Microbially Induced Calcite Precipitation Employing Environmental Isolates
Abstract
:1. Introduction
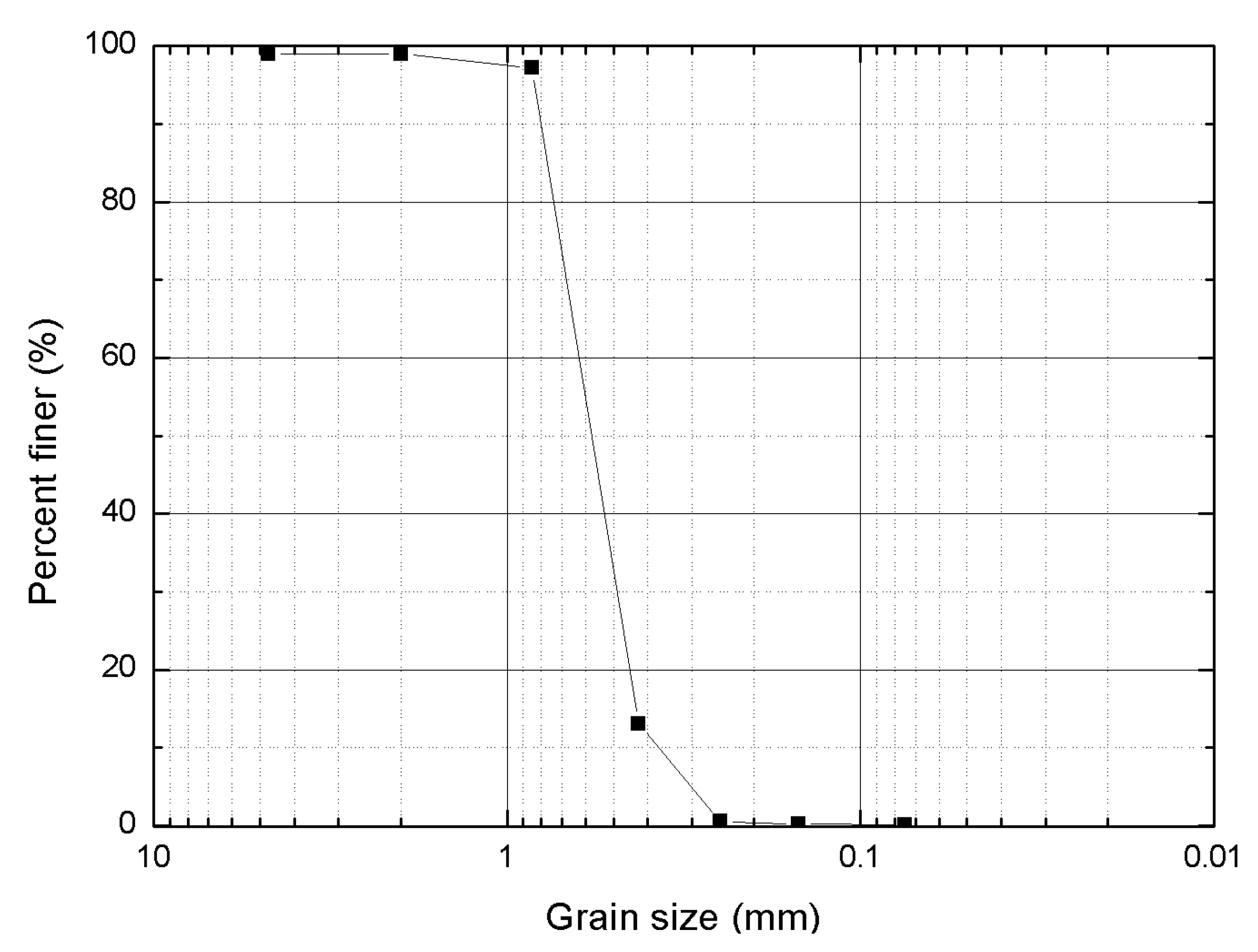
2. Materials and Methods
2.1. Isolation and Maintenance of Microbial Strains
2.2. Measurement of Urease Activity
2.3. Bacterial Identification by 16S rRNA Sequencing
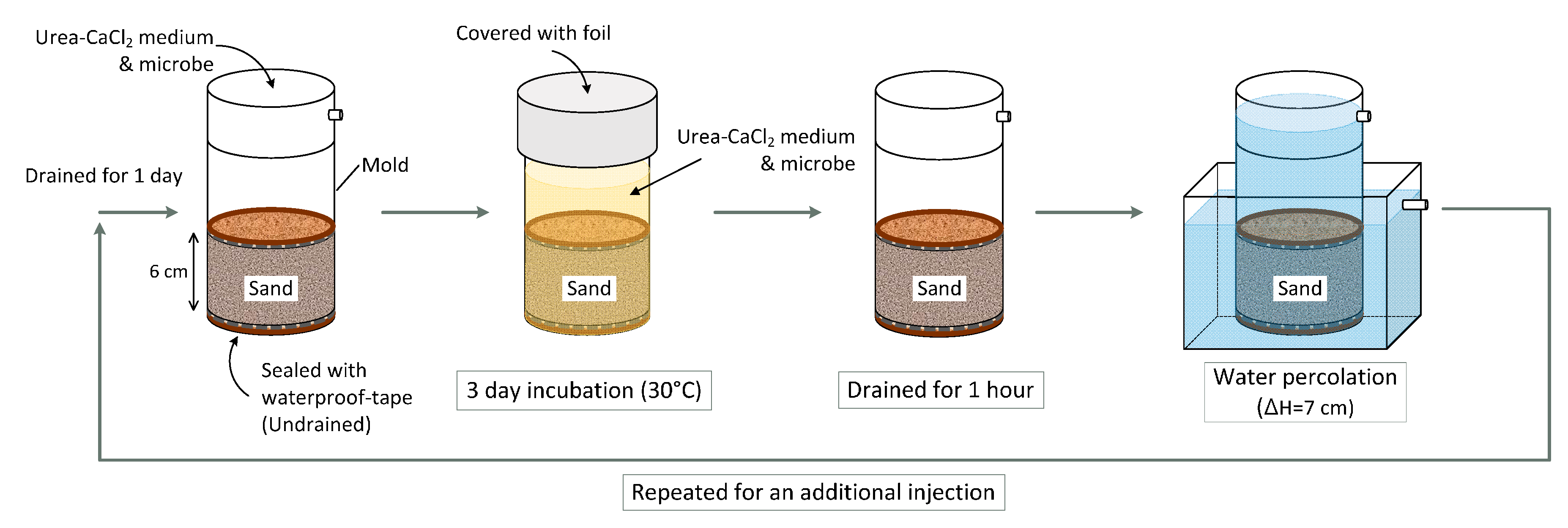
2.4. Bacterial Inoculation and MICP Treatment
2.5. Calcite Precipitation
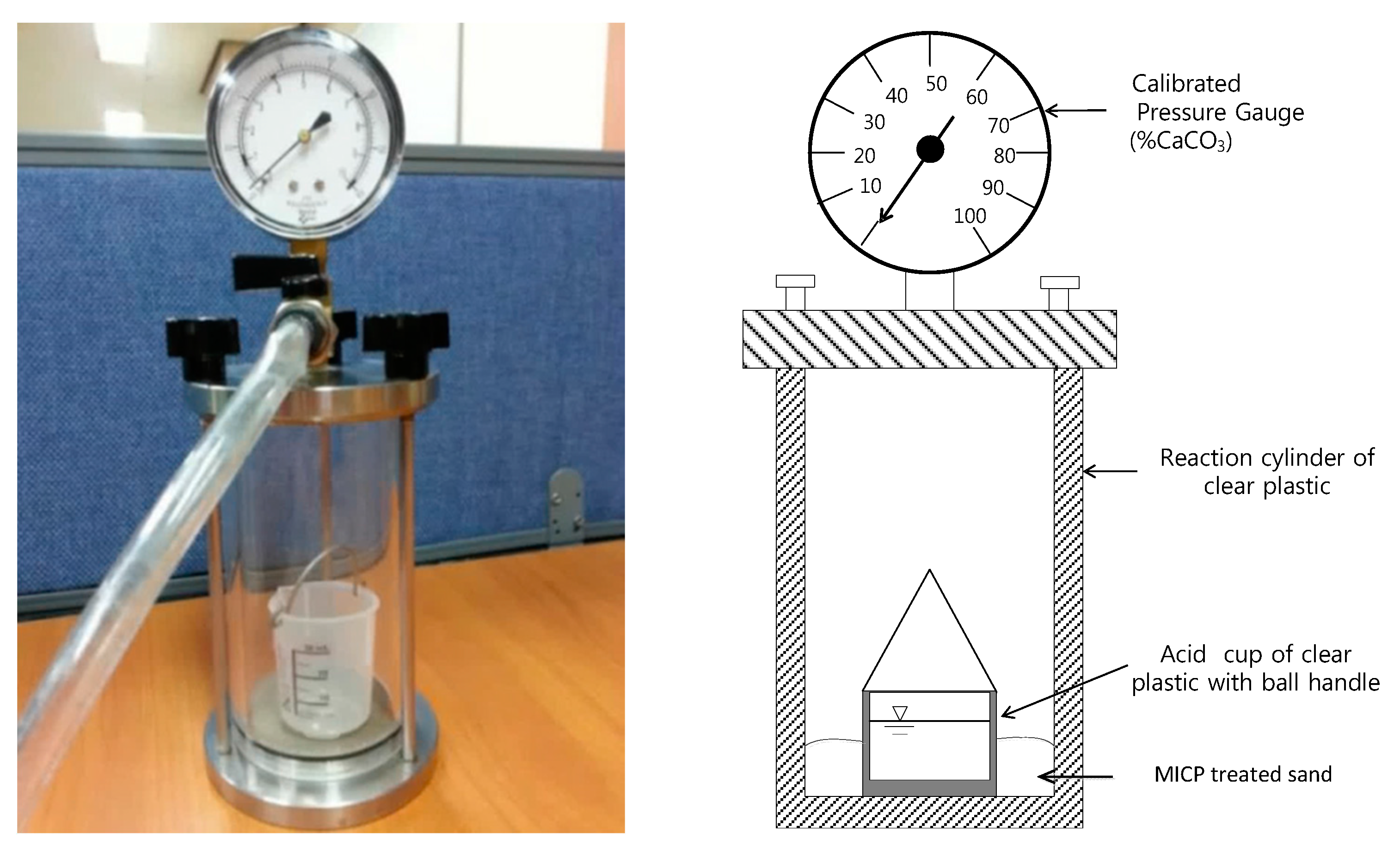
2.6. Calcium Carbonate Measurements
2.7. Scanning Electron Microscopy (SEM) & Energy Dispersive Spectroscopy (EDS)
3. Results and Discussion
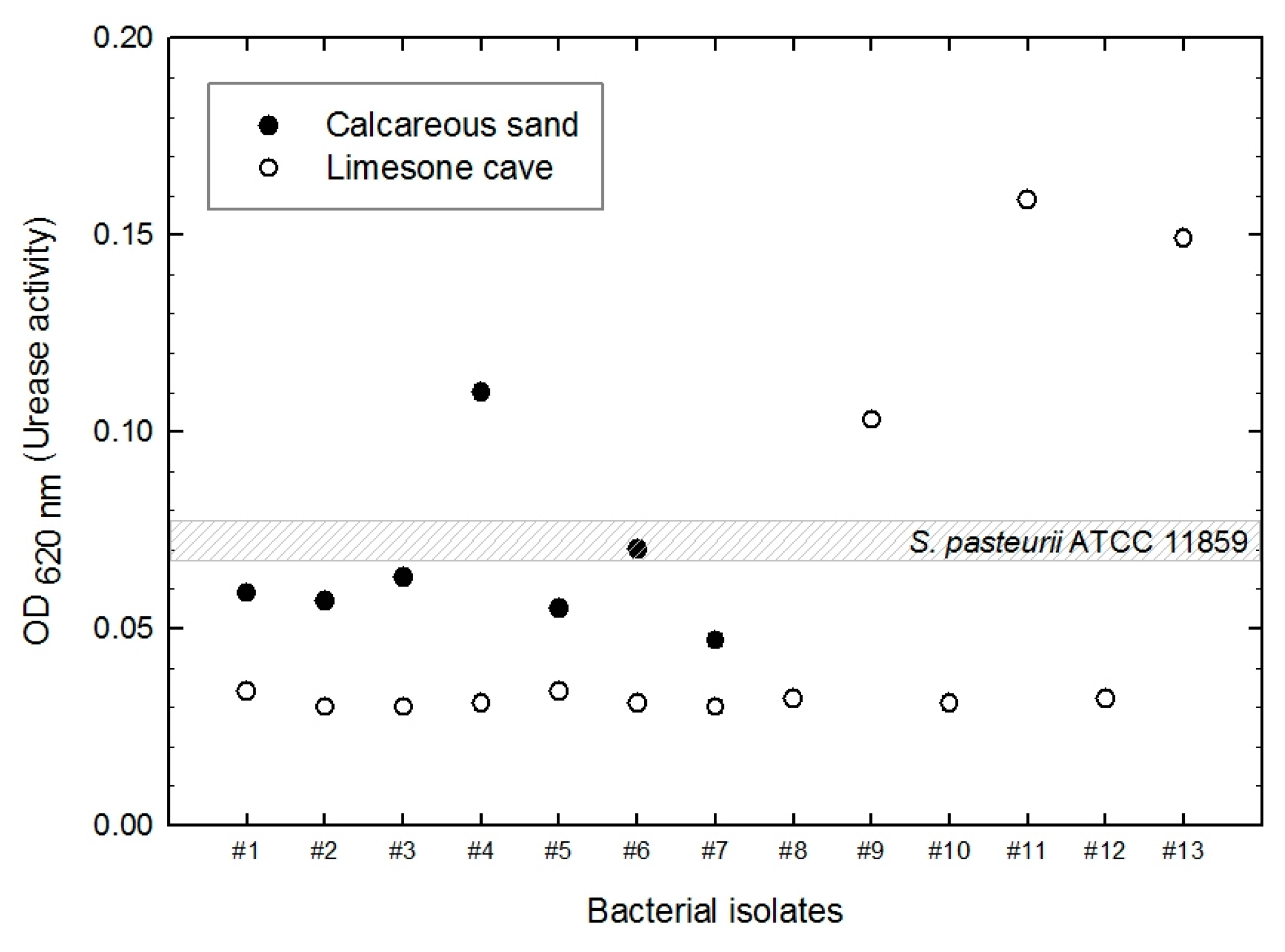
3.1. Bacterial Isolation Based on Urease Activity Determinations
3.2. Calcium Carbonate Measurements
