1. Introduction
Restriction on the use of lead based solder in the electronic industry has led to extensive developments in lead free solders. Driven by the necessity to improve the reliability of lead free electronic products and by the trend towards miniaturization, researchers are putting intense efforts into improving the properties of Sn based solders. Sn-Ag-Cu (SAC solder) alloys have been a favored replacement for Sn-Pb solders. However, the currently used ternary eutectic (T
m = 217 °C) or near eutectic SAC solders have some drawbacks. These SAC solders were reported to have relatively high amount of undercooling (10–30 °C), as β-Sn requires large undercooling to nucleate and solidify. This large undercooling promotes the formation of large, plate-like Ag
3Sn structures that have been reported to cause joint embrittlement and reliability problem [
1]. Research has been done on solders with lower silver content of, e.g., Sn-1.0Ag-0.5Cu (SAC105), in an attempt to inhibit the formation of Ag
3Sn and also to reduce the solder cost, as the price of Ag has increased dramatically in recent years [
2,
3,
4,
5]. Although it has been reported that SAC105 performs better in drop tests, it has a higher liquidus temperature, which requires a higher reflow profile than the eutectic Sn-3.8Ag-0.7Cu (SAC387) alloy [
5]. It also performs poorly in thermal cycling tests [
6]. These drawbacks have prompted researchers to modify the solder content by adding small amounts of other alloying elements to further improve its reliability and lower its melting temperature.
Fourth minor alloying elements (0.05–0.1 wt.%) that have been added into SAC solder include Ni, Co, Fe, Mn, Zn, Ti, Ce, In and Al [
7,
8,
9,
10]. The effects of the fourth alloying element on the microstructure and mechanical properties have been investigated. Improvements in mechanical properties such as shear strength [
10], tensile strength [
7], impact resistance [
5], and creep resistance [
7] have been reported. It has been observed that the addition of fourth alloying elements imparts their influence on the mechanical properties by modifying the microstructure of the solder. It was observed that the fourth alloying element modify the microstructure, by: (i) refining the microstructure of the solder matrix; (ii) suppressing brittle intermetallic compound (IMC) formation; and (iii) changing the morphology of the IMC in solder matrix/interface [
11].
The addition of aluminum has attracted much interest in recent years. Research on Al addition as nanoparticles or minor alloying element has been reported [
12,
13,
14,
15,
16,
17,
18]. Amagai [
12] observed that the addition of 0.05% Al nanoparticles did not have any significant effect on the interfacial IMC. As for the addition of aluminum as minor alloying element, a few works on the effect of Al on the mechanical properties, microstructure and solder/Cu substrate interfacial reaction have been reported [
2,
9,
11,
14,
15,
16,
17,
18]. Previous studies have shown that minor addition of Al gives promising results in suppressing undercooling of β-Sn and reduced formation of brittle phase Ag
3Sn in SAC solder matrix [
14]. It was found that Al addition (0.05 wt.%) results in excellent shear strength retention after thermal aging at 150 °C for up to 1000 h. Faizul et al. have investigated the effect of Al (0.1–2 wt.%) on the tensile strength of SAC105 [
12,
19]. They found that with 0.1 wt.% Al addition, Al reduced the yield strength and ultimate tensile strength (UTS) and promoted ductile fracture. When Al addition was more than 0.1 wt.%, the yield strength and ultimate tensile strength of the solder increased as a function of Al content and brittle fracture modes were seen.
A couple of studies dealt with the effects of Al on the bulk microstructure and interfacial IMC between solder and copper substrate [
15,
16,
18]. With the addition of Al into Sn-Ag (SA) solder, Al
2Cu IMC and ambiguous Al-Cu IMC were found in the bulk microstructure of SA solder on copper substrate after reflow [
16,
18]. On the other hand, only ambiguous Al-Cu IMC (Anderson et al. reported it as δ-Cu
33Al
17) was found in the bulk microstructure of SAC [
14,
19]. Though the reported effect of Al on the bulk microstructure seen by other researchers seems consistent, there are contradictory reports on the effects of Al addition on the interfacial solder/Cu reaction. With addition of 0.5 wt.% of Al into SA solder, Xia et al. observed a spalled layer of Al
2Cu compound layer near the interface and the suppression of the growth of the Cu
6Sn
5 layer. When Al was added up to 1 wt.%, a layer of Al
2Cu compound was formed at the solder/Cu interface, which completely replaced Cu
6Sn
5 layer [
15]. On the other hand, Kotadia et al. reported that spherical Al-Cu IMC spalled away from the solder matrix when Al was depleted in the solder and there was no suppression of the growth of interfacial Cu
6Sn
5 layer when 0.5 to 2.0 wt.% of Al was added in to SA solder [
18]. Li et al. have reported that with the addition of 1 wt.% Al into SAC387 solder, layered δ-Al
2Cu
3 formed at the interface after 10 min reflow and the growth of the Cu
6Sn
5 layer was reduced [
16]. Dhaffer et al. have investigated the effect of 0.1.wt.% Al addition on the interfacial reaction by dipping method, they reported that 0.1% Al addition have suppressed the growth of Cu
6Sn
5 layer [
20]. Until now, no detailed study has been done for the effect of minor Al addition (<1 wt.%) on interfacial reaction of SAC/Cu.
