Inhibition of Cariogenic Plaque Formation on Root Surface with Polydopamine-Induced-Polyethylene Glycol Coating
Abstract
:1. Introduction
2. Results
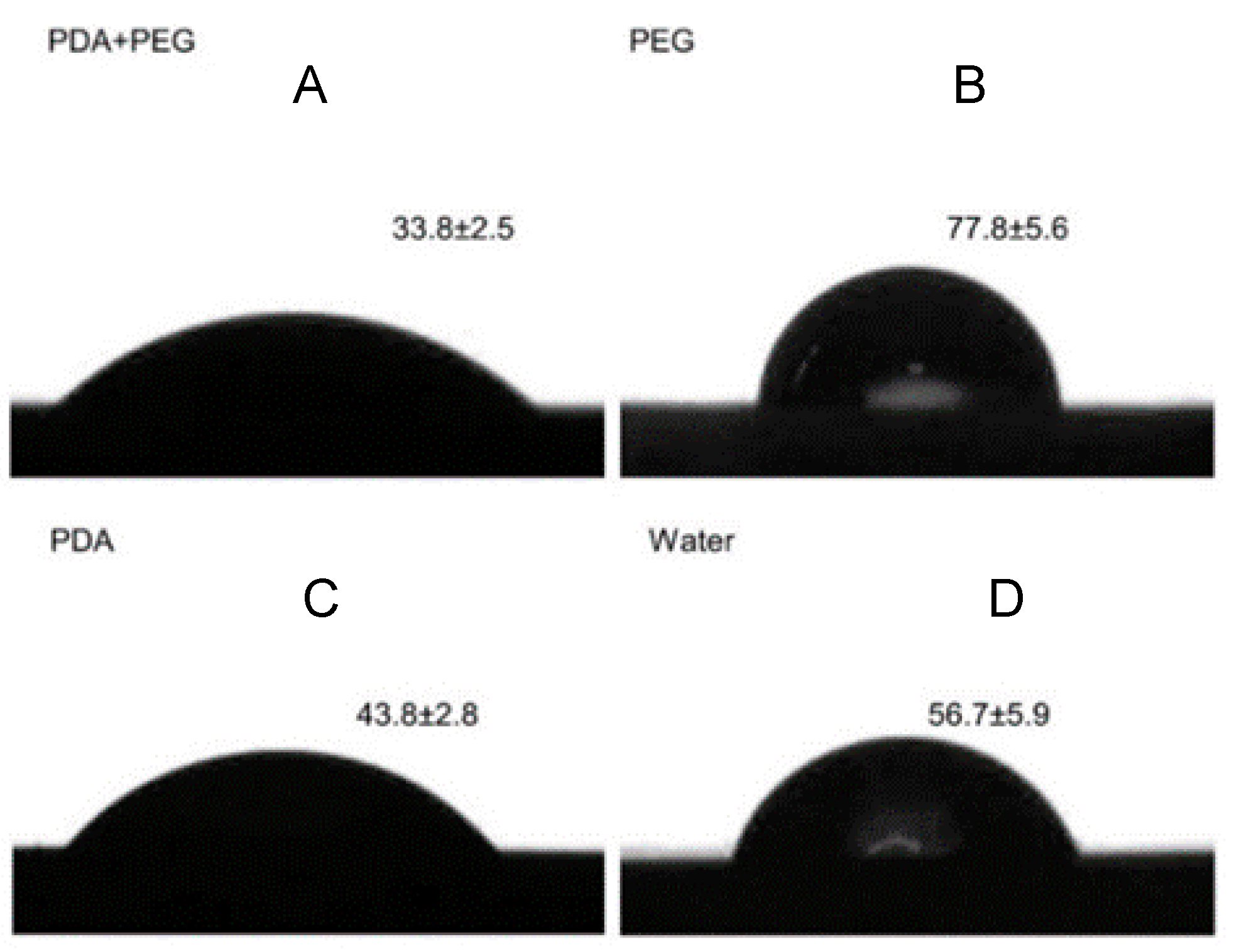
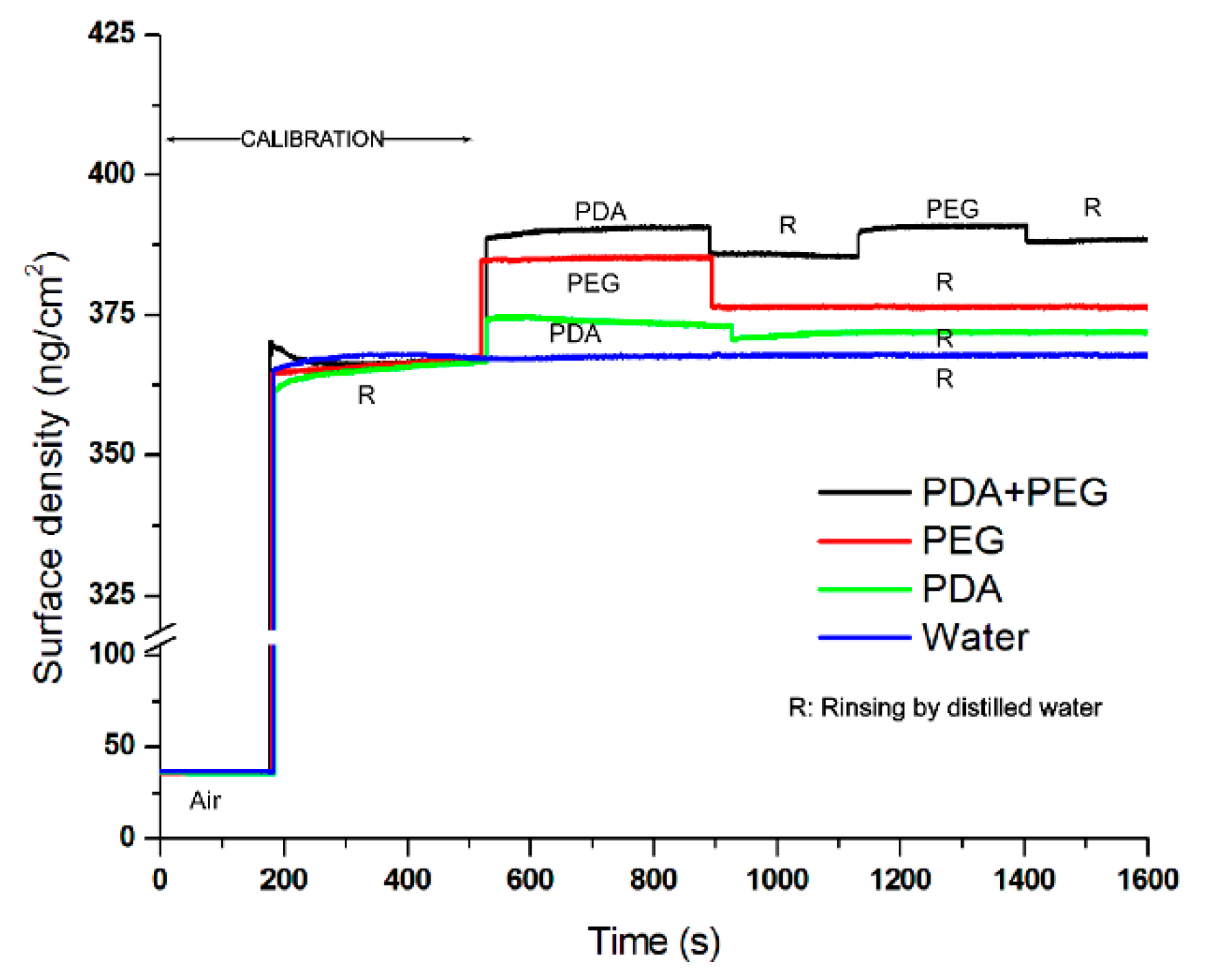
2.1. Characterization of the Polydopamine-Induced-PEG
2.2. Mucin Absorption
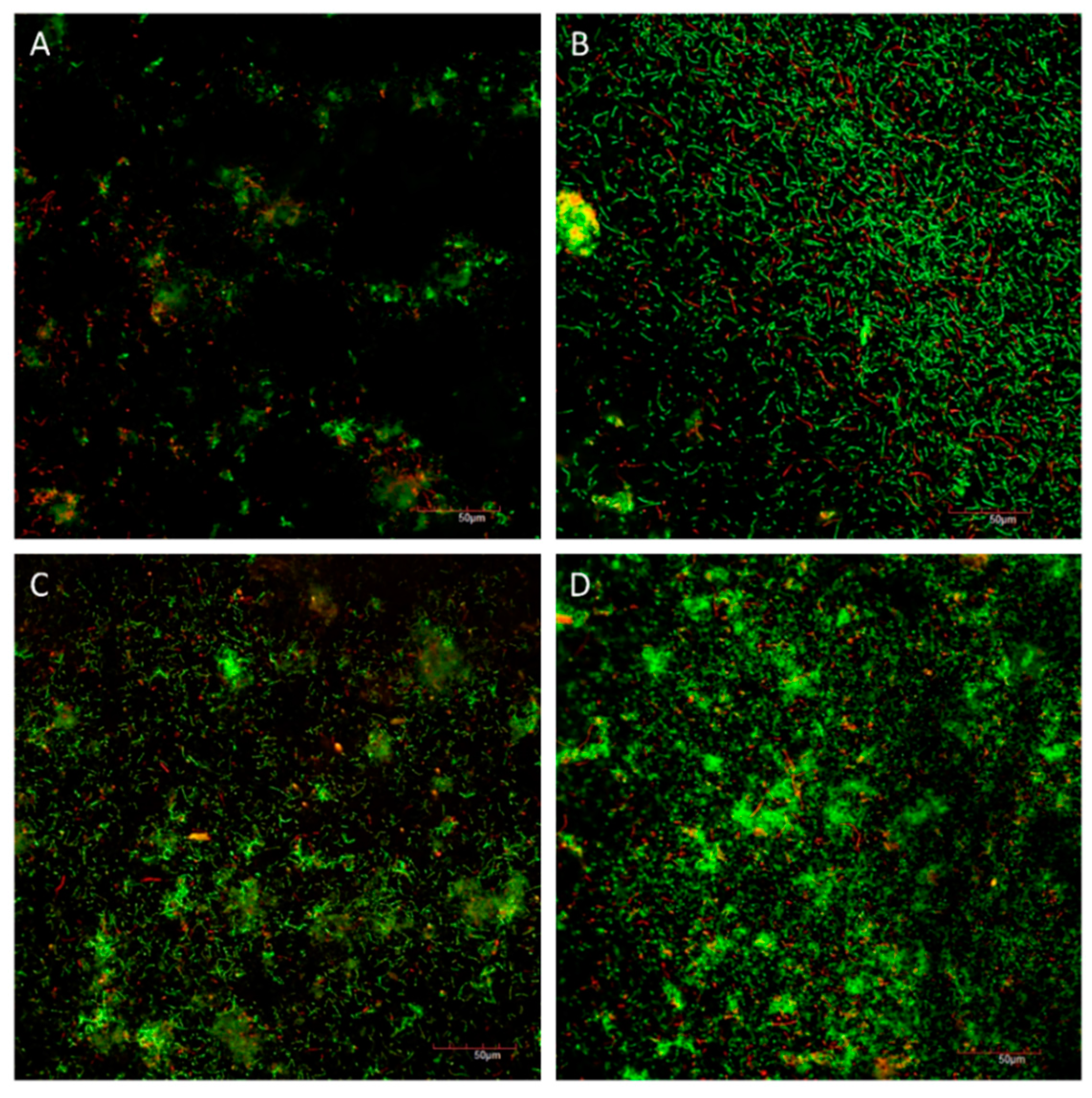
2.3. Development of the Cariogenic Biofilm
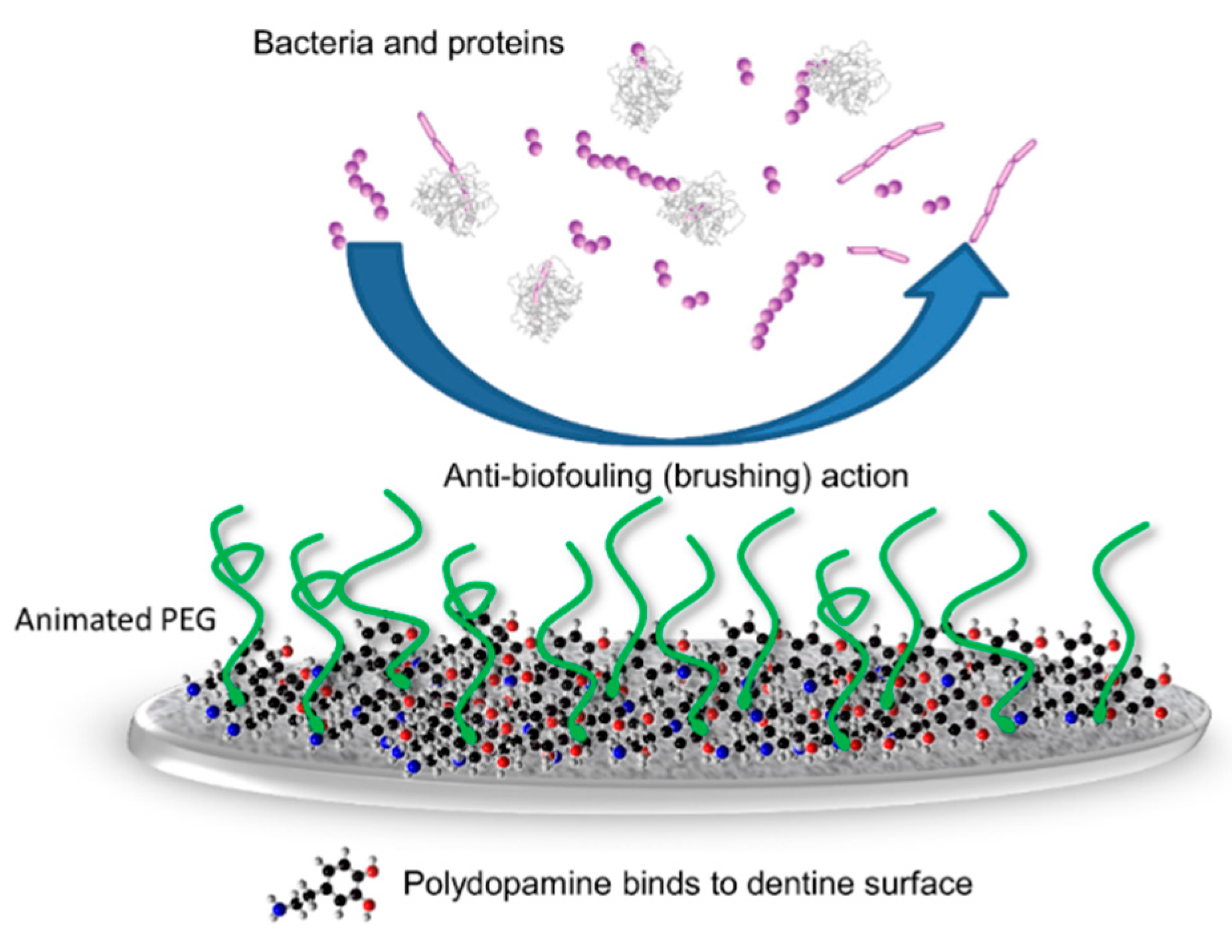
3. Discussion
4. Materials and Methods
4.1. Dentine Block and Hydroxyapatite Disk Preparation
4.2. Experimental Design
4.3. Characterization of the Polydopamine-Induced-PEG
4.3.1. Contact Angle Measurement
4.3.2. Quartz Crystal Microbalance
4.3.3. Fourier Transform Infrared Measurement
4.4. Absorption of Mucin
4.5. Development of the Cariogenic Biofilm
4.5.1. Morphology of the Cariogenic Biofilm
4.5.2. Viability of the Cariogenic Biofilm
4.5.3. Kinetics of the Cariogenic Biofilm
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PEG | polyethylene glycol |
| QCM | quartz crystal microbalance |
| ATCC | American Type Culture Collection |
| PBS | phosphate buffer solution |
| BHI | brain heart infusion |
| SEM | scanning electron microscopy |
| CFU | colony-forming unit |
| ANOVA | analysis of variance |
| DOPA | 3,4-dihydroxy-L-phenylalanine |
References
- Fejerskov, O. Concepts of dental caries and their consequences for understanding the disease. Community Dent. Oral. Epidemiol. 