Preparation and Properties of Electrospun Poly (Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CNCs
2.3. Preparation of PVP/CNC/Ag Nanocomposites
3. Characterization
3.1. Characterization of Electrospinning Suspensions
3.2. Characterization of PVP/CNC/Ag Nanocomposites
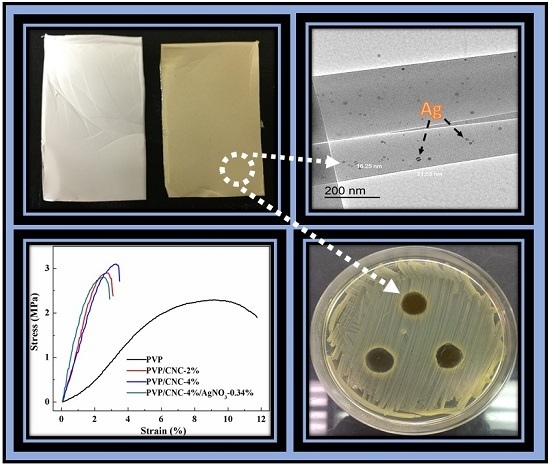
3.2.1. Field Emission-Scanning Electron Microscopy (FE-SEM) Analysis
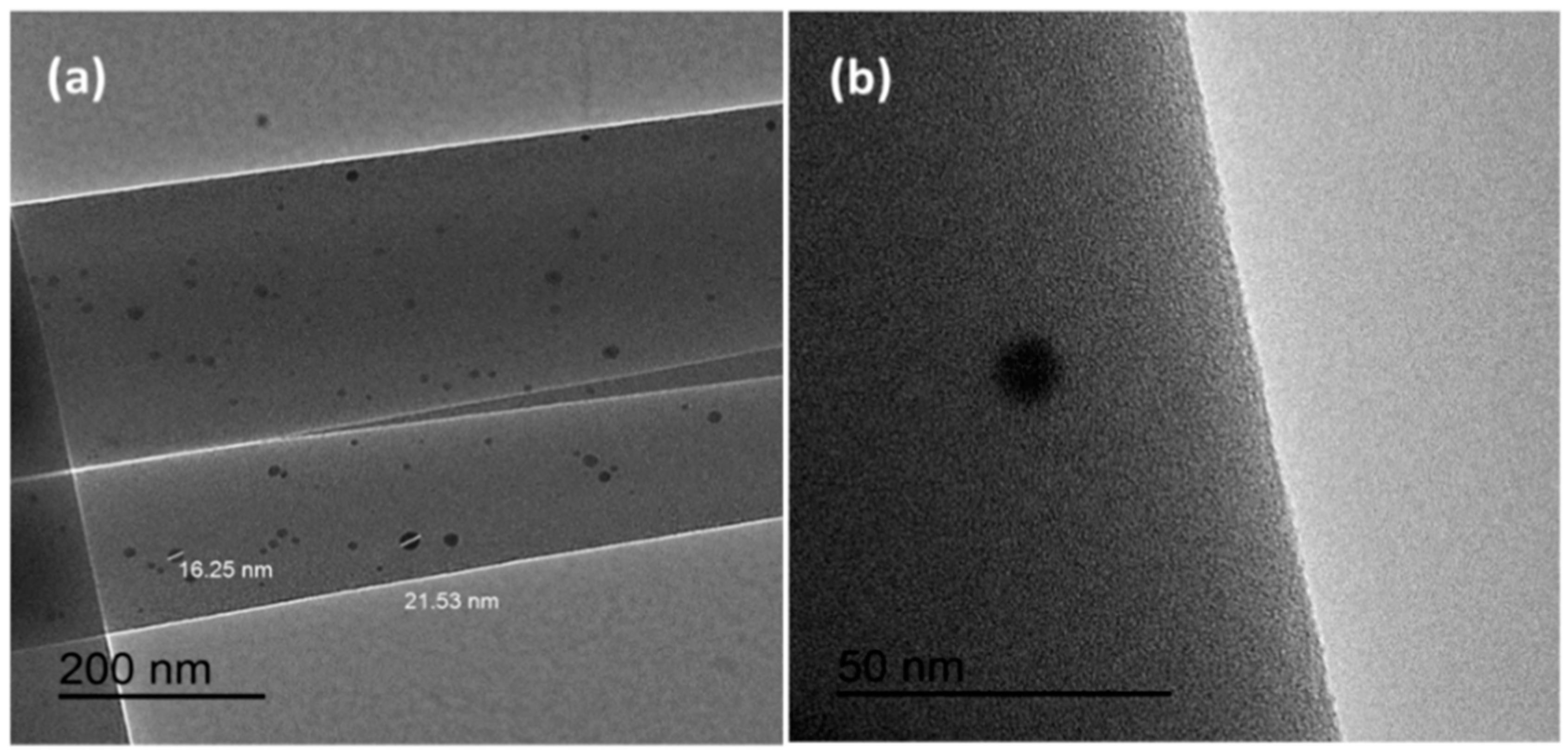
3.2.2. Transmission Electron Microscopy (TEM)
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. Thermogravimetric Analysis (TGA)
3.2.5. Mechanical Properties
3.2.6. Antimicrobial Performance
4. Results and Discussions
4.1. Properties of PVP/CNC/AgNO3 Suspension
4.2. Morphologies of Fibrous Mats
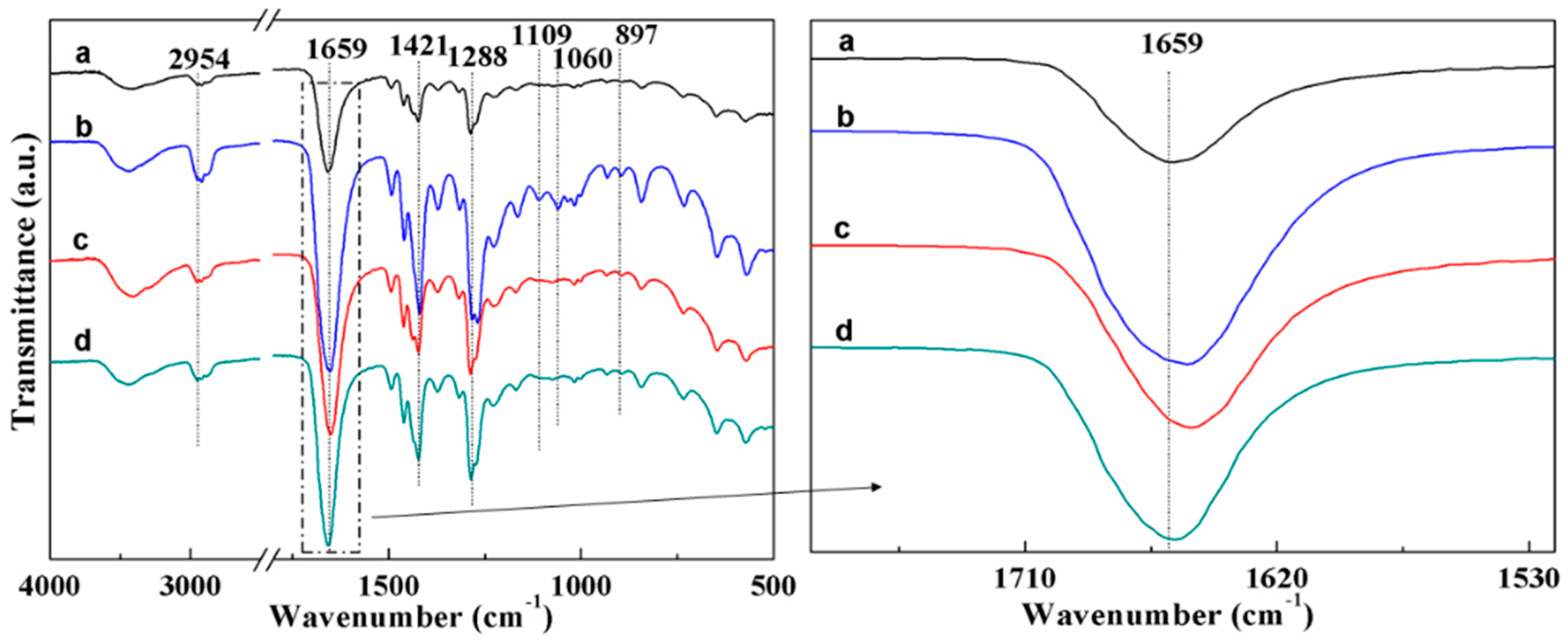
4.3. FTIR Analysis
4.4. Thermal Properties
4.5. Mechanical Properties
4.6. Antimicrobial Performance
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bourourou, M.; Holzinger, M.; Bossard, F.; Hugenell, F.; Maaref, A.; Cosnier, S. Chemically reduced electrospun polyacrilonitrile-carbon nanotube nanofibers hydrogels as electrode material for bioelectrochemical applications. Carbon 2015, 87, 233–238. [Google Scholar]
- Hong, K.H. Preparation and properties of electrospun poly(vinyl alcohol)/silver fiber web as wound dressings. Polym. Eng. Sci. 2007, 47, 43–49. [Google Scholar]
- Baptista, A.C.; Botas, A.M.; Almeida, A.P.C.; Nicolau, A.T.; Falcão, B.P.; Soares, M.J.; Leitão, J.P.; Martins, R.; Borges, J.P.; Ferreira, I. Down conversion photoluminescence on PVP/Ag-nanoparticles electrospun composite fibers. Opt. Mater. 2015, 39, 278–281. [Google Scholar]
- Nasouri, K.; Shoushtari, A.M.; Mojtahedi, M.R.M. Effects of polymer/solvent systems on electrospun polyvinylpyrrolidone nanofiber morphology and diameter. Polym. Sci. Ser. A 2015, 57, 747–755. [Google Scholar]