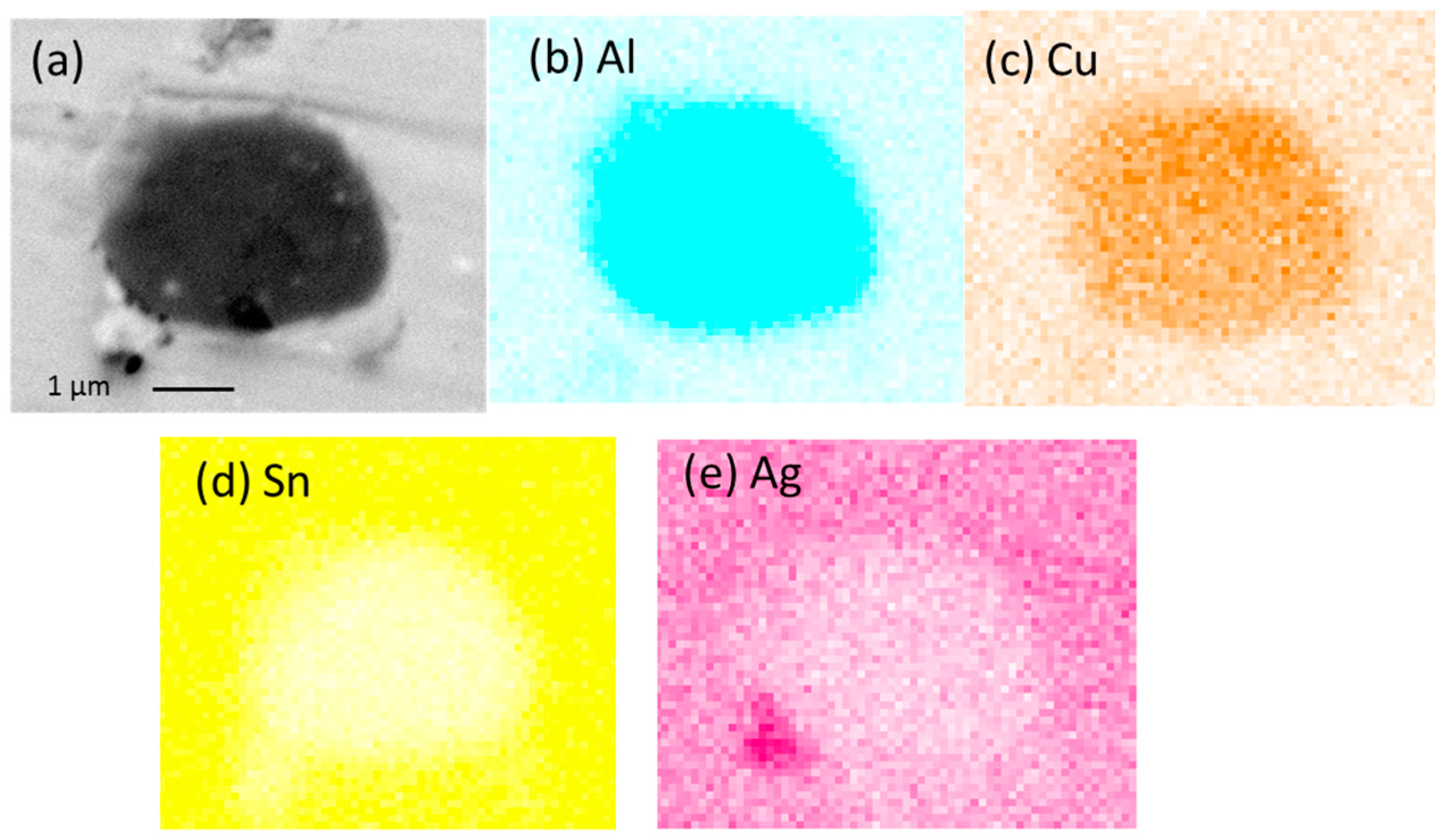
Nanoindentation as a mechanical testing method has attracted a great deal of attraction in many fields of research as it has the ability to measure the properties of sample in extremely small scales such as thin films and coating in nanometer range [
21]. This technique is well suited to investigate the mechanical properties of IMCs, which have thicknesses of only several nanometers/micrometers. Cu-Al IMC was reported to form in SAC even with addition of as low as 0.05 wt.% [
22]. Besides, it has been reported that Al is likely to substitute into Cu
6Sn
5 as well [
14] Previous studies with addition such as Ni, and Mn have shown that solubility of minor alloying element in Cu
6Sn
5 could alter the nanomechanical properties of Cu
6Sn
5 [
14]. Thus, understanding the mechanical properties of Cu-Al IMC and Cu
6Sn
5 is essential in the understanding of deformation behavior and failure mechanisms in lead-free solder joints.
The present work investigates the effect of Al on the interfacial IMC between SAC105 solder alloy and copper substrate. This work concentrates on the effect of the lower percentage of Al (0.1–0.5 wt.%) on the Cu–Sn reaction during reflow and isothermal aging. As has been noted earlier [
15,
16], absence of Cu in the solder led to the formation of Al
2Cu and Al-Cu IMC near/at the interface during reflow, and the IMCs tended to spall away from the solder matrix when Al was depleted in solder, thus complicating the situation at the interface. With the presence of Cu in SAC solder, it is expected that Al will react with Cu in the bulk and thus provide a more simplified scenario at the interface, which may lead to a better understanding of the effects of Al. This study concentrates on the lower percentage of Al (0.1%–0.5%), as higher percentages may lead to formation of Al
2Cu and other IMCs at the interface. Furthermore, this study investigates the nanomechanical properties of Cu-Al IMC particles and also examines whether addition of Al would affect the nanomechanical properties of Cu
6Sn
5.