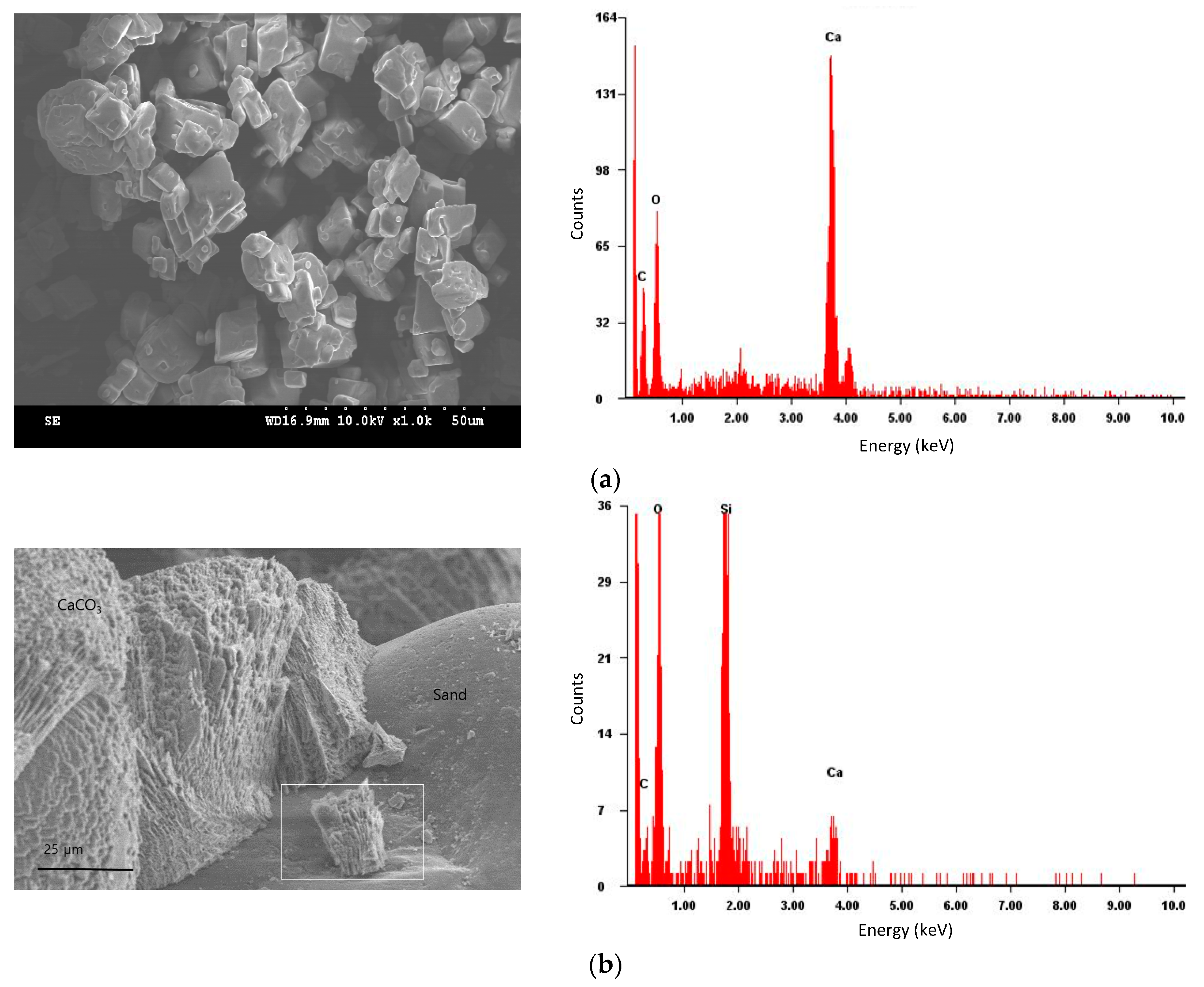
3.3. Scanning Electron Microscopy (SEM) & Energy Dispersive Spectroscopy (EDS)
4. Conclusions
- Four isolates (S. saprophyticus, S. globispora, B. lentus, and Sporosarcina sp.) were selected from 20 environmental microbes that precipitated calcium carbonate and displayed higher urease activity than S. pasteurii.
- Relative density of cohesionless soils significantly affected the amount of calcite precipitation, which was measured to be approximately 40%–50% higher in 80% relative density soil than that in 60% relative density soil.
- The amount of calcium carbonate precipitation was greatest for Sporosarcina sp., followed by S. saprophyticus, B. lentus, S. pasteurii, and