- Nasouri, K.; Shoushtari, A.M.; Mojtahedi, M.R.M. Evaluation of effective electrospinning parameters controlling polyvinylpyrrolidone nanofibers surface morphology via response surface methodology. Fibers Polym. 2015, 16, 1941–1954. [Google Scholar] [CrossRef]
- Gupta, D.; Jassal, M.; Agrawal, A.K. Electrospinning of poly(vinyl alcohol)-based boger fluids to understand the role of elasticity on morphology of nanofibers. Ind. Eng. Chem. Res. 2015, 54, 1547–1554. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Electrospinning chitosan/poly(ethylene oxide) solutions with essential oils: Correlating solution rheology to nanofiber formation. Carbohydr. Polym. 2016, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, A.; Ajji, A. Fabrication of polyaniline/poly(ethylene oxide)/non-covalently functionalized graphene nanofibers via electrospinning. Synth. Metals 2015, 200, 7–15. [Google Scholar] [CrossRef]
- Dufficy, M.K.; Geiger, M.T.; Bonino, C.A.; Khan, S.A. Electrospun ultrafine fiber composites containing fumed silica: From solution rheology to materials with tunable wetting. Langmuir ACS J. Surf. Coll. 2015, 31, 12455–12463. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by adding polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J. 2007, 43, 1112–1122. [Google Scholar] [CrossRef]
- Castillo-Ortega, M.M.; Nájera-Luna, A.; Rodríguez-Félix, D.E.; Encinas, J.C.; Rodríguez-Félix, F.; Romero, J.; Herrera-Franco, P.J. Preparation, characterization and release of amoxicillin from cellulose acetate and poly(vinyl pyrrolidone) coaxial electrospun fibrous membranes. Mater. Sci. Eng. C 2011, 31, 1772–1778. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Li, M.; Wang, S.; Zhang, C.; Yang, Q. Electrospinning method for the preparation of silver chloride nanoparticles in PVP nanofiber. Appl. Surf. Sci. 2008, 254, 4520–4523. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Sun, G.; Zhang, G.; Liu, H.; Du, J.; Yang, S.; Bai, J.; Yang, Q. Fabrication of Au/PVP nanofiber composites by electrospinning. J. Appl. Polym. Sci. 2007, 105, 3618–3622. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, G.; Dong, F.; Liu, Q.; Nie, W. Preparation and characterization of electrospun poly(ε-caprolactone)/poly(vinyl pyrrolidone) nanofiber composites containing silver particles. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Li, C.; Zhang, Y.; Zhang, J. Ag nanoparticle-embedded one-dimensional β-CD/PVP composite nanofibers prepared via electrospinning for use in antibacterial material. Colloid Polym. Sci. 2012, 290, 667–672. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-nps/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Wu, Q.; Song, K.; Qing, Y.; Wu, Y. Cellulose nanoparticles as modifiers for rheology and fluid loss in bentonite water-based fluids. ACS Appl. Mater. Interfaces 2015, 7, 5006–5016. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanoparticles: Structure-morphology-rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Gabr, M.H.; Phong, N.T.; Okubo, K.; Uzawa, K.; Kimpara, I.; Fujii, T. Thermal and mechanical properties of electrospun nano-celullose reinforced epoxy nanocomposites. Polym. Test. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Wanasekara, N.D.; Santos, R.P.O.; Douch, C.; Frollini, E.; Eichhorn, S.J. Orientation of cellulose nanocrystals in electrospun polymer fibres. J. Mater. Sci. 2015, 51, 218–227. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospun bionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind. Crops Prod. 2016, in press. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Jiang, L.; Wang, X.; Payne, S.A.; Zhu, J.Y.; Li, R. Comparison between cellulose nanocrystal and cellulose nanofibril reinforced poly(ethylene oxide) nanofibers and their novel shish-kebab-like crystalline structures. Macromolecules 2014, 47, 3409–3416. [Google Scholar] [CrossRef]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, R.; Liu, J.; Lan, Y.; Zhang, Y.; Xue, W.; Zhang, Y. Preparation and properties of PLGA nanofiber membranes reinforced with cellulose nanocrystals. Colloids Surf. B Biointerfaces 2015, 132, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, C.; Yue, Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical properties and in vitro degradation of electrospun bio-nanocomposite mats from PLA and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; Zhang, X.; McCord, M. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. Eur. Polym. J. 2011, 47, 1402–1409. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Shang, S.; Song, Z. Synthesis and characterization of Ag–Pd alloy nanoparticles/carboxylated cellulose nanocrystals nanocomposites. Carbohydr. Polym. 2011, 83, 38–43. [Google Scholar] [CrossRef]
- Borůvková, K.; Wiener, J. Polymer films based on cellulose containing silver nanoparticles and vitamin C. ACC J. 2015, 21, 15–23. [Google Scholar] [CrossRef]
- Quiros, J.; Borges, J.P.; Boltes, K.; Rodea-Palomares, I.; Rosal, R. Antimicrobial electrospun silver-, copper- and zinc-doped polyvinylpyrrolidone nanofibers. J. Hazard. Mater. 2015, 299, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Xiao, X.; Liu, X.; Qian, B.; Ma, Z.; Ye, S.; Chen, D.; Qiu, J. Preparation and characterization of Ag nanoparticle-embedded polymer electrospun nanofibers. J. Nanopart. Res. 2009, 12, 1319–1329. [Google Scholar] [CrossRef]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Arias