3. Discussion
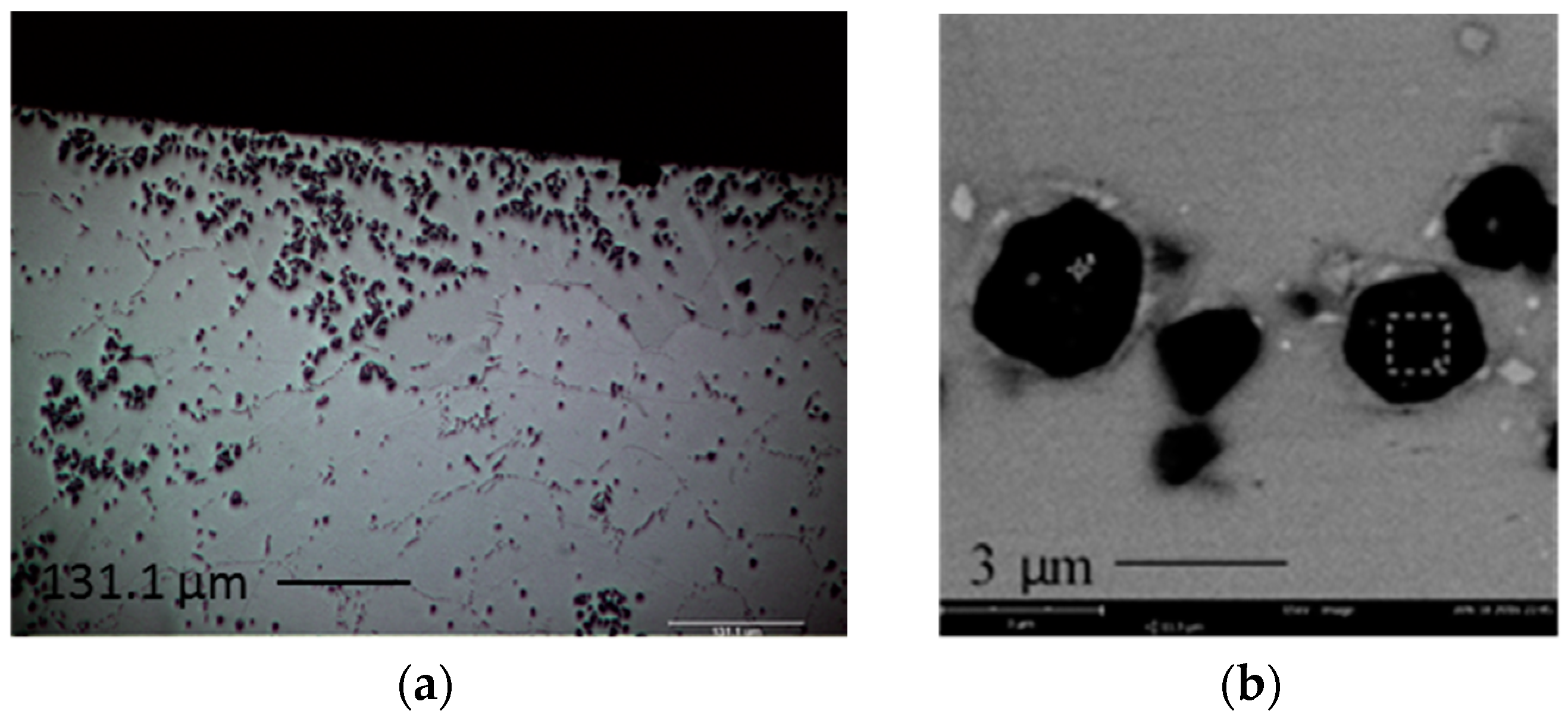
From the DSC curves (
Figure 1), it is found that when 0.1 wt.% of Al was added to the solder, the endothermic curve remains similar to that of SAC105. Both SAC105 and SAC105 + 0.1Al exhibit twin peaks endothermic curve. The onset melting temperature of SAC105 + 0.1Al is the same as that of SAC105, which is ~217 °C. This corresponds to Sn-Ag-Cu ternary eutectic temperature. When the percentage of Al is increased to 0.3 wt.% and 0.5 wt.%, the onset of melting temperature shifts to ~221 °C (Sn-Ag eutectic temperature) [
30]. The DSC curves of SAC105 + 0.3Al and SAC + 0.5Al solders are similar to the DSC curve of non-eutectic Sn-Ag solder [
31]. This shows that free Cu atoms are not available in SAC105 + 0.3Al and SAC + 0.5Al solders as Sn-Ag-Cu solder alloy always shows the onset melting at ~217 °C [
32]. One possible explanation for this is the formation of Cu
3Al
2 compound, which has reduced the available Cu in the bulk solder to react with Sn and Ag. The Cu
3Al
2 compound already formed in the as-received samples, which were supplied in the as-cast condition. As seen in
Figure 2b–d, Cu
3Al
2 is formed in the as-cast solder. Cu
3Al
2 has a higher melting temperature of 960 °C [
33]. Thus, during the melting of solders for up to 300 °C, the stable Cu
3Al
2 IMC did not react and this lowers the activity of Cu in solder melts. The lack of free Cu atom in the solder is further indicated by the shift of a prominent peak in the DSC curves of SAC105 and SAC105 + 0.1Al. The ~228 °C (Sn-Cu eutectic temperature) peak in SAC105 and SAC105 + 0.5Al has shifted to ~231 °C (T
m of Sn = 232 °C) in SAC105 + 0.3Al and SAC105 + 0.5Al. This shift indicates that the deficiency of Cu atom in SAC105 + 0.3Al and SAC105 + 0.5Al has reduced the strong eutectic reaction of Sn-Cu, L → Cu
6Sn
5 + Sn. With Al addition, the peak indicating Sn melting is seen, as fractions of un-melted Sn remaining in the solder melted when temperature increased to 232 °C [
30].
Table 1 shows that SAC105 has the largest undercooling of ~17 °C. This is well within range of undercooling values, 10–30 °C reported for Sn-Ag-Cu solder [
1]. This is because β-Sn requires large undercooling to induce nucleation and solidification [
1]. The presence of Al in SAC105 has reduced the undercooling significantly. It is seen that with addition of Al up to 0.5%, undercooling has reduced to 1–5 °C. Kotadia et al. and Anderson et al. have observed the effect of Al in reducing undercooling of Sn-3.5Ag and Sn-3.5Ag-0.95Cu solder to 7 °C and 4 °C respectively [
14,
34]. The addition of minor alloying element into solder has been one of the effective ways of reducing undercooling by promoting nucleation of β-Sn. Minor alloying atoms which have a much higher melting temperature can exist in molten Sn and provide heterogeneous site for β-Sn nucleation. In the case of Al as minor alloying, Cu
3Al
2 compound is formed even when the addition of Al is as low as 0.1%. During exothermic reaction in DSC, the existing Al-Cu intermetallic compounds act as a preferential site to promote heterogeneous nucleation of β-Sn, and thus lowering the undercooling of SAC105.