1997, 25, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental materials with antibiofilm properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.O.; Griffin, P.M.; Swann, J.L.; Zlobin, N. Estimating rates of new root caries in older adults. J. Dent. Res. 2004, 83, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Fejerskov, O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J. Dent. Res. 2004, 83, C35–C38. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Lussi, A.; Seemann, R.; Neuhaus, K.W. Prevention of crown and root caries in adults. Periodontol. 2000 2011, 55, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Fishburn, C.S. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Guo, S.; Janczewski, D.; Velandia, F.J.; Teo, S.L.; Vancso, G.J. Multilayers of fluorinated amphiphilic polyions for marine fouling prevention. Langmuir 2014, 30, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [PubMed]
- Shimotoyodome, A.; Koudate, T.; Kobayashi, H.; Nakamura, J.; Tokimitsu, I.; Hase, T.; Inoue, T.; Matsukubo, T.; Takaesu, Y. Reduction of Streptococcus mutans adherence and dental biofilm formation by surface treatment with phosphorylated polyethylene glycol. Antimicrob. Agents Chemother. 2007, 51, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Chu, C.H.; Lo, E.C.; Samaranayake, L.P. Preventing root caries development under oral biofilm challenge in an artificial mouth. Med. Oral. Patol. Oral. Cir. Bucal. 