Chavez, L.H.; Elimelech, M. Antimicrobial electrospun biopolymer nanofiber mats functionalized with graphene oxide-silver nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, H.; Zhang, J.; Zhao, Y.; Yuan, X. Preparation and antibacterial activity of electrospun chitosan/poly(ethylene oxide) membranes containing silver nanoparticles. Colloid Polym. Sci. 2009, 287, 1425–1434. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M. Co-spinning of silver nanoparticles with nisin increases the antimicrobial spectrum of PDLLA: PEO nanofibers. Curr. Microbiol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wu, Q.; Zhang, Z.; Ren, S.; Lei, T.; Negulescu, I.I.; Zhang, Q. Porous carbon nanofibers from electrospun biomass tar/polyacrylonitrile/silver hybrids as antimicrobial materials. ACS Appl. Mater. Interfaces 2015, 7, 15108–15116. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wu, Q.; Zhang, Z.; Ren, S.; Lei, T.; Dooley, K.M.; Liu, D.; Janes, M.E. Fabricating electrospun nanofibers with antimicrobial capability: A facile route to recycle biomass tar. Fuel 2015, 150, 123–130. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Crespí-Llorens, D.; Vicente, P.; Viedma, A. Generalized reynolds number and viscosity definitions for non-newtonian fluid flow in ducts of non-uniform cross-section. Exp. Therm. Fluid Sci. 2015, 64, 125–133. [Google Scholar] [CrossRef]
- Pontrelli, G.; Gentili, D.; Coluzza, I.; Pisignano, D.; Succi, S. Effects of non-linear rheology on electrospinning process: A model study. Mech. Res. Commun. 2014, 61, 41–46. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Zhang, D.; Karki, A.B.; Rutman, D.; Young, D.P.; Wang, A.; Cocke, D.; Ho, T.H.; Guo, Z. Electrospun polyacrylonitrile nanocomposite fibers reinforced with Fe3O4 nanoparticles: Fabrication and property analysis. Polymer 2009, 50, 4189–4198. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; De Hoop, C.F.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanocrystals and polyanionic cellulose as additives in bentonite water-based drilling fluids: Rheological modeling and filtration mechanisms. Ind. Eng. Chem. Res. 2016, 55, 133–143. [Google Scholar] [CrossRef]
- Cacciotti, I.; Fortunati, E.; Puglia, D.; Kenny, J.M.; Nanni, F. Effect of silver nanoparticles and cellulose nanocrystals on electrospun poly(lactic) acid mats: Morphology, thermal properties and mechanical behavior. Carbohydr. Polym. 2014, 103, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.-J.; Lee, H.K.; Jeong, E.H.; Park, W.H.; Youk, J.H. Preparation of polymer nanofibers containing silver nanoparticles by using poly(n-vinylpyrrolidone). Macromol. Rapid Commun. 2005, 26, 1903–1907. [Google Scholar] [CrossRef]
- Wu, J.C.-C.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.; Cooney, R.P. Effect of polyvinylpyrrolidone on storage stability, anti-oxidative and anti-bacterial properties of colloidal polyaniline. Synth. Metals 2016, 217, 202–209. [Google Scholar] [CrossRef]
- César, N.R.; Pereira-da-Silva, M.A.; Botaro, V.R.; de Menezes, A.J. Cellulose nanocrystals from natural fiber of the macrophyte typha domingensis: Extraction and characterization. Cellulose 2015, 22, 449–460. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, C.; Xing, C.; Shi, X.; Matuana, L.M.; Zhou, H.; Ma, X. Fabrication and characteristics of cellulose nanofibril films from coconut palm petiole prepared by different mechanical processing. Ind. Crops Prod. 2015, 65, 96–101. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Chen, D.; Liu, M.; Chen, Q.; Ge, L.; Fan, B.; Wang, H.; Lu, H.; Yang, D.; Zhang, R.; Yan, Q.; et al. Large-scale synthesis and enhanced visible-light-driven photocatalytic performance of hierarchical Ag/AgCl nanocrystals derived from freeze-dried PVP–Ag+ hybrid precursors with porosity. Appl. Catal. B Environ. 2014, 144, 394–407. [Google Scholar] [CrossRef]
- Khan, S.B.; Alamry, K.A.; Bifari, E.N.; Asiri, A.M.; Yasir, M.; Gzara, L.; Ahmad, R.Z. Assessment of antibacterial cellulose nanocomposites for water permeability and salt rejection. J. Ind. Eng. Chem. 2015, 24, 266–275. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.; Song, K.; Lei, T.; Wu, Y. Poly(vinylidene fluoride)/cellulose nanocrystals composites: Rheological, hydrophilicity, thermal and mechanical properties. Cellulose 2015, 22, 2431–2441. [Google Scholar] [CrossRef]
- Bao, J.; Clarke, L.I.; Gorga, R.E. Effect of constrained annealing on the mechanical properties of electrospun poly(ethylene oxide) webs containing multiwalled carbon nanotubes. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 787–796. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H.; Aghazadeh, M. Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int. J. Biol. Macromol. 2015, 79, 269–277. [Google Scholar] [CrossRef] [PubMed]
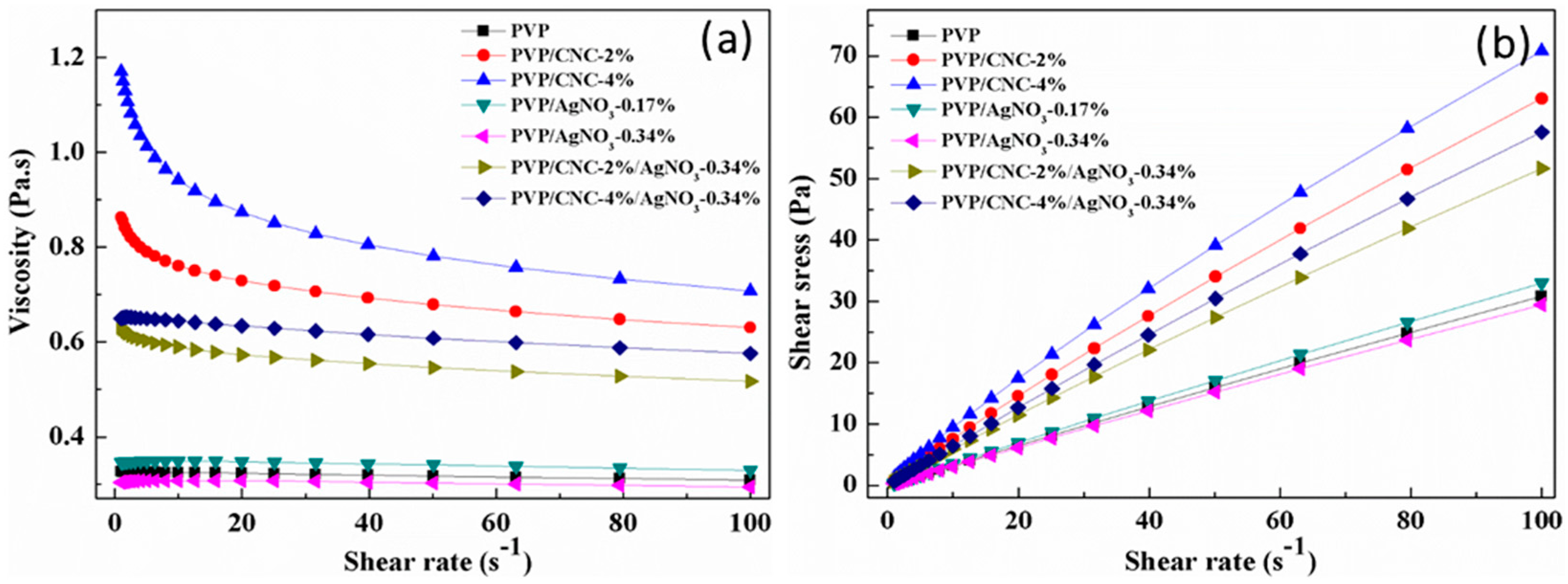
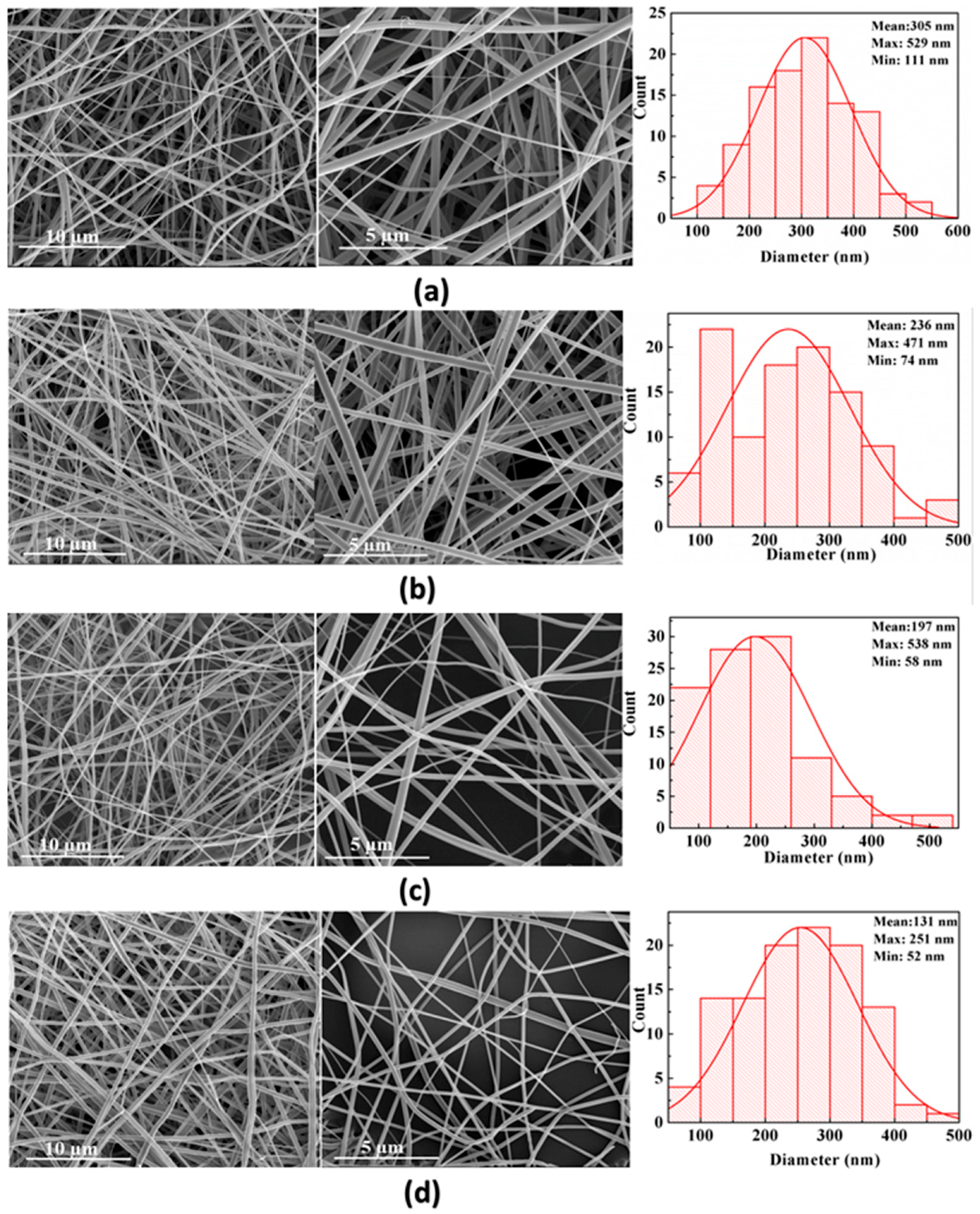



| Samples | Composition | Electrical Conductivity (µs·cm−1) | Viscosity (Pa·s) | Average Fiber Diameters (nm) |
|---|---|---|---|---|
| 1 | PVP | 45.3 ± 0.17 | 0.3228 | 305 ± 31 |
| 2 | PVP/CNC-2% | 55.2 ± 0.19 | 0.7185 | 236 ± 40 |
| 3 | PVP/CNC-4% | 60.2 ± 0.20 | 0.8512 | 197 ± 41 |
| 4 | PVP/AgNO3-0.17% | 53.8 ± 0.22 | 0.3467 | 214 ± 35 |
| 5 | PVP/AgNO3-0.34% | 59.9 ± 0.23 | 0.3075 | 193 ± 43 |
| 6 | PVP/CNC-2%/AgNO3-0.34% | 84.8 ± 0.23 | 0.5675 | 151 ± 45 |
| 7 | PVP/CNC-4%/AgNO3-0.34% | 157.7 ± 0.24 | 0.6228 | 131 ± 46 |