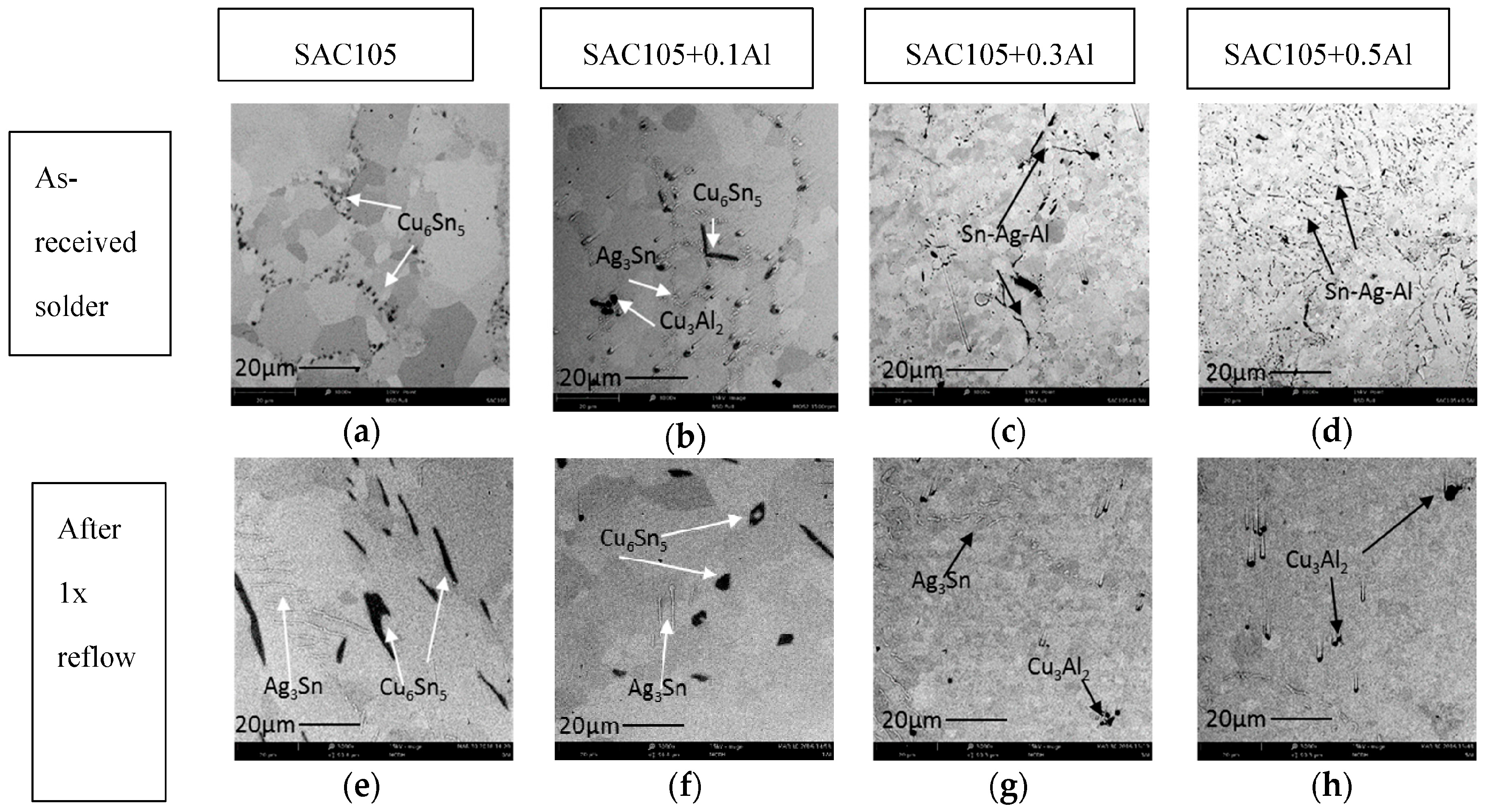
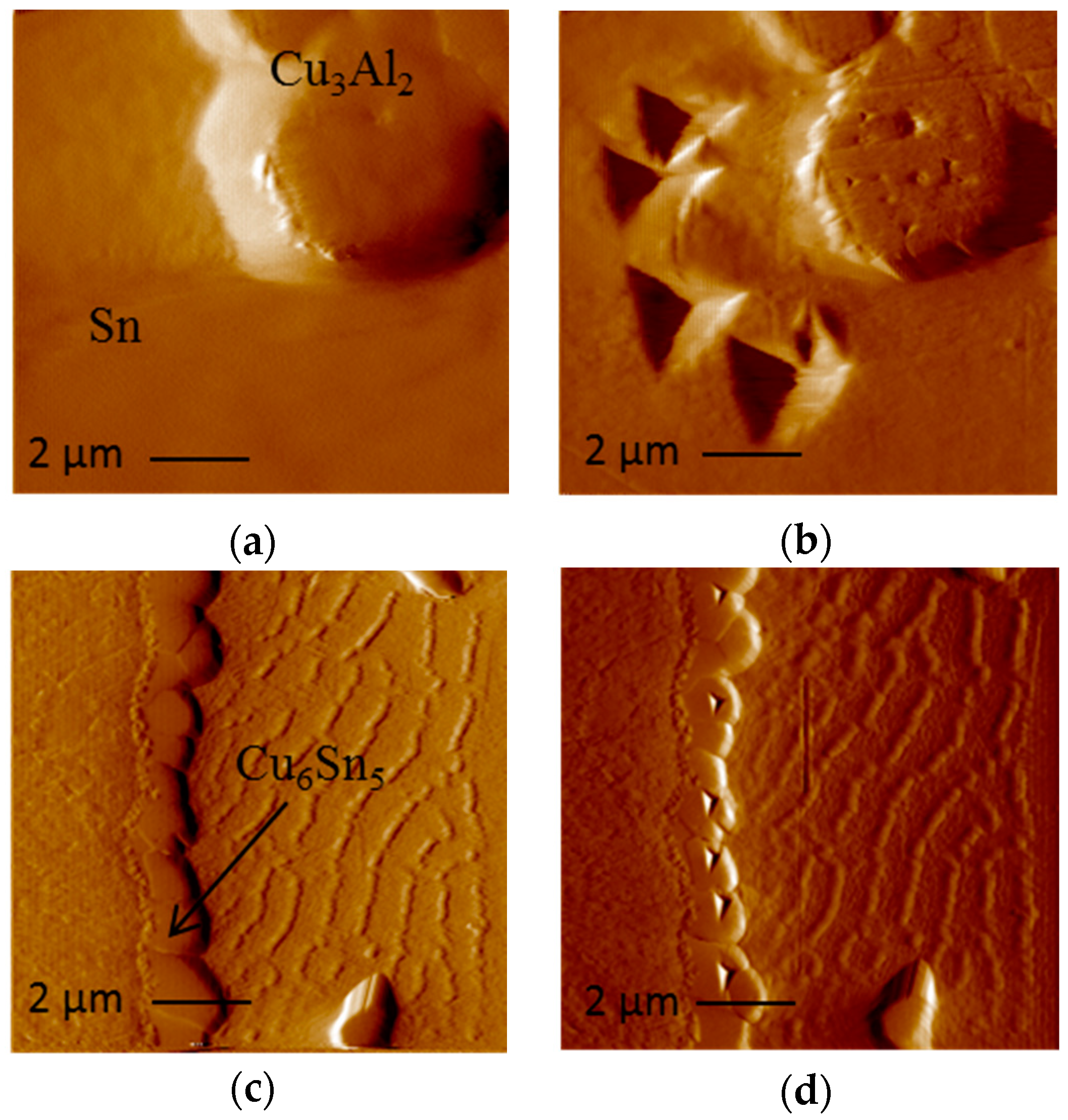
Segregated Cu
3Al
2 IMC was found near the top surface of the sample with addition of Al after 1 × reflow. One of the possible reasons could be that, during reflow, solders are melted on the Cu substrates at 250 °C, the Cu
3Al
2 particles are not wet by the molten solder, thus they are not drawn into the melt as the molten solder particles solidify. This causes rejection of the Cu
3Al
2, which remain near the surface/edges of the solder after reflow. Another possible reason for the segregation of Cu
3Al
2 particles at the edge/surface could be attributed to the buoyancy effects. The density of Cu
3Al
2 particles is 6.278 g/cm
3, which is lower than the density of liquid Sn, 6.99 g/cm
3 [
35]. Thus, during reflow, the less dense Cu
3Al
2 particles migrate to the surface/edges. Kotadia et al. have reported the segregation of Al
2Cu in SA solder. They suggested that the segregation of Al rich phase and Al-Cu compound is caused by Stokes and Marangoi motion, which is due to large stable liquid miscibility gap in binary Sn-Al and ternary Sn-Ag-Al [
18].