S. globispora. The difference between the greatest and smallest was about 17% and 23% for 60% and 80% relative density soil, respectively.
- The urease activity was highest in B. lentus, followed by Sporosarcina sp., S. saprophyticus, S. globispora, and S. pasteurii. It appears that the urease activity weakly correlates with calcium carbonate precipitation.
- SEM analysis identified a crystalline substance on the sand grain surface after MICP treatment. The substance was verified as the calcium carbonate with EDS.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferris, F.G.; Stehmeier, L.G. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1992, 36. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Santamarina, J.C. Biological considerations in geotechnical engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Chou, C.-W.; Seagren, E.A.; Aydilek, A.H.; Lai, M. Biocalcification of sand through ureolysis. J. Geotech. Geoenviron. Eng. 2011, 137, 1179–1189. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Keykha, H.A.; Huat, B.B.; Asadi, A. Electro-biogrouting stabilisation of soft soil. Environ. Geotech. 2015, 2, 292–300. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; da Costa, M.S.; Costa, J.N.; Nobre, M.F. Comparison of the ability of two bacteria to improve the behavior of sandy soil. J. Mater. Civ. Eng. 2014, 27. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Zhang, H.; Amini, F. Factors effecting improvement of engineering properties of micp-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26. [Google Scholar] [CrossRef]
- Stabnikov, V.; Naeimi, M.; Ivanov, V.; Chu, J. Formation of water-impermeable crust on sand surface using biocement. Cem. Concr. Res. 2011, 41, 1143–1149. [Google Scholar] [CrossRef]
- Tobler, D.J.; Maclachlan, E.; Phoenix, V.R. Microbially mediated plugging of porous media and the impact of differing injection strategies. Ecol. Eng. 2012, 42, 270–278. [Google Scholar] [CrossRef]
- Van Paassen, L.; Harkes, M.; van Zwieten, G.; van der Zon, W.; van der Star, W.; van Loosdrecht, M. Scale up of biogrout: A biological ground reinforcement method. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; pp. 2328–2333.
