Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation, Thermogravimetric Analysis, X-ray Diffraction, Fourier Transform Infrared Spectroscopy, in Vitro Degradation Studies and in Vitro Fibroblast Adhesion Studies
2.3. Mineralization of Films with Calcium Carbonate
2.4. Mineralization of Films with Calcium Phosphate
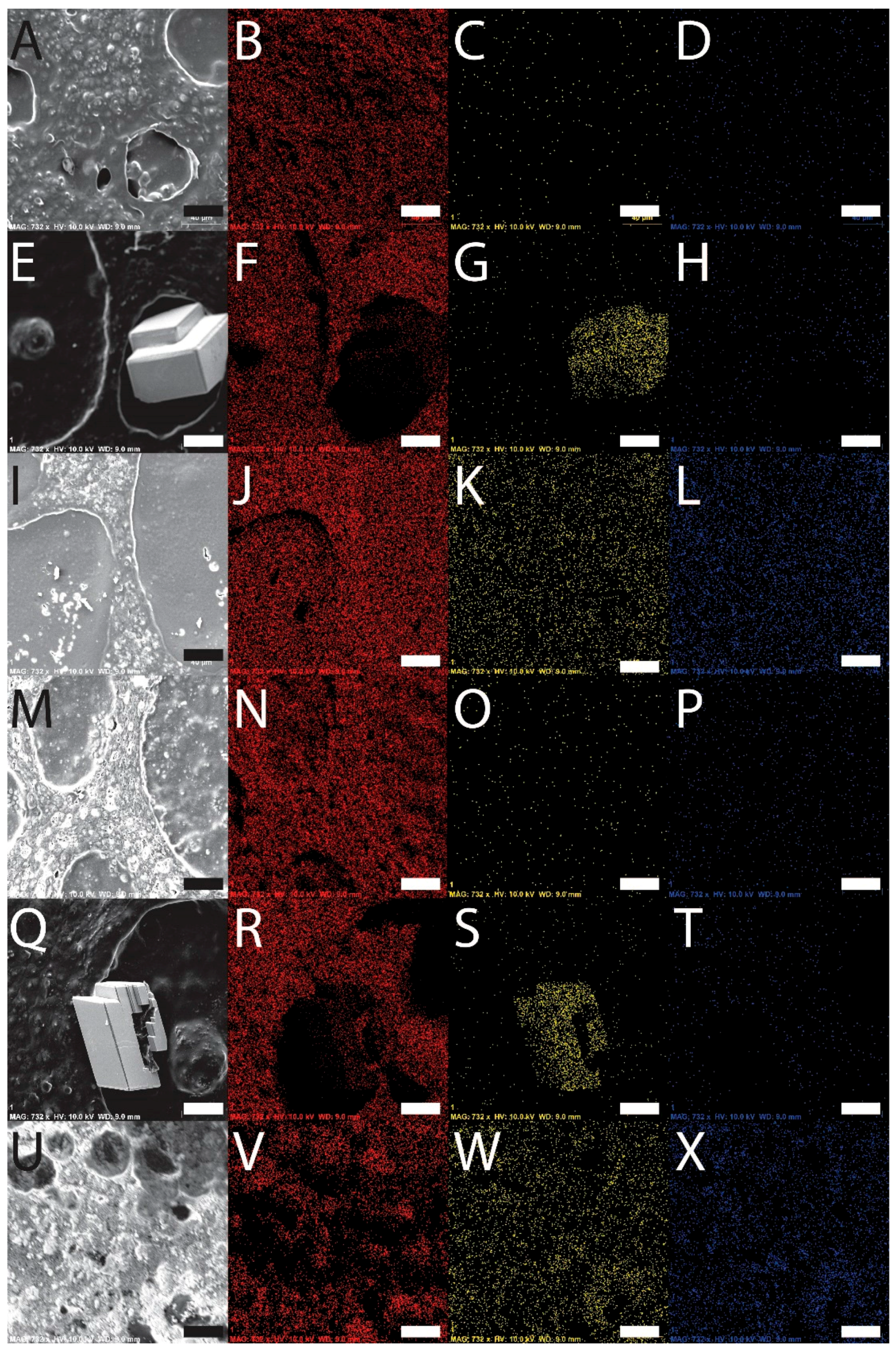
2.5. Scanning Electron Microscopy and Energy Dispersive Spectroscopy
2.6. Stem Cell Culture and Qualitative and Quantitative Studies of Alkaline Phosphatase Activity
3. Results and Discussion
3.1. Film Preparation and Characterization
3.2. In Vitro Degradation Studies
3.3. In Vitro Fibroblast Adhesion Studies
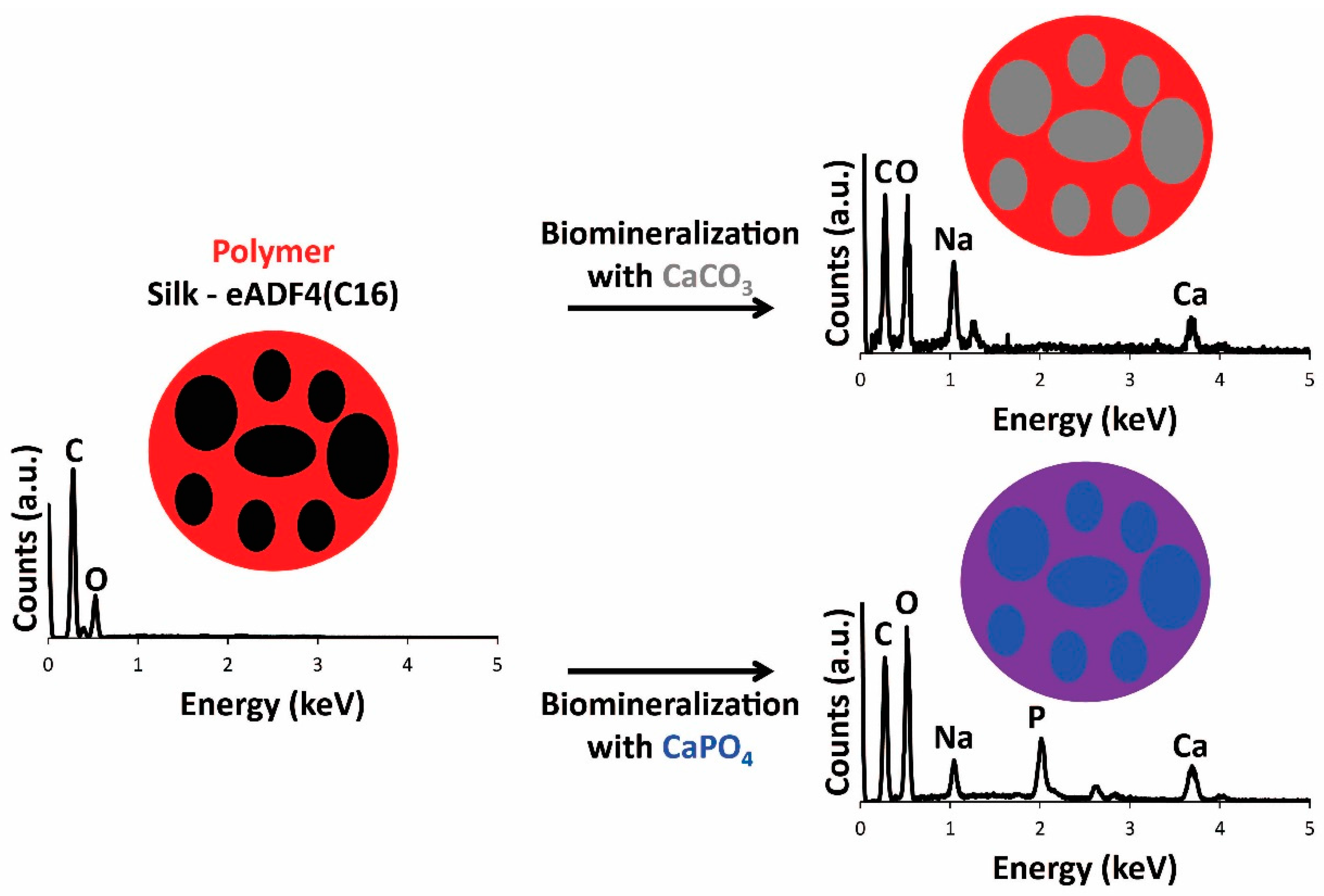
3.4. Film Biomineralization with Calcium Carbonate or Calcium Phosphate
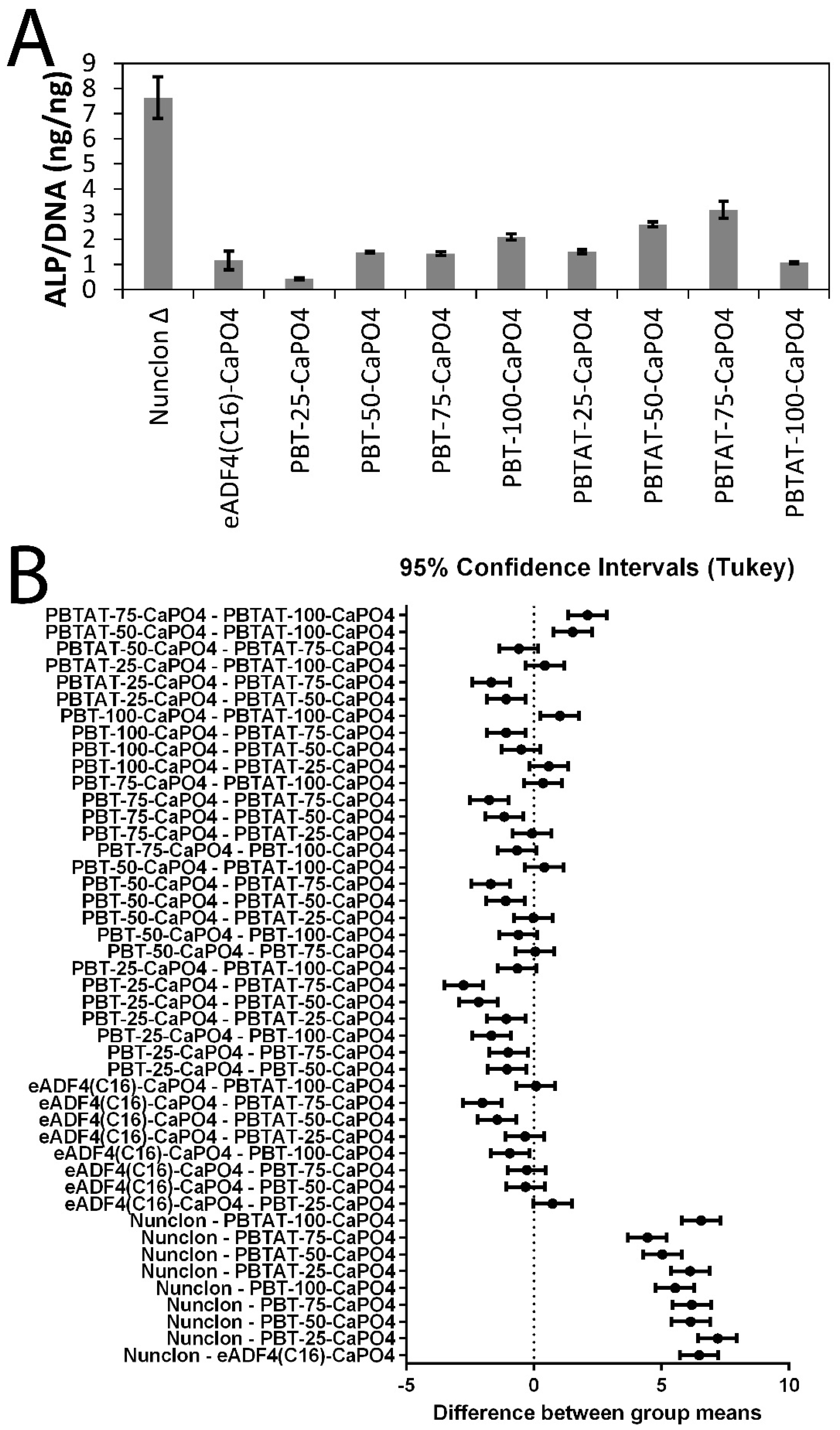
3.5. In Vitro Stem Cell Culture
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qiu, Z.Y.; Cui, Y.; Tao, C.-S.; Zhang, Z.-Q.; Tang, P.F.; Mao, K.-Y.; Wang, X.-M.; Cui, F.-Z. Mineralized Collagen: Rationale, Current Status, and Clinical Applications. Materials 2015, 8, 4733–4750. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Novel Bioactive Titanate Layers Formed on Ti Metal and Its Alloys by Chemical Treatments. Materials 2010, 3, 48–63. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, T. Chitin Scaffolds in Tissue Engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Di, L.; Ren, Q.-S.; Wang, J.-Y. Applications and Degradation of Proteins Used as Tissue Engineering Materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate Cements and Concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanodimensional and Nanocrystalline Apatites and Other Calcium Orthophosphates in Biomedical Engineering, Biology and Medicine. Materials 2009, 2, 1975–2045. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate-Based Bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef]