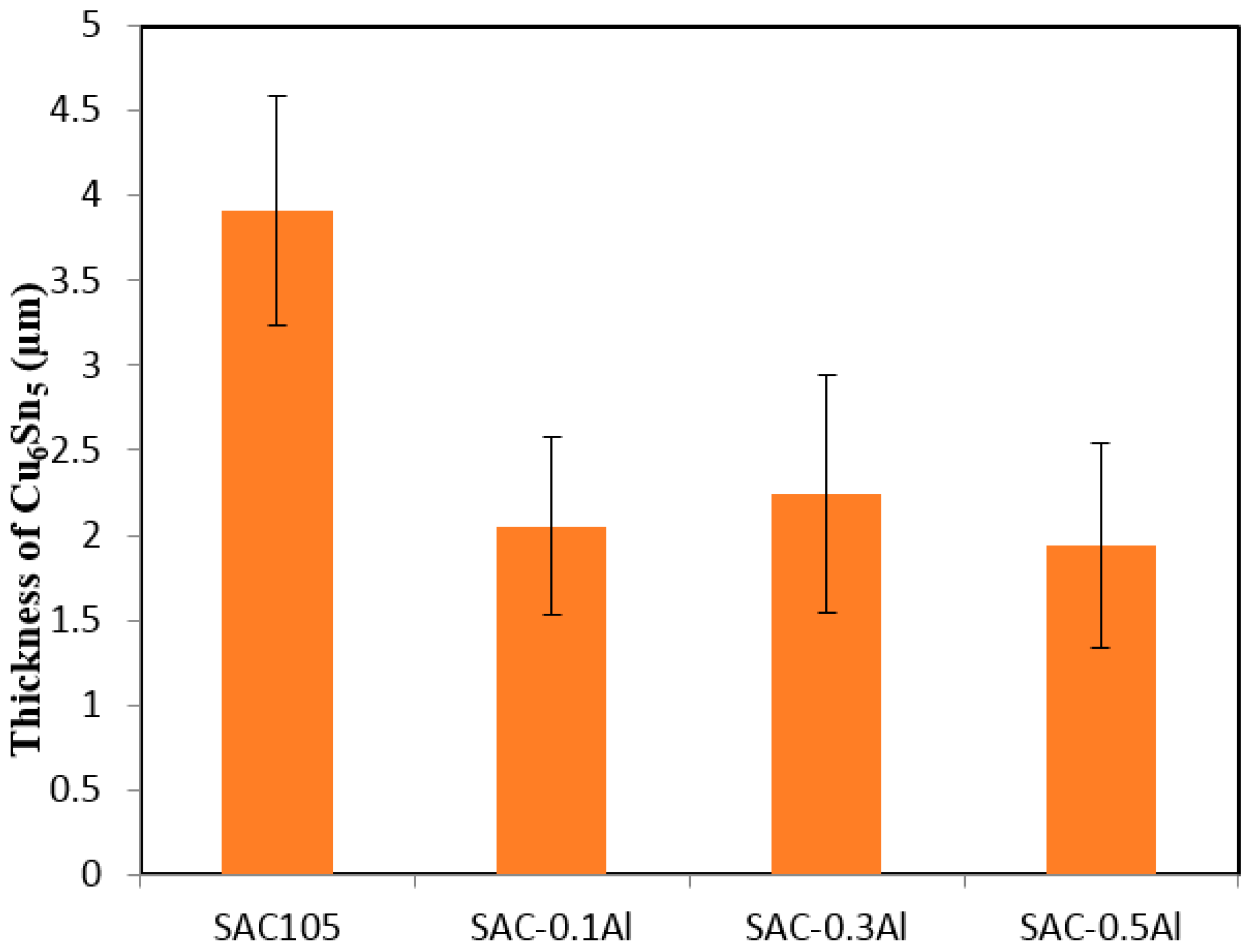
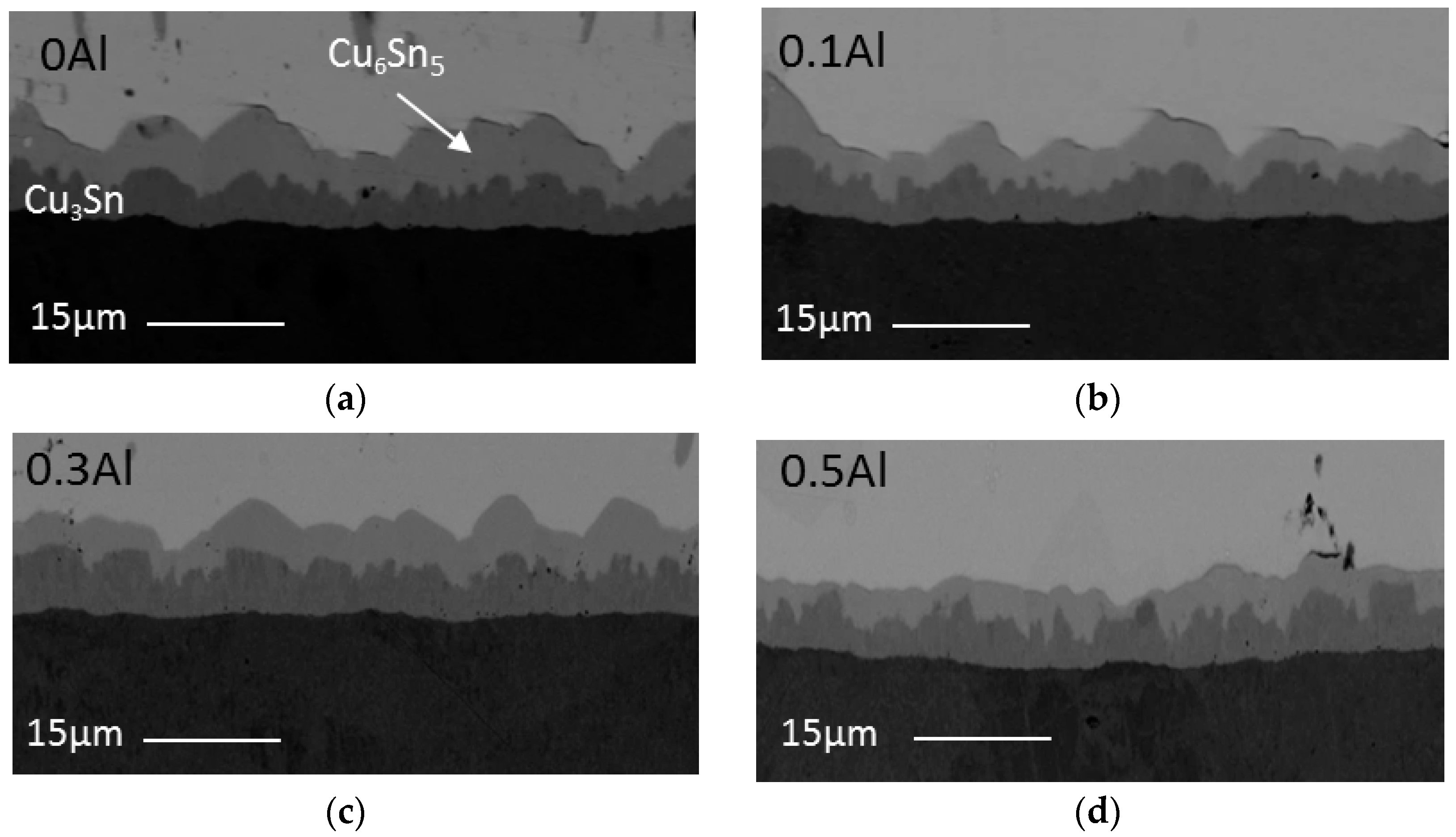
Minor alloying elements which could affect the growth of Cu-Sn compound are divided into two categories: (i) elements that show marked solubility in either one or both of the Cu-Sn IMCs; and (ii) elements that do not significantly dissolve in either of the Cu–Sn IMCs [
11]. The effect of elements in category 1 on IMC growth could be explained by using thermodynamic argument. These elements stabilize Cu
6Sn
5 and decrease the growth of Cu
3Sn. The elements in category 2 do not have a prominent effect on IMC as they only affect the growth of IMC layers indirectly. It can be seen from
Figure 4 and
Figure 5 that the addition of Al has reduced the growth of Cu
6Sn
5. Though Anderson et al. have suggested Al has some solubility in Cu
6Sn
5, no trace of Al could be found in Cu
6Sn
5 in this study. In this study, the addition of up to 0.5 wt.% Al did not alter the scallop morphology of Cu
6Sn
5 [
14]. Scallop type IMC formation is promoted by higher value of the IMC/liquid solder interfacial energy.
There is very limited amount of information available on the influence of Al on the Cu–Sn reaction. Li et al. have reported the suppression of Cu
6Sn
5 IMC growth with addition of 1% Al. They suggested that the suppression is due to the formation of an Al-Cu IMC layer at the interface, which acts as a barrier for Cu and Sn diffusion [
16]. However, Al-Cu IMC layer is not found at the interface in this study as the amount of Al added is less (≤5%). Dhaffer et al. have also reported suppression of Cu
6Sn
5 IMC growth with addition of 0.1% Al during dip soldering and solid state reaction [
20]. The Cu
3Al
2 IMC found at the Cu
6Sn