2013, 18, e557–e563. [Google Scholar] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Ogaki, R.; Laursen, A.O.; Lovmand, J.; Sutherland, D.S.; Stadler, B. Polydopamine/liposome coatings and their interaction with myoblast cells. ACS Appl. Mater. Interfaces 2011, 3, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Hwang, N.S.; Yeom, J.; Park, S.Y.; Messersmith, P.B.; Choi, I.S.; Langer, R.; Anderson, D.G.; Lee, H. One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine (Lond.) 2011, 6, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly (DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.B. Dopamine-induced mineralization of calcium carbonate vaterite microspheres. Langmuir 2010, 26, 14730–14736. [Google Scholar] [CrossRef] [PubMed]
- Pantera, E.A., Jr.; Schuster, G.S. Sterilization of extracted human teeth. J. Dent. Educ. 1990, 54, 283–285. [Google Scholar] [PubMed]
- Parsell, D.E.; Stewart, B.M.; Barker, J.R.; Nick, T.G.; Karns, L.; Johnson, R.B. The effect of steam sterilization on the physical properties and perceived cutting characteristics of extracted teeth. J. Dent. Educ. 1998, 62, 260–263. [Google Scholar] [PubMed]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Lo, E.C.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, M.E.; Costerton, J.W. Molecular genetics analyses of biofilm formation in oral isolates. Periodontol. 2000 2006, 42, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Cousins, B.G.; Doherty, P.J.; Whitelock, J.M.; Simmons, A.; Williams, R.L.; Milthorpe, B.K. The effect of silica nanoparticulate coatings on serum protein adsorption and cellular response. Biomaterials 2006, 27, 4856–4862. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.; Rakhmatullina, E.; Bleek, K.; Boye, S.; Yuan, J.; Volkel, A.; Gräwert, M.; Cheaib, Z.; Eick, S.; Günter, C.; et al. Poly(ethylene oxide)-b-poly(3-sulfopropyl methacrylate) block copolymers for calcium phosphate mineralization and biofilm inhibition. Biomacromolecules 2014, 15, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Tirrell, M.; Kokkoli, E.; Biesalski, M. The role of surface science in bioengineered materials. Surf. Sci. 2002, 500, 61–83. [Google Scholar] [CrossRef]
- Rosenberg, M.; Judes, H.; Weiss, E. Cell surface hydrophobicity of dental plaque microorganisms in situ. Infect. Immun. 1983, 42, 831–834. [Google Scholar] [PubMed]
- Statz, A.; Finlay, J.; Dalsin, J.; Callow, M.; Callow, J.A.; Messersmith, P.B. Algal antifouling and fouling-release properties of metal surfaces coated with a polymer inspired by marine mussels. Biofouling 2006, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Wilder, R. Antimicrobial mouthrinse as part of a comprehensive oral care regimen. Safety and compliance factors. J. Am. Dent. Assoc. 2006, 137, 22S–26S. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Song, Q.; Zhou, Y.; Zhang, L.; Wang, J.; Chen, J.; Leng, Y.X.; Li, S.Y.; Huang, N. Immobilization of selenocystamine on TiO2 surfaces for in situ catalytic generation of nitric oxide and potential application in intravascular stents. Biomaterials 2011, 32, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ji, T.; Liang, K.; Zhu, L. Mussel-inspired soft-tissue adhesive based on poly(diol citrate) with catechol functionality. J. Mater. Sci. Mater. Med. 