- Harrison, R.; Criss, Z.K.; Feller, L.; Modi, S.P.; Hardy, J.G.; Schmidt, C.E.; Suggs, L.J.; Murphy, M.B. Mechanical properties of α-tricalcium phosphate-based bone cements incorporating regenerative biomaterials for filling bone defects exposed to low mechanical loads. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.E.; McDevitt, T.C. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater. Sci. 2015, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Holderegger, C.; Schmidlin, P.R.; Weber, F.E.; Mohn, D. Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review. Materials 2015, 8, 4912–4931. [Google Scholar] [CrossRef]
- Raucci, M.G.; Guarino, V.; Ambrosio, L. Biomimetic Strategies for Bone Repair and Regeneration. J. Funct. Biomater. 2012, 3, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Jahan, K.; Tabrizian, M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomater. Sci. 2016, 4, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and Biodegradable Nanocomposites and Hybrid Biomaterials for Bone Regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, R.; Azhari, R.; Qanadilo, H.F.; Mahmood, T.A.; Triantafyllou, M.S.; Langer, R. Tissue engineering of fish skin: Behavior of fish cells on poly(ethylene glycol terephthalate)/poly(butylene terephthalate) copolymers in relation to the composition of the polymer substrate as an initial step in constructing a robotic/living tissue hybrid. Tissue Eng. 2004, 10, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Licht, R.; de Boer, J.; de Wijn, J.R.; van Blitterswijk, C.A. Fiber diameter and texture of electrospun PEOT/PBT scaffolds influence human mesenchymal stem cell proliferation and morphology, and the release of incorporated compounds. Biomaterials 2006, 27, 4911–4922. [Google Scholar] [CrossRef] [PubMed]