5/Sn interface (
Figure 8) could account for the suppression of Cu
6Sn
5. This is seen in previous work where Zn found at the Cu
6Sn
5/Sn interface, hindered the flow of copper atoms to the solder thereby slowing down the IMC growth [
36]. Thus, the segregation of Al atoms at the IMC/Sn interface may have similar effect on the growth of IMC, by hindering the flow of Cu or Sn atom. With minor Al addition, most of the Al reacts with Cu in the bulk solder to form Cu
3Al
2. Hence, Al does not form a layer of compound at the interface. Reduction of free Cu atom in the bulk solder could also be attributed to the retardation of Cu
6Sn
5 growth. When the amount of Al in the solder increased (≥0.5 wt.% Al in SA, ≥1 wt.% Al in SAC), it formed a layer of Cu-Al compound at the solder/Cu interface [
15,
16]. By their presence at the interface, Cu
3Al
2 IMC hinders the flow of Cu or Sn atom to the solder thereby retarding IMC growth during reflow.
During isothermal aging, the Cu
6Sn
5 IMC layer grows by interdiffusion of Cu and Sn and reaction with each other, while the Cu
3Sn IMC forms and grows by reactions between the Cu substrate and Cu
6Sn
5 IMC layer, as given in the equation below [
11]:
The presence of Cu3Al2 IMC at the Cu6Sn5/Sn interface hinders the flow of Cu or Sn atom to the solder, however it does not affect the reaction in Equation (1). Cu3Sn grows by consuming Cu6Sn5 that is formed during reflow. Thus, the thickness of Cu3Sn was not significantly affected by the addition of Al in solder. On the other hand, with slower interdiffusion of Cu and Sn at the interface (due to presence of Cu3Al2) and Cu3Sn formation by consumption of Cu6Sn5, the thickness of Cu6Sn5 was reduced in SAC105 + Al solder.
