stainless Steels. Materials, 9(7), 606. https://doi.org/10.3390/ma9070606