| Samples | Models | |||||
|---|---|---|---|---|---|---|
| BP | PL | |||||
| τo | μ | R2 | K | n | R2 | |
| PVP | 0.127 | 0.311 | 0.9997 | 0.353 | 0.97 | 0.9999 |
| PVP/CNC-2% | 1.011 | 0.639 | 0.9980 | 0.951 | 0.91 | 0.9999 |
| PVP/CNC-4% | 1.813 | 0.717 | 0.9962 | 1.280 | 0.87 | 0.9999 |
| PVP/AgNO3-0.17% | 0.149 | 0.333 | 0.9996 | 0.384 | 0.97 | 0.9999 |
| PVP/AgNO3-0.34% | 0.105 | 0.297 | 0.9997 | 0.334 | 0.97 | 0.9999 |
| PVP/CNC-2%/AgNO3-0.34% | 0.556 | 0.523 | 0.9989 | 0.694 | 0.94 | 0.9999 |
| PVP/CNC-4%/AgNO3-0.34% | 0.520 | 0.584 | 0.9990 | 0.753 | 0.94 | 0.9999 |
| Composition | T10% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|
| PVP | 396.0 | 430.5 | 4.0 |
| PVP/CNC-2% | 385.8 | 429.3 | 7.1 |
| PVP/CNC-4% | 375.8 | 431.0 | 6.6 |
| PVP/CNC-4%/AgNO3-0.34% | 391.6 | 426.8 | 9.2 |
| CNC | 292.5 | 348.3 | – |
| Sample | Elongation at Break (%) | Ultimate Tensile Strength (MPa) |
|---|---|---|
| PVP | 9.10 ± 0.2 | 2.30 ± 0.2 |
| PVP/CNC-2% | 2.70 ± 0.2 | 2.90 ± 0.2 |
| PVP/CNC-4% | 3.25 ± 0.2 | 3.10 ± 0.1 |
| PVP/CNC-4%/AgNO3-0.34% | 2.50 ± 0.3 | 2.81 ± 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and Properties of Electrospun Poly (Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers. Materials 2016, 9, 523. https://doi.org/10.3390/ma9070523
Huang S, Zhou L, Li M-C, Wu Q, Kojima Y, Zhou D. Preparation and Properties of Electrospun Poly (Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers. Materials. 2016; 9(7):523. https://doi.org/10.3390/ma9070523
Chicago/Turabian StyleHuang, Siwei, Ling Zhou, Mei-Chun Li, Qinglin Wu, Yoichi Kojima, and Dingguo Zhou. 2016. "Preparation and Properties of Electrospun Poly (Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers" Materials 9, no. 7: 523. https://doi.org/10.3390/ma9070523