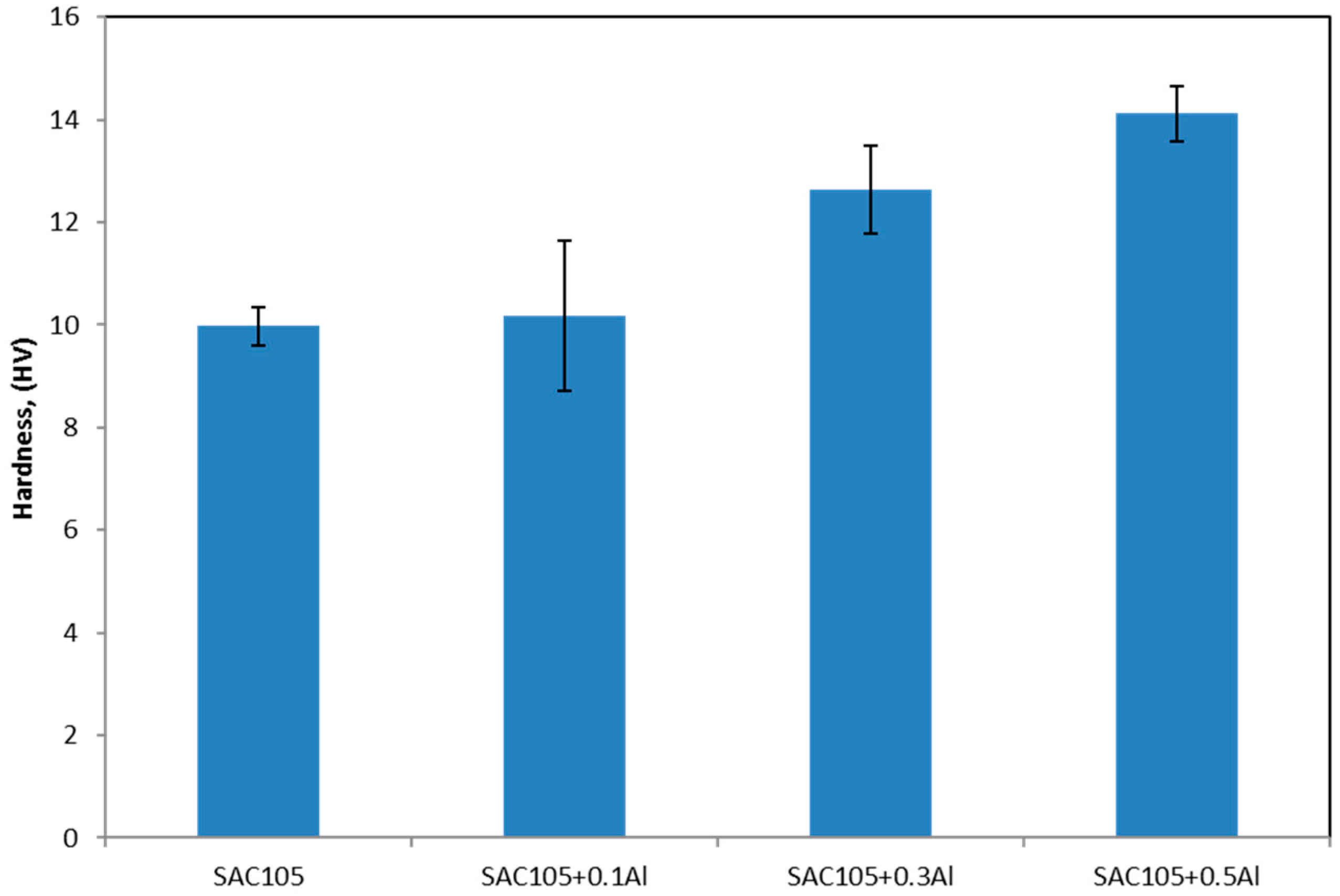
The addition of Al has increased the hardness of bulk solder. This could be due to the grain refinement of Sn, which can be seen in
Figure 3. Kim et al. has also reported that addition of Al as low as 0.01 wt.% could refine the Sn grain of Sn-Cu solder [
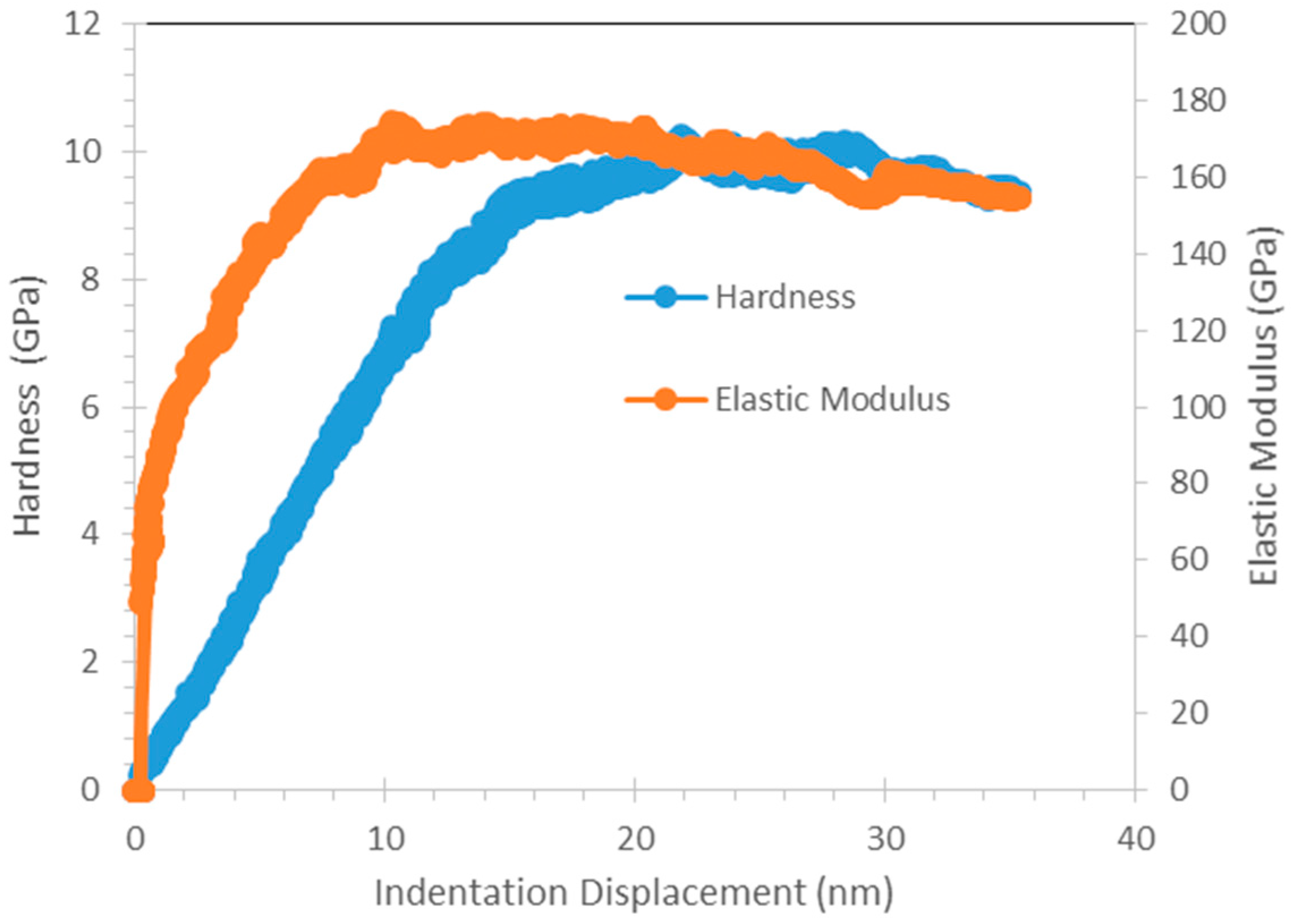
8]. From nanoindentation, Cu
3Al
2 that are found in all SAC + Al solder exhibits higher hardness than other elements in SAC105 + Al. This seems promising in strengthening the solder joints. However, its high elastic modulus should be considered as well, as high elastic modulus could be detrimental for impact testing. Thus, further testing need to be done to further verify the effects of strengthening effects of Cu
3Al
2.
