Preparation of Layer-by-Layer Films Composed of Polysaccharides and Poly(Amidoamine) Dendrimer Bearing Phenylboronic Acid and Their pH- and Sugar-Dependent Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
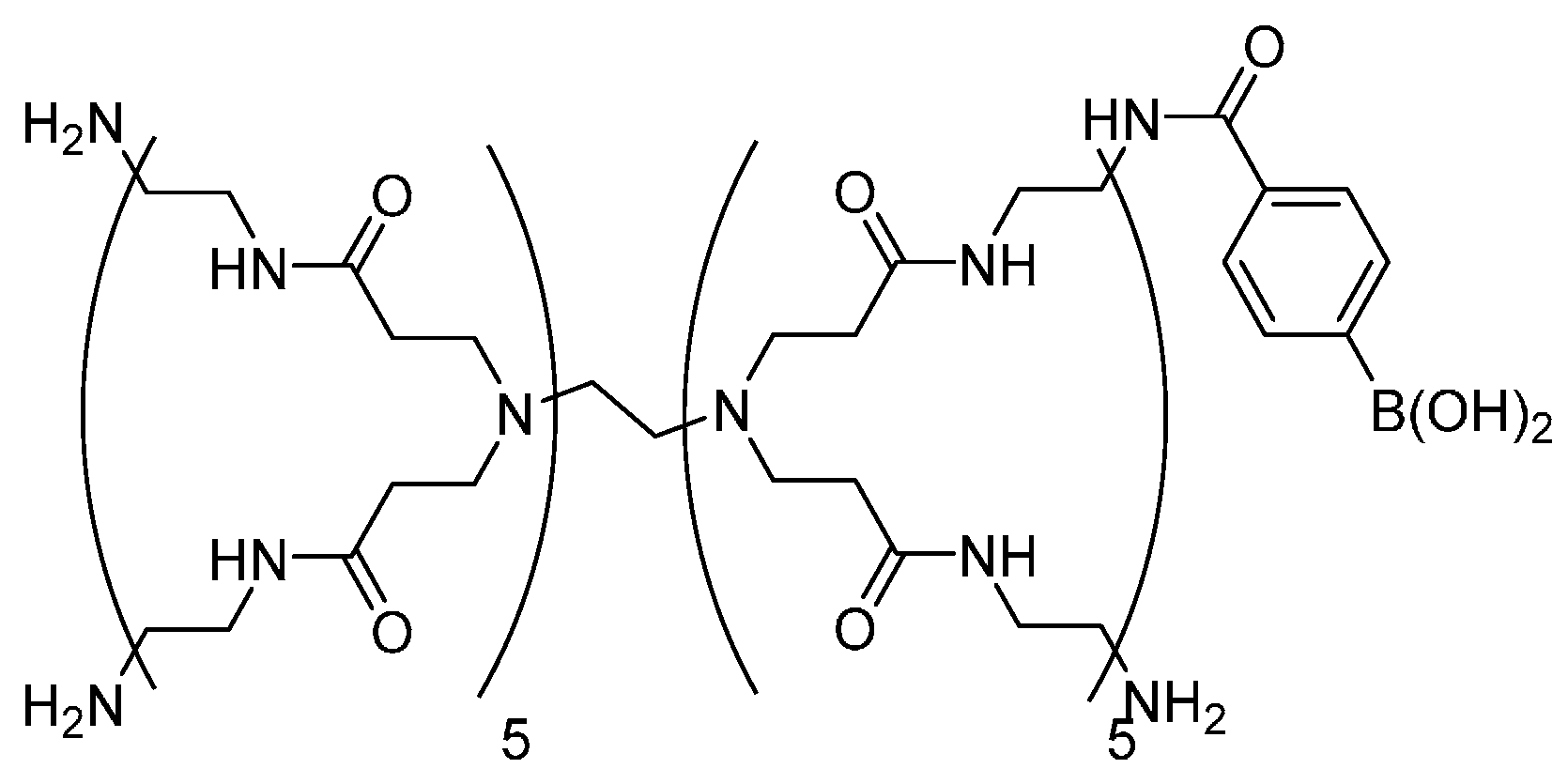
2.2. Preparation of PBA-PAMAM
2.3. Preparation of LbL Films
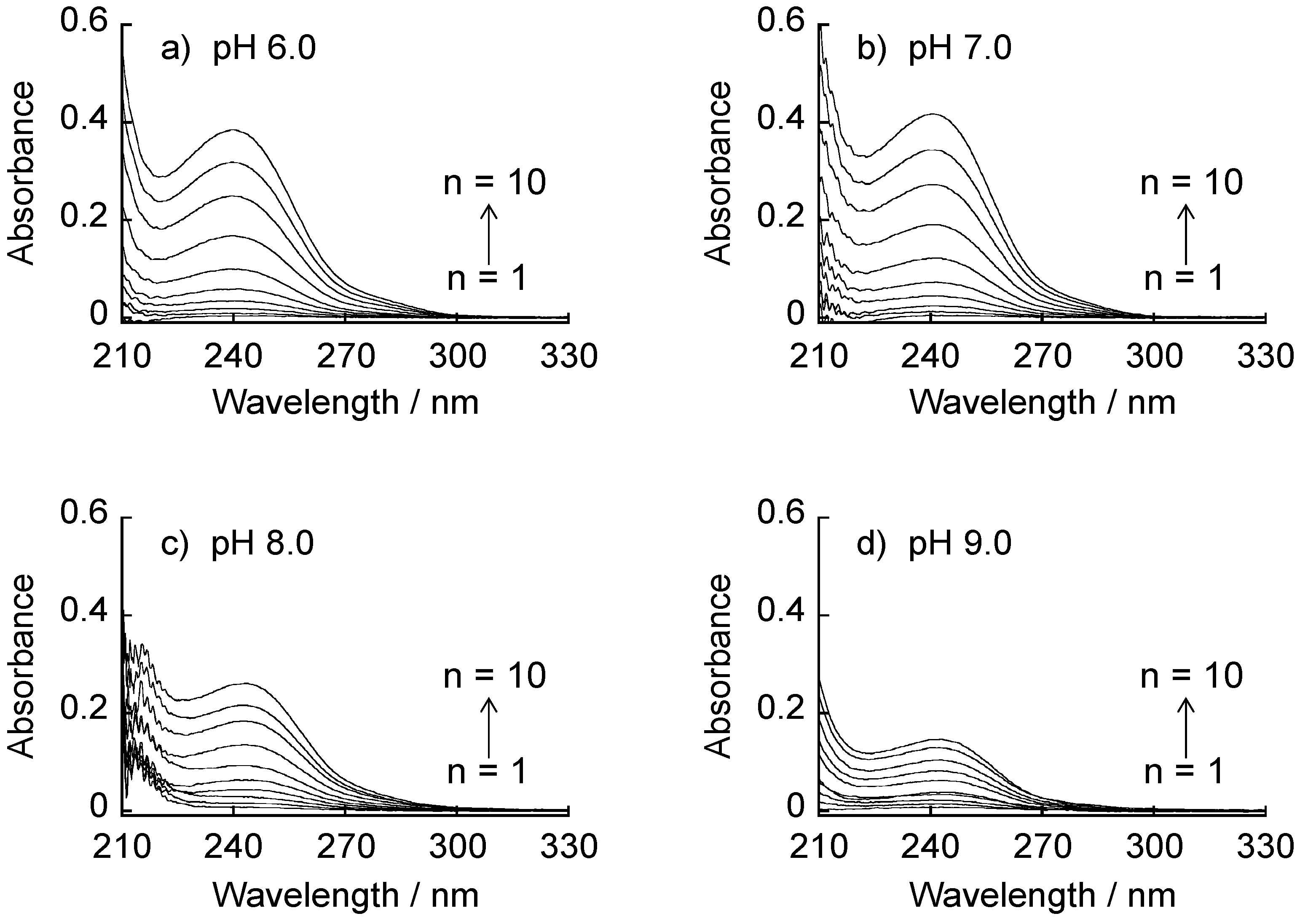
2.4. pH-Dependent Stability
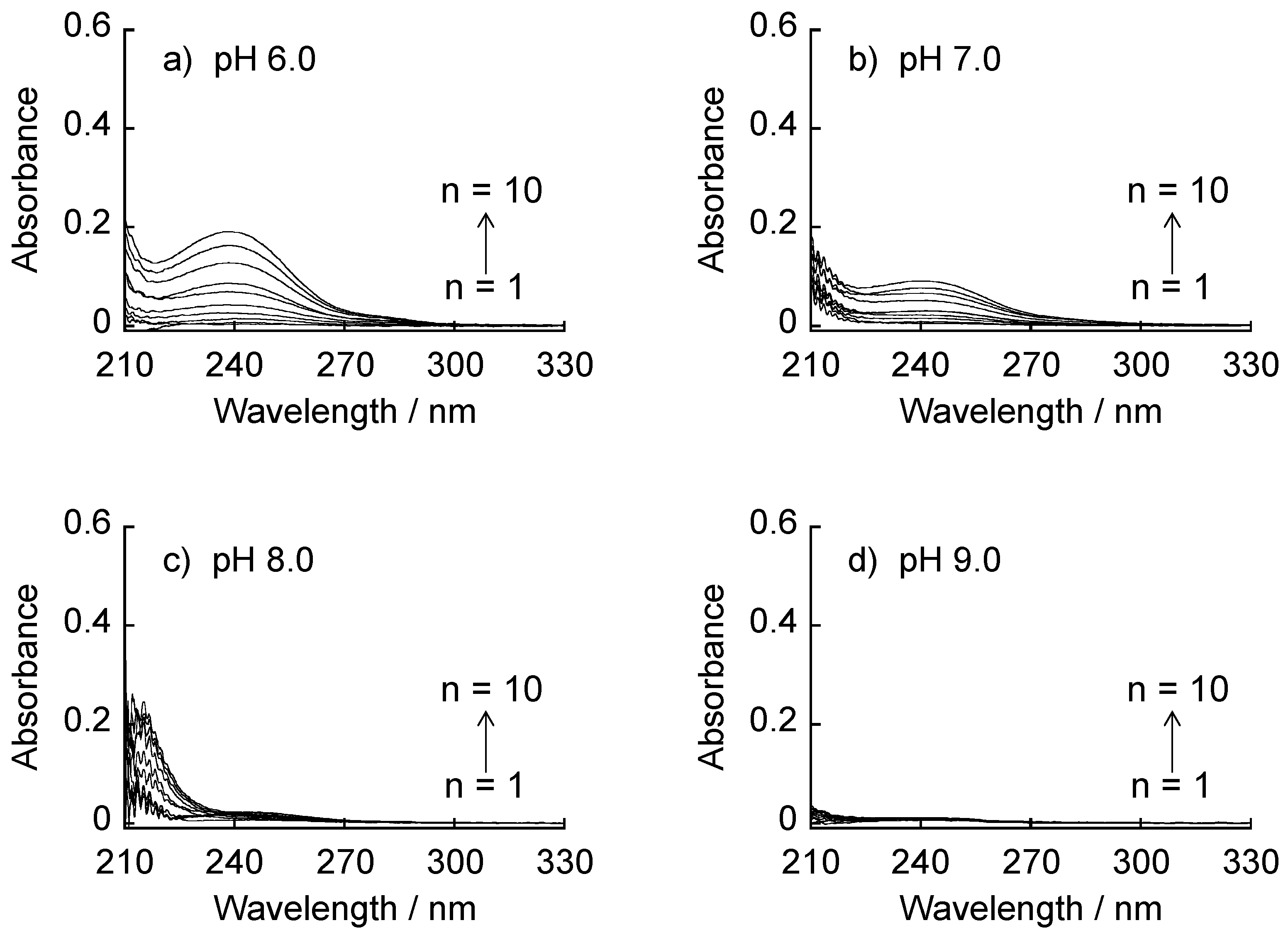
2.5. Sugar-Induced Decomposition
3. Results and Discussion
3.1. Preparation of LbL Film
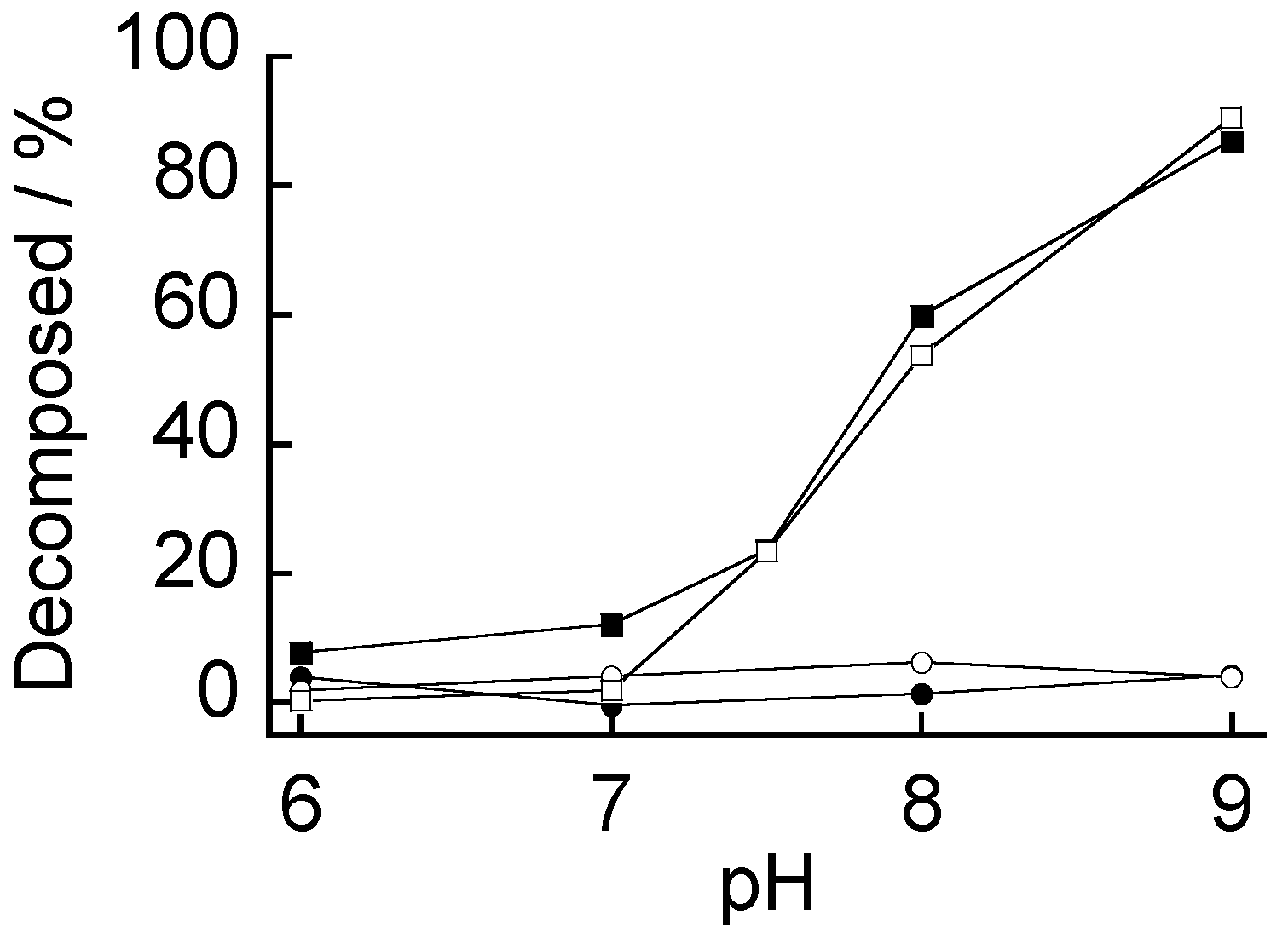
3.2. pH-Dependent Stability
3.3. Sugar-Induced Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Lin, X.; Yang, M.; Jeong, H.; Chang, M.; Hong, J. Durable superhydrophilic coatings formed for anti-biofouling and oil-water separation. J. Membr. Sci. 2016, 506, 22–30. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Shi, H.; Song, Z.; Zhao, Z.; Anzai, J.; Chen, Q. Multi-walled carbon nanotubes-based glucose biosensor prepared by a layer-by-layer technique. Mater. Sci. Eng. C 2006, 26, 113–117. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Qi, Z.; Lu, D. In situ study of self-assembled nanocomposite films by spectral SPR sensor. Mater. Sci. Eng. C 2015, 51, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, K.; Anzai, J. Layer-by-layer polyelectrolyte films containing insulin for pH-triggered release. J. Mater. Chem. 2010, 20, 1546–1552. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Anzai, J. Dendrimers in layer-by-layer assemblies: Synthesis and applications. Molecules 2013, 18, 8440–8460. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte multilayers: Toward cell studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J. Layer-by-layer assembled thin films composed of carboxyl-terminated poly(amidoamine) dendrimer as a pH-sensitive nano-device. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Liu, Y.; Gao, W.; Hu, N. Layer-by-layer assembly of collagen and electroactive myoglobin. Bioelectrochemistry 2010, 79, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 18, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Anzai, J.; Kobayashi, Y.; Suzuki, Y.; Takeshita, H.; Chen, Q.; Osa, T.; Hoshi, T.; Du, X. Enzyme sensors prepared by layer-by-layer deposition of enzymes on a platinum electrode through avidin-biotin interaction. Sens. Actuators B 1998, 52, 3–9. [Google Scholar] [CrossRef]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun. 2002, 164–165. [Google Scholar] [CrossRef]
- Silva, J.M.; Caridale, S.G.; Costa, R.R.; Alves, N.M.; Groth, T.; Picart, C.; Reis, R.L.; Mano, J.F. pH Responsiveness of multilayered films and membranes made of polysaccharides. Langmuir 2015, 31, 11318–11328. [Google Scholar] [CrossRef] [PubMed]
- Aytar, B.S.; Prausnitz, M.R.; Lynn, D.M. Rapid release of plasmid DNA from surfaces coated with polyelectrolyte multilayers promoted by the application of electrochemical potentials. ACS Appl. Mater. Interfaces 2012, 4, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suzuki, I.; Anzai, J. Preparation of polyelectrolyte-layered assemblies containing cyclodextrin and their binding properties. Langmuir 2003, 19, 7406–7412. [Google Scholar] [CrossRef]
- Rani, A.; Oh, K.A.; Koo, H.; Lee, H.J.; Park, M. Multilayer films of cationic graphene-polyelectrolytes and anionic graphene-polyelectrolytes fabricated using layer-by-layer self-assembly. Appl. Surf. Sci. 2011, 257, 4982–4989. [Google Scholar] [CrossRef]
- Watahiki, R.; Sato, K.; Suwa, K.; Niina, S.; Egawa, Y.; Seki, T.; Anzai, J. Multilayer films composed of phenylboronic acid-modified dendrimers sensitive to glucose under physiological conditions. J. Mater. Chem. B 2014, 2, 5809–5817. [Google Scholar] [CrossRef]