- Claase, M.B.; Olde Riekerink, M.B.; de Bruijn, J.D.; Grijpma, D.W.; Engbers, G.H.; Feijen, J. Enhanced bone marrow stromal cell adhesion and growth on segmented poly(ether ester)s based on poly(ethylene oxide) and poly(butylene terephthalate). Biomacromolecules 2003, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.; Mahmood, T.; Gupta, P.; Claase, M.B.; Grijpma, D.W.; Riesle, J.; van Blitterswijk, C.A.; Langer, R. The different behaviors of skeletal muscle cells and chondrocytes on PEGT/PBT block copolymers are related to the surface properties of the substrate. J. Biomed. Mater. Res. 2001, 54, 47–58. [Google Scholar] [CrossRef]
- Claase, M.B.; Grijpma, D.W.; Mendes, S.C.; De Bruijn, J.D.; Feijen, J. Porous PEOT/PBT scaffolds for bone tissue engineering: Preparation, characterization, and in vitro bone marrow cell culturing. J. Biomed. Mater. Res. A 2003, 64, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Claase, M.B.; de Bruijn, J.D.; Grijpma, D.W.; Feijen, J. Ectopic bone formation in cell-seeded poly(ethylene oxide)/poly(butylene terephthalate) copolymer scaffolds of varying porosity. J. Mater. Sci. Mater. Med. 2007, 18, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Van Dijkhuizen-Radersma, R.; Roosma, J.R.; Sohier, J.; Péters, F.L.; van den Doel, M.; van Blitterswijk, C.A.; de Groot, K.; Bezemer, J.M. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications: An in vivo evaluation. J. Biomed. Mater. Res. A 2004, 71, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, A.A.; van Apeldoorn, A.A.; Hayen, H.; de Bruijn, J.D.; Karst, U.; Grijpma, D.W.; Feijen, J. In vivo and in vitro degradation of poly(ether ester) block copolymers based on poly(ethylene glycol) and poly(butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shuai, Y.; He, W.; Min, S.; Zhu, L. Preparation of Porous Scaffolds from Silk Fibroin Extracted from the Silk Gland of Bombyx mori (B. mori). Int. J. Mol. Sci. 2012, 13, 7762–7775. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Kim, B.-S.; Kim, I.