- Van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M. Potential soil reinforcement by biological denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Cuthbert, M.O.; McMillan, L.A.; Handley-Sidhu, S.; Riley, M.S.; Tobler, D.J.; Phoenix, V.R. A field and modeling study of fractured rock permeability reduction using microbially induced calcite precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643. [Google Scholar] [CrossRef] [PubMed]
- Van der Star, W.; van Wijngaarden, W.; van Paassen, L.; van Baalen, L.; van Zwieten, G. Stabilization of gravel deposits using microorganisms. In Proceedings of the 15th European Conference on Soil Mechanics and Geotechnical Engineering, Athens, Greece, 5–9 October 2011; pp. 85–90.
- Van Paassen, L. Bio-mediated ground improvement: From laboratory experiment to pilot applications. In Proceedings of the Geo-Frontiers 2011@ Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; pp. 4099–4108.
- Van Paassen, L.; van Hemert, W.; van der Star, W.; van Zwieten, G.; van Baalen, L. Direct shear strength of biologically cemented gravel. In Proceeding of the GeoCongress, Oakland, CA, USA, 25–29 March 2012.
- Burbank, M.; Weaver, T.; Lewis, R.; Williams, T.; Williams, B.; Crawford, R. Geotechnical tests of sands following bioinduced calcite precipitation catalyzed by indigenous bacteria. J. Geotech. Geoenviron. Eng. 2013, 139, 968–977. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Fujita, Y.; Taylor, J.L.; Gresham, T.L.; Delwiche, M.E.; Colwell, F.S.; McLing, T.L.; Petzke, L.M.; Smith, R.W. Stimulation of microbial urea hydrolysis in groundwater to enhance calcite precipitation. Environ. Sci. Technol. 2008, 42, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Tobler, D.J.; Cuthbert, M.O.; Greswell, R.B.; Riley, M.S.; Renshaw, J.C.; Handley-Sidhu, S.; Phoenix, V.R. Comparison of rates of ureolysis between sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite. Geochim. Cosmochim. Acta 2011, 75, 3290–3301. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of caco 3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 1 May 2014).
- Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM D2487; ASTM International: West Conshohocken, PA, USA, 2011.
- Standard Test Method for Rapid Determination of Carbonate Content of Soils; ASTM D4373; ASTM International: West Conshohocken, PA, USA, 2014.


| Sample | No. | Microbes | Identity |
|---|---|---|---|
| Calcareous sand | 4 | Staphylococcus saprophyticus subsp. saprophyticus | 99% |
| Limestone cave soils | 9 | Sporosarcina globispora | 98% |
| 11 | Bacillus lentus strain NCIMB8773 | 99% | |
| 13 | Sporosarcina sp. | 99% |
| Microbes | Precipitated Calcite | Rank in Urease Activity | |||
|---|---|---|---|---|---|
| Relative Density = 60% | Relative Density = 80% | ||||
| Amount of Calcite (g/100 cm3) | Rank | Amount of Calcite (g/100 cm3) | Rank | ||
| Sporosarcina sp. | 0.585 | 1 | 0.905 | 1 | 2 |
| S. saprophyticus | 0.583 | 2 | 0.805 | 2 | 3 |
| B. lentus | 0.559 | 3 | 0.794 | 3 | 1 |
| S. pasteurii | 0.519 | 4 | 0.756 | 4 | 5 |
| S. globispora | 0.487 | 5 | 0.700 | 5 | 4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Youn, H. Microbially Induced Calcite Precipitation Employing Environmental Isolates. Materials 2016, 9, 468. https://doi.org/10.3390/ma9060468
Kim G, Youn H. Microbially Induced Calcite Precipitation Employing Environmental Isolates. Materials. 2016; 9(6):468. https://doi.org/10.3390/ma9060468
Chicago/Turabian StyleKim, Gunjo, and Heejung Youn. 2016. "Microbially Induced Calcite Precipitation Employing Environmental Isolates" Materials 9, no. 6: 468. https://doi.org/10.3390/ma9060468
APA StyleKim, G., & Youn, H. (2016). Microbially Induced Calcite Precipitation Employing Environmental Isolates. Materials, 9(6), 468. https://doi.org/10.3390/ma9060468