- Suwa, K.; Nagasaka, M.; Niina, S.; Egawa, Y.; Seki, T.; Anzai, J. Sugar response of layer-by-layer films composed of poly(vinyl alcohol) and poly(amidoamine) dendrimer bearing 4-aminophenylboronic acid. Colloid Polym. Sci. 2015, 293, 1043–1048. [Google Scholar] [CrossRef]
- Suwa, K.; Sato, K.; Anzai, J. Preparation of multilayer films consisting of glucose oxidase and poly(aminoamine) dendrimer and their stability. Colloid Polym. Sci. 2015, 293, 2713–2718. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid—Diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Ding, Z.; Guan, Y.; Zhang, Y.; Zhu, X.X. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 2009, 5, 2302–2309. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Xu, Y.; Li, Y.; An, T.; Su, Z.; Peng, B.; Lin, Y.; Wang, Q. Construction of glycoprotein multilayers using the layer-by-layer assembly technique. J. Mater. Chem. 2012, 22, 17954–17960. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Y.; Zhang, Y. Dynamically bonded layer-by-layer films for self-regulated insulin release. J. Mater. Chem. 2012, 22, 16299–16305. [Google Scholar] [CrossRef]
- Scott, R.W.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Maureira, A.; Rivas, B.L. Metal ions recovery with alginic acid coupled to ultrafiltration membrane. Eur. Polym. J. 2009, 45, 573–581. [Google Scholar] [CrossRef]