-S. Fabrication and Biocompatibility of Electrospun Silk Biocomposites. Membranes 2011, 1, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Ghezzi, C.E.; Saballos, R.J.; Kaplan, D.L.; Schmidt, C.E. Supracolloidal Assemblies as Sacrificial Templates for Porous Silk-Based Biomaterials. Int. J. Mol. Sci. 2015, 16, 20511–20522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Dawson, R.A.; Chirila, T.V.; Shadforth, A.M.A.; Hogerheyde, T.A.; Edwards, G.A.; Harkin, D.G. Treatment of Silk Fibroin with Poly(ethylene glycol) for the Enhancement of Corneal Epithelial Cell Growth. J. Funct. Biomater. 2015, 6, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Liu, J.M.-J.; Chua, C.-K.; Chou, S.-M.; Shyu, V.B.-H.; Chen, J.-P. Cartilage Tissue Engineering with Silk Fibroin Scaffolds Fabricated by Indirect Additive Manufacturing Technology. Materials 2014, 7, 2104–2119. [Google Scholar] [CrossRef]
- Bray, L.J.; Suzuki, S.; Harkin, D.G.; Chirila, T.V. Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes. J. Funct. Biomater. 2013, 4, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.G.; Geissler, S.A.; Aguilar, D.; Villancio-Wolter, M.K.; Mouser, D.J.; Sukhavasi, R.C.; Cornelison, R.C.; Tien, L.W.; Preda, R.C.; Hayden, R.S.; et al. Instructive Conductive 3D Silk Foam-Based Bone Tissue Scaffolds Enable Electrical Stimulation of Stem Cells for Enhanced Osteogenic Differentiation. Macromol. Biosci. 2015, 15, 1490–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; Suzuki, S.; Alzonne, R.; Edwards, G.A.; Richardson, N.; Chirila, T.V.; Harkin, D.G. Incorporation of Human Recombinant Tropoelastin into Silk Fibroin Membranes with the View to Repairing Bruch’s Membrane. J. Funct. Biomater. 2015, 6, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Humenik, M.; Smith, A.M.; Scheibel, T. Recombinant Spider Silks—Biopolymers with Potential for Future Applications. Polymers 2011, 3, 640–661. [Google Scholar] [CrossRef]
- Widhe, M.; Johansson, J.; Hedhammar, M.; Rising, A. Invited review current progress and limitations of spider silk for biomedical applications. Biopolymers 2012, 97, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, C.; Hedhammar, M.; Feinstein, R.; Nordling, K.; Kratz, G.; Johansson, J.; Huss, F.; Rising, A. Tissue Response to Subcutaneously Implanted Recombinant Spider Silk: Anin vivo Study. Materials 2009, 2, 1908–1922. [Google Scholar] [CrossRef]
- Dinjaski, N.; Kaplan, D.L. Recombinant protein blends: Silk beyond natural design. Curr. Opin. Biotechnol. 2015, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, M.A.; Nogueira, G.M.; Weska, R.F.; Beppu, M.M. Preparation and Characterization of Insoluble Silk Fibroin/Chitosan Blend Films. Polymers 2010, 2, 719–727. [Google Scholar] [CrossRef]
- Cai, Z.; Mo, X.; Zhang, K.; Fan, L.; Yin, A.; He, C.; Wang, H. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, R.; Scheibel, T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry 2004, 26, 13604–13612. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Scheibel, T.R. Production and processing of spider silk proteins. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3957–3963. [Google Scholar] [CrossRef]
- Schacht, K.; Scheibel, T. Processing of recombinant spider silk proteins into tailor-made materials for biomaterials applications. Curr. Opin. Biotechnol. 2014, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, E.; Schacht, K.; Jüngst, T.; Groll, J.; Scheibel, T. Biofabrication of 3D constructs: Fabrication technologies and spider silk proteins as bioinks. Pure Appl. Chem. 2015, 87, 737–749. [Google Scholar] [CrossRef]
- Hardy, J.G.; Pfaff, A.; Leal-Egaña, A.; Müller, A.H.E.; Scheibel, T.R. Glycopolymer Functionalization of Engineered Spider Silk Protein-based Materials for Improved Cell Adhesion. Macromol. Biosci. 2014, 14, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Leal-Egaña, A.; Scheibel, T.R. Engineered Spider Silk Protein-Based Composites for Drug Delivery. Macromol. Biosci. 2013, 13, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 28 June 2016).
- Nandakumar, A.; Yang, L.; Habibovic, P.; van Blitterswijk, C. Calcium phosphate coated electrospun fiber matrices as scaffolds for bone tissue engineering. Langmuir 2010, 26, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yang, G. Crystallization, and morphology of poly(trimethylene terephthalate)/poly(ethylene oxide terephthalate) segmented block copolymers. Polymer 2010, 51, 1516–1523. [Google Scholar] [CrossRef]
- Müller-Herrmann, S.; Scheibel, T. Enzymatic Degradation of Films, Particles, and Nonwoven Meshes Made of a Recombinant Spider Silk Protein. ACS Biomater. Sci. Eng. 2015, 1, 247–259. [Google Scholar] [CrossRef]
- Radtke, C.; Allmeling, C.; Waldmann, K.-H.; Reimers, K.; Thies, K.; Schenk, H.C.; Hillmer, A.; Guggenheim, M.; Brandes, G.; Vogt, P.M. Spider Silk Constructs Enhance Axonal Regeneration and Remyelination in Long Nerve Defects in Sheep. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liu, X.; Liu, S.; Zhang, A.; Lu, Q.; Kaplan, D.L.; Zhu, H. Biomineralization regulation by nano-sized features in silk fibroin proteins: Synthesis of water-dispersible nano-hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, Z.; Lu, Q.; Huang, Y.; Kaplan, D.L.; Zhu, H. Hierarchical biomineralization of calcium carbonate regulated by silk microspheres. Acta Biomater. 2013, 9, 6974–6980. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, F.C.; Coelfen, H. Controlling Mineral Morphologies and Structures in Biological and Synthetic Systems. Chem. Rev. 2008, 108, 11, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.-W.; Ma, Y.; Coelfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.W.P.; Patwardhan, S.V.; Belton, D.J.; Kitchel, B.; Anastasiades, D.; Huang, J.; Naik, R.R.; Perry, C.C.; Kaplan, D.L. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 9428–9433. [Google Scholar]
- Mieszawska, A.J.; Nadkarni, L.D.; Perry, C.C.; Kaplan, D.L. Nanoscale control of silica particle formation via silk-silica fusion proteins for bone regeneration. Chem. Mater. 2010, 22, 5780–5785. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, L.; Luo, Y.; Schlaad, H.; Seitz, M.; Colfen, H.; Gaub, H.E. Quantitative single molecule measurements on the interaction forces of poly(l-glutamic acid) with calcite crystals. J. Am. Chem. Soc. 2007, 129, 15364–15371. [Google Scholar] [CrossRef] [PubMed]
- Casse, O.; Shkilnyy, A.; Linders, J.; Mayer, C.; Haussinger, D.; Volkel, A.; Thunemann, A.F.; Dimova, R.; Colfen, H.; Meier, W.; et al. Solution Behavior of Double-Hydrophilic Block Copolymers in Dilute Aqueous Solution. Macromolecules 2012, 45, 4772–4777. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.G.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]

| Film | Mass Ratio Protein:Polymer | Continuous Phase | Fibroblast Adhesion Relative to Nunclon® Δ Surface (%) | Figure |
|---|---|---|---|---|
| eADF4(C16) | 100:0 | eADF4(C16) | 72.0 ± 8.0 | S1 and [7] |
| PBT-25 | 75:25 | eADF4(C16) | 55.5 ± 5.9 | S2 |
| PBT-50 | 50:50 | PBT | 58.9 ± 8.0 | S3 |
| PBT-75 | 25:75 | PBT | 69.8 ± 10.0 | S4 |
| PBT-100 | 0:100 | PBT | 75.8 ± 3.5 | S5 |
| PBTAT-25 | 75:25 | eADF4(C16) | 76.9 ± 6.6 | S6 |
| PBTAT-50 | 50:50 | PBTAT | 104.5 ± 4.4 | S7 |
| PBTAT-75 | 25:75 | PBTAT | 76.4 ± 2.4 | S8 |
| PBTAT-100 | 0:100 | PBTAT | 69.3 ± 2.4 | S9 |
| Untreated Nunclon® | Not applicable | Not applicable | 74.0 ± 6.2 | S11 |
| Nunclon® Δ Surface | Not applicable | Not applicable | 100.0 ± 7.5 | [47] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardy, J.G.; Torres-Rendon, J.G.; Leal-Egaña, A.; Walther, A.; Schlaad, H.; Cölfen, H.; Scheibel, T.R. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials 2016, 9, 560. https://doi.org/10.3390/ma9070560
Hardy JG, Torres-Rendon JG, Leal-Egaña A, Walther A, Schlaad H, Cölfen H, Scheibel TR. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials. 2016; 9(7):560. https://doi.org/10.3390/ma9070560
Chicago/Turabian StyleHardy, John G., Jose Guillermo Torres-Rendon, Aldo Leal-Egaña, Andreas Walther, Helmut Schlaad, Helmut Cölfen, and Thomas R. Scheibel. 2016. "Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering" Materials 9, no. 7: 560. https://doi.org/10.3390/ma9070560