In spite of the incorporation by only a small fraction, Al is clearly seen to have influenced decisively on the hardness, undercooling of solder and the interfacial characteristics during reflow. However, the amount of Al must be kept below 0.3 wt.% in order to avoid large Cu3Al2 agglomeration, which could affect the performance of solder.
4. Materials and Methods
SAC105-xAl (where x = 0, 0.1, 0.3, 0.5 wt.%) were fabricated by Beijing Compo Advanced Technology Co. Ltd. (Beijing, China). All the solder alloys were prepared in rod shape (0.7 cm diameter, 15 cm length). The solder alloy was then cut into thin disks with 1 mm thickness by using electric discharge machining (EDM, A500W, Sodick, Schaumburg, IL, USA).
As-received solder was prepared for micro-examination by standard metallographic technique which included, cutting, mounting, grinding (up to 3000 grit paper) and polishing (diamond paste with size 9 µm, 6 µm, 3 µm, 1 µm and colloidal silica with size 0.2 μm). The microstructure of the as-received SAC105-Al solders was characterized by Field emission scanning electron microscope (FESEM, FEI-FEG450, FEI, Houston, TX, USA) and intermetallic compound (IMC) composition was investigated by energy dispersive X-ray spectroscope (EDS, EDAX-Genesis Utilities, EDAX, Mahwah, NJ, USA). Differential scanning calorimeter, DSC (DSC Q20, TA Instruments, New Castle, DE, USA) was used to evaluate the effect of the addition of Al on the onset melting and onset solidification temperature of the SAC105 solder. Each of the samples was weighed to approximately 10 mg and placed on an aluminum pan and covered with a lid. It was then heated from 25 °C to 300 °C and then cooled down to room temperature at a rate of 10 °C/min. For each composition, DSC test was repeated 3 times to ensure the reproducibility of the DSC results.
For reflow, commercial grade polycrystalline copper (Cu) sheets (30 mm × 30 mm × 0.3 mm) were used as substrates. Before soldering, Cu sheets were polished with 2000 Grit silica carbide paper, washed with detergent and soaked in 10 vol.% H2SO4 solution for 15–30 min to remove any oxide film present. These were then rinsed with distilled water followed by drying with acetone. Sparkle Flux WF-6317 (Senju Metal Industry, Tokyo, Japan) was then evenly placed on the Cu substrate. SAC-xAl thin disc (diameter = 6.5 mm, thickness = 1.24 mm) were placed in the middle of copper substrates which had been covered with Flux WF-6317. Reflow was carried out in an oven at 250 °C for 60 s. After the reflow process, the residual flux was cleaned and removed by rinsing the sample under running distilled water. After that, the reflowed samples were prepared by standard metallographic specimen preparation for microstructural investigation. The microstructure of the bulk solder and the morphology of the IMCs formed at the solder/substrate interface were investigated by FESEM and the composition of the IMCs was investigated by EDS. IMC thickness was calculated by dividing the IMC area by the length of the IMC using a built-in image analyzer software in an Olympus SZX10 (Olympus, Tokyo, Japan) stereoscope. For each experimental condition, thickness values were measured on 5 micrographs and the average IMC thickness is reported. For the top view observation of the interfacial IMCs, the solders were etched in a mixture of 93% CH3OH, 5% HNO3, and 2% HCl to remove the solder matrix and expose the interfacial intermetallic compound.
Vickers hardness measurement was performed to investigate the relationship between microstructure and microhardness. The Vickers hardness values were obtained as [
36]:
where φ is the indenter apex angle, P is the applied load and d is the average length of diagonals. The applied load and loading period are 1 kgf and 5 s, respectively. For each specimen, five points were tested and the mean values were obtained.
Nanoindentation testing was done using Hysitron Ubi-750 (Hysitron, Minneapolis, MI, USA). Two modes of indentation were conducted on the samples: Quasi-static and Continuous Dynamic Measurement. Quasi-static indentation was conducted with a maximum load of 500 μN, holding time of 2 s, loading rate and unloading rate of 16.67 μN/s and continuous dynamic measurement test was conducted with a maximum of 1000 μN, holding time of 2 s and a loading rate and unloading rate of 16.67 μN/s. For each element, 5 points were tested and the mean values were obtained. The load–displacement data obtained were analyzed using the method proposed by Oliver and Pharr [
37] to determine the hardness and elastic modulus as functions of the displacement of the indenter. For quasi static nanoindentation testing, hardness (H) and reduced modulus (E) can be obtained. The elastic modulus E of the material being indented is related to the reduced modulus using the following equation [
38]:
where v is the Poisson’s ratio of the indented material (usually assumed to be 0.3 if unknown), and v
i and E
i are the Poisson’s ratio and elastic modulus of the indenter tip material, respectively. The elastic modulus E
i and Poisson’s ratio v
i of the Berkovich indenter used in this study are 1141 GPa and 0.07 respectively. For continuous dynamic measurement, results for the hardness and complex modulus as a function of indentation depth can be obtained.
Complex modulus, which is also known as dynamic modulus, is a ratio of stress strain under vibratory conditions. It could be defined by the equation below [
39]:
where E* is the reduced complex modulus, E′ is the reduced storage modulus (or elastic modulus), E″ is the loss modulus and i is the imaginary unit.