2016, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hirota, K.; Goto, T.; Yumoto, H.; Miyake, Y.; Ichikawa, T. Biofilm formation of Candida albicans on implant overdenture materials and its removal. J. Dent. 2012, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Terraf, L.; Mendoza, L.M.; Tomás, J.; Silva, C.; Nader-Macías, M.E.F. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J. Appl. Microbiol. 2014, 117, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Mei, L.; Seneviratne, C.J.; Lo, E.C. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int. J. Paediatr. Dent. 2012, 22, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Ito, L.; Cao, Y.; Lo, E.C.; Li, Q.L.; Chu, C.H. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J. Dent. 2014, 42, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhitomirsky, I.; King, N.M. Electrophoretic deposition of silica–hyaluronic acid and titania–hyaluronic acid nanocomposites. J. Alloys Compd. 2011, 509, S510–S513. [Google Scholar] [CrossRef]
- Chen, F.; Rice, K.C.; Liu, X.M.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm. Res. 2010, 27, 2356–2364. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, M.L.; Li, Q.-L.; Chu, C.H. Inhibition of Cariogenic Plaque Formation on Root Surface with Polydopamine-Induced-Polyethylene Glycol Coating. Materials 2016, 9, 414. https://doi.org/10.3390/ma9060414
Mei ML, Li Q-L, Chu CH. Inhibition of Cariogenic Plaque Formation on Root Surface with Polydopamine-Induced-Polyethylene Glycol Coating. Materