Solvent-Induced Polymorphism of Iron(II) Spin Crossover Complexes
Abstract
:1. Introduction
2. Results and Discussion
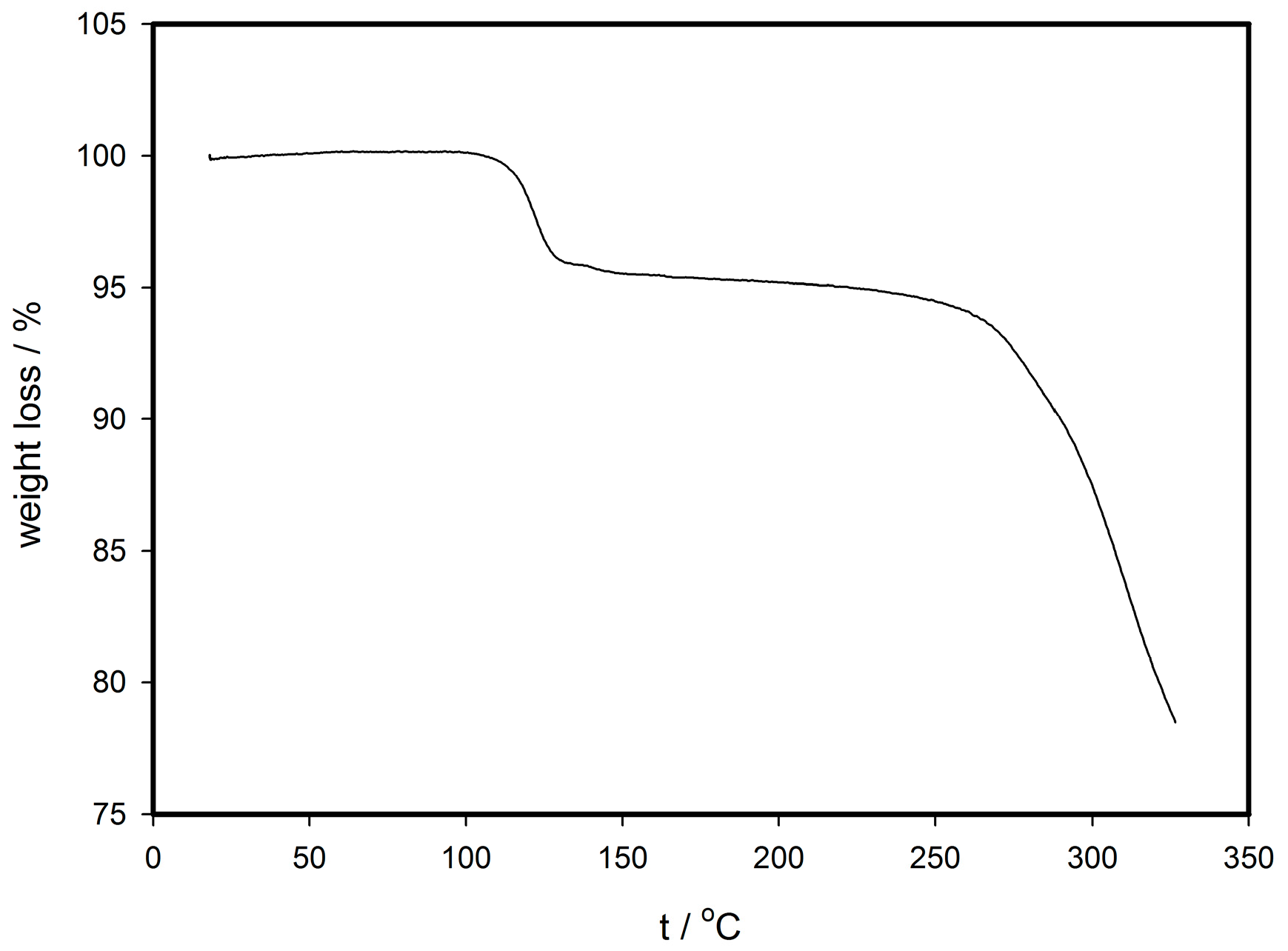
2.1. Synthesis, Crystallization and Thermogravimetry Analysis
2.2. Structural Investigation
2.3. Magnetic Properties of Compounds (1) and (2)
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. Magnetic Susceptibility Measurement
3.4. Single-Crystal Diffraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Nemec, I.; Herchel, R.; Boca, R.; Travnicek, Z.; Svoboda, I.; Fuess, H.; Linert, W. Tuning of spin crossover behaviour in iron(III) complexes involving pentadentate Schiff bases and pseudohalides. Dalton Trans. 2011, 40, 10090–10099. [Google Scholar] [CrossRef] [PubMed]
- Ksenofontov, V.; Gaspar, A.B.; Gütlich, P. Pressure Effect Studies on Spin Crossover and Valence Tautomeric Systems. In Spin Crossover in Transition Metal Compounds III; Gütlich, P., Goodwin, H.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; ISSN: 0340-1022. [Google Scholar]
- Bousseksou, A.; Varret, F.; Goiran, M.; Boukheddaden, K.; Tuchagues, J.P. The Spin Crossover Phenomenon under High Magnetic Field. In Spin Crossover in Transition Metal Compounds III; Gütlich, P., Goodwin, H.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; ISSN: 0340-1022. [Google Scholar]
- Meded, V.; Bagrets, A.; Fink, K.; Chandrasekar, R.; Ruben, M.; Evers, F.; Bernand-Mantel, A.; Seldenthuis, J.S.; Beukman, A.; van der Zant, H.S.J. Electrical control over the Fe(II) spin crossover in a single molecule: Theory and experiment. Phys. Rev. B 2011, 83, 245415. [Google Scholar] [CrossRef]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-Temperature Electrical Addressing of a Bistable Spin-Crossover Molecular System. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A. Light-Induced Spin Crossover and the High-Spin → Low-Spin Relaxation. In Spin Crossover in Transition Metal Compounds II; Gütlich, P., Goodwin, H.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; ISSN: 0340-1022. [Google Scholar]
- Collison, D.; Garner, C.D.; McGrath, C.M.; Mosselmans, J.F.W.; Roper, M.D.; Seddon, J.M.W.; Sinn, E.; Young, N.A. Soft X-ray induced excited spin state trapping and soft X-ray photochemistry at the iron L2,3 edge in [Fe(phen)2(NCS)2] and [Fe(phen)2(NCSe)2] (phen = 1,10-phenanthroline). J. Chem. Soc. Dalton Trans. 1997, 4371–4376. [Google Scholar] [CrossRef]