- Levy, T.; Déjugnat, C.; Sukhorukov, G.B. Polymer microcapsules with carbohydrate-sensitive properties. Adv. Funct. Mater. 2008, 18, 1586–1594. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Sukhishvili, S.A. Polyelectrolyte multilayers of weak polyacid and cationic copolymer: Competition of hydrogen-bonding and electrostatic interactions. Macromolecules 2003, 36, 9950–9956. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Izumrudov, V.A.; Sukhishvili, S.A. Electrostatic layer-by-layer self-assembly of poly(carboxybetaine)s: Role of zwitterions in film growth. Macromolecules 2007, 40, 3663–3668. [Google Scholar] [CrossRef]
- Westwood, M.; Noel, T.R.; Parker, R. Environmental responsiveness of polygalacturonic acid-based multilayers to variation of pH. Biomacromolecules 2011, 12, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qian, L.; Huang, H.; Yang, X. Buildup of gold nanoparticle multilayer thin films based on the covalent-bonding interaction between boronic acids and polyols. J. Colloid Interface Sci. 2006, 295, 583–588. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.G.; Jones, A.M.; Demeester, J.; De Smedt, S.C. Glucose-responsive polyelectrolyte capsules. Langmuir 2006, 22, 5070–5074. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Patil, S. Glucose-triggered drug delivery from boronate mediated layer-by-layer self-assembly. ACS Appl. Mater. Interfaces 2010, 2, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef]
- Qi, W.; Yan, X.; Fei, J.; Wang, A.; Cui, Y.; Li, J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials 2009, 30, 2799–2806. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Suwa, K.; Anzai, J.-i. Preparation of Layer-by-Layer Films Composed of Polysaccharides and Poly(Amidoamine) Dendrimer Bearing Phenylboronic Acid and Their pH- and Sugar-Dependent Stability. Materials 2016, 9, 425. https://doi.org/10.3390/ma9060425
Yoshida K, Suwa K, Anzai J-i. Preparation of Layer-by-Layer Films Composed of Polysaccharides and Poly(Amidoamine) Dendrimer Bearing Phenylboronic Acid and Their pH- and Sugar-Dependent Stability. Materials. 2016; 9(6):425. https://doi.org/10.3390/ma9060425
Chicago/Turabian StyleYoshida, Kentaro, Keisuke Suwa, and Jun-ichi Anzai. 2016. "Preparation of Layer-by-Layer Films Composed of Polysaccharides and Poly(Amidoamine) Dendrimer Bearing Phenylboronic Acid and Their pH- and Sugar-Dependent Stability" Materials 9, no. 6: 425. https://doi.org/10.3390/ma9060425