- Boča, R. Theoretical Foundations of Molecular Magnetism; Elsevier: Amsterdam, The Netherlands, 1999; ISBN ISBN: 9780080542713. [Google Scholar]
- Kershaw Cook, L.J.; Kulmaczewski, R.; Mohammed, R.; Dudley, S.; Barrett, S.A.; Little, M.A.; Deeth, R.J.; Halcrow, M.A. A Unified Treatment of the Relationship Between Ligand Substituents and Spin State in a Family of Iron(II) Complexes. Angew. Chem. Int. Ed. 2016, 55, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Kershaw Cook, L.J.; Mohammed, R.; Sherborne, G.; Roberts, T.D.; Alvarez, S.; Halcrow, M.A. Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements. Coor. Chem. Rev. 2015, 289–290, 2–12. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Nemec, I.; Herchel, R.; Trávníček, Z. The relationship between the strength of hydrogen bonding and spin crossover behaviour in a series of iron(III) Schiff base complexes. Dalton Trans. 2015, 44, 4474–4484. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.; Kröber, J.; Jay, C. Spin Transition Molecular Materials for displays and data recording. Adv. Mater. 1992, 4, 718–728. [Google Scholar] [CrossRef]
- Krober, J.; Codjovi, E.; Kahn, O.; Grolibre, F.; Jay, C. A spin transition system with a thermal hysteresis at room temperature. J. Am. Chem. Soc. 1993, 115, 9810–9811. [Google Scholar] [CrossRef]
- Niel, V.; Martinez-Agudo, J.M.; Carmen Munoz, M.; Gaspar, A.B.; Real, J.A. Cooperative Spin Crossover Behavior in Cyanide-Bridged Fe(II)−M(II) Bimetallic 3D Hofmann-like Networks (M = Ni, Pd, and Pt). Inorg. Chem. 2001, 40, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Bréfuel, N.; Shova, S.; Wolny, J.A.; Dahan, F.; Verelst, M.; Paulsen, H.; Trautwein, A.X.; Tuchagues, J.P. A Range of Spin-Crossover Temperature T1/2>300 K Results from Out-of-Sphere Anion Exchange in a Series of Ferrous Materials Based on the 4-(4-Imidazolylmethyl)-2-(2-imidazolylmethyl)imidazole (trim) Ligand, [Fe(trim)2]X2 (X = F, Cl, Br, I): Comparison of Experimental Results with Those Derived from Density Functional Theory Calculations. Chem. Eur. J. 2006, 12, 7421–7432. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, I.; Krummenacher, I.; Hewitt, I.J.; Lan, Y.; Mereacre, V.; Powell, A.K.; Höfer, P.; Harmer, J.; Breher, F. Syntheses, Structures and Electronic Properties of Zwitterionic Iron(II) and Cobalt(II) Complexes Featuring Ambidentate Tris(pyrazolyl)methanide Ligands. Chem. Eur. J. 2009, 15, 4350–4365. [Google Scholar] [CrossRef] [PubMed]
- Shatruk, M.; Dragulescu-Andrasi, A.; Kristen, E.; Chambers, K.E.; Stoian, S.A.; Bominaar, E.L.; Achim, C.; Dunbar, K.R. Properties of Prussian Blue Materials Manifested in Molecular Complexes: Observation of Cyanide Linkage Isomerism and Spin-Crossover Behavior in Pentanuclear Cyanide Clusters. J. Am. Chem. Soc. 2007, 129, 6104–6116. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; DiPasquale, A.; Arnold, L.; Rheingold, A.L.; Miller, J.S. Room-Temperature Spin Crossover Observed for [(TPyA)FeII(DBQ2-)FeII(TPyA)]2 + [TPyA = Tris(2-pyridylmethyl)amine; DBQ2- = 2,5-Di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate]. Inorg. Chem. 2007, 46, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Ui, M.; Yokota, M.; Han, L.; Maeda, A.; Kishida, H.; Okamoto, H.; Oshio, H. Two-Step Spin Conversion in a Cyanide-Bridged Ferrous Square. Angew. Chem. Int. Ed. 2005, 44, 6484–6487. [Google Scholar] [CrossRef] [PubMed]
- Šalitroš., I.; Madhu, N.T.; Boča, R.; Pavlik, J.; Ruben, M. Room-temperature spin-transition iron compounds. Monatsh. Chem. 2009, 140, 695–733. [Google Scholar] [CrossRef]
- Kilner, C.A.; Halcrow, M.A. An iron(II) complex of 2,6-di(pyrazol-1-yl)pyrazine that crystallises in three forms, two of which exhibit an unusual angular Jahn–Teller distortion. Polyhedron 2006, 25, 235–240. [Google Scholar] [CrossRef]
- Chandrasekar, R.; Schramm, F.; Fuhr, O.; Ruben, M. An Iron(II) Spin-Transition Compound with Thiol Anchoring Groups. Eur. J. Inorg. Chem. 2008, 2649–2653. [Google Scholar] [CrossRef]
- Madhu, N.T.; Salitros, I.; Schramm, F.; Klyatskaya, S.; Fuhr, O.; Ruben, M. Above room temperature spin transition in a series of iron(II) bis(pyrazolyl)pyridine compounds. Compte Rendus Chim. 2008, 11, 1166. [Google Scholar] [CrossRef]
- Šalitroš, I.; Fuhr, O.; Eichhöfer, A.; Kruk, R.; Pavlik, J.; Dlháň, L.; Boča, R.; Ruben, M. The interplay of iron(II) spin transition and polymorphism. Dalton Trans. 2012, 41, 5163–5171. [Google Scholar] [CrossRef]
- Šalitroš, I.; Pogány, L.; Ruben, M.; Boča, R.; Linert, W. Polymorphism dependent light induced spin transition. Dalton Trans. 2014, 43, 16584–16587. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, R.; Lazar, H.; Barrett, S.A.; Kilner, C.A.; Asthana, S.; Carbonera, C.; Létard, J.-F.; Halcrow, M.A. Thermal and light-induced spin-transitions in iron(II) complexes of 2,6-bis(4-halopyrazolyl)pyridines: The influence of polymorphism on a spin-crossover compound. Dalton Trans. 2009, 6656–6666. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Bergenti, I.; Milita, S.; Kengne, J.C.; Gentili, D.; Ruani, G.; Salitros, I.; Meded, V.; Ruben, M. Thin Deposits and Patterning of Room-Temperature-Switchable One-Dimensional Spin-Crossover Compounds. Langmuir 2011, 27, 4076–4081. [Google Scholar] [CrossRef] [PubMed]
- Devid, E.J.; Martinho, P.N.; Kamalakar, M.V.; Salitros, I.; Prendergast, U.; Dayen, J.-F.; Meded, V.; Lemma, T.; González-Prieto, R.; Evers, F.; et al. Spin Transition in Arrays of Gold Nanoparticles and Spin Crossover Molecules. ACS Nano 2015, 9, 4496–4507. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.D.; Tinant, B.; Muffler, K.; Wolny, J.A.; Schunemann, V.; Garcia, Y. Relevance of supramolecular interactions, texture and lattice occupancy in the designer iron(II) spin crossover complexes. J. Solid State Chem. 2009, 182, 1365–1376. [Google Scholar] [CrossRef]
- Šalitroš, I.; Pavlik, J.; Boča, R.; Fuhr, O.; Rajadurai, C.; Ruben, M. Supramolecular lattice-solvent control of iron(II) spin transition parameters. Cryst. Eng. Commun. 2010, 12, 2361–2368. [Google Scholar] [CrossRef]
- Rajadurai, C.; Qu, Z.; Fuhr, O.; Gopalan, B.; Kruk, R.; Ghafari, M.; Ruben, M. Lattice-solvent controlled spin transitions in iron(II) complexes. Dalton Trans. 2007, 3531–3537. [Google Scholar] [CrossRef] [PubMed]
- Kershaw Cook, L.J.; Shepherd, H.J.; Comyn, T.P.; Baldé, C.; Cespedes, O.; Chastanet, G.; Halcrow, M.A. Decoupled Spin Crossover and Structural Phase Transition in a Molecular Iron(II) Complex. Angew. Chem. Int. Ed. 2015, 21, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- ; where ϕi is value of N-Fe-N octahedron angle.
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.F.; Chasseau, D.; Ksenofontov, V.; Gaspar, A.B.; Gütlich, P. Structural Aspects of Spin Crossover. Example of the [FeIILn(NCS)2] Complexes. In Spin Crossover in Transition Metal Compounds II; Gütlich, P., Goodwin, H.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; ISSN: 0340-1022. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond; IUCr Oxford Science Publication: Oxford, UK, 1999; ISBN ISBN: 9780198509707. [Google Scholar]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. Sect. A 1990, 660, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97 (Release 97-2) Program for the refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]



| Compound (1) [Fe(L)2](BF4)2·CH3CN | Compound (2) [Fe(L)2](BF4)2·2CH3CN | ||
|---|---|---|---|
| Formula | C28H19B2Br2F8FeN11 | C30H22B2Br2F8FeN12 | C30H22B2Br2F8FeN12 |
| Formula weight/g·mol−1 | 898.83 | 939.89 | 939.89 |
| Crystal color | Red | orange | orange |
| Temperature/K | 180(2) | 180(2) | 293(2) |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | monoclinic | monoclinic |
| Space group | P-1 | C2/c | C2/c |
| a/Å | 11.324(2) | 24.948(5) | 25.311(5) |
| b/Å | 11.742(2) | 20.260(4) | 20.702(4) |
| c/Å | 14.187(3) | 18.360(4) | 18.395(4) |
| α/° | 83.29(3) | 90.00 | 90.00 |
| β/° | 84.40(3) | 129.92(3) | 129.81(3) |
| γ/° | 66.38(3) | 90.00 | 90.00 |
| V/Å3 | 1713.9(6) | 7117(3) | 7404(3) |
| Z, ρcalc/g·cm−3 | 2, 1.742 | 8, 1.754 | 8, 1.686 |
| μ (Mo-Kα)/mm−1 | 2.855 | 2.755 | 2.648 |
| F(000) | 884 | 3712 | 3712 |
| Crystal size/mm | 0.35 × 0.33 × 0.16 | 0.41 × 0.32 × 0.24 | 0.41 × 0.32 × 0.24 |
| θ range for the data collection/° | 1.45 to 25.64 | 1.46 to 25.62 | 1.34 to 25.79 |
| Final R indices [I > 2σ(I)] * | R1 = 0.0371 wR2 = 0.0929 | R1 = 0.0375 wR2 = 0.0852 | R1 = 0.0516 wR2 = 0.1314 |
| R indices (all data) * | R1 = 0.0453 wR2 = 0.0964 | R1 = 0.0525 wR2 = 0.0902 | R1 = 0.0707 wR2 = 0.1417 |
| GoF on F2 | 1.046 | 1.048 | 1.035 |
| CCDC deposit number | 846337 | 846338 | 846339 |
| (1) 180(2) K | (2) 180(2) K | (2) 293(2) K |
|---|---|---|
| Fe1-N1 = 1.973(2) | Fe1-N1 = 1.971(3) | Fe1-N1 = 2.133(4) |
| Fe1-N3 = 1.887(2) | Fe1-N3 = 1.906(3) | Fe1-N3 = 2.104(3) |
| Fe1-N5 = 1.972(2) | Fe1-N5 = 1.981(3) | Fe1-N5 = 2.151(4) |
| Fe1-N6 = 1.973(3) | Fe1-N6 =1.981(3) | Fe1-N6 =2.173(4) |
| Fe1-N8 = 1.895(2) | Fe1-N8 = 1.900(3) | Fe1-N8 = 2.098(3) |
| Fe1-N10 = 1.962(2) Npz-Fe-Npy * = 80.1 N3-Fe-Fe8 = 179.3 | Fe1-N10 = 1.998(3) Npz-Fe-Npy = 79.9 N3-Fe-Fe8 = 173.0 | Fe1-N10 = 2.160(4) Npz-Fe-Npy = 74.1 N3-Fe-Fe8 = 167.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šalitroš, I.; Fuhr, O.; Ruben, M. Solvent-Induced Polymorphism of Iron(II) Spin Crossover Complexes. Materials 2016, 9, 585. https://doi.org/10.3390/ma9070585
Šalitroš I, Fuhr O, Ruben M. Solvent-Induced Polymorphism of Iron(II) Spin Crossover Complexes. Materials. 2016; 9(7):585. https://doi.org/10.3390/ma9070585
Chicago/Turabian StyleŠalitroš, Ivan, Olaf Fuhr, and Mario Ruben. 2016. "Solvent-Induced Polymorphism of Iron(II) Spin Crossover Complexes" Materials 9, no. 7: 585. https://doi.org/10.3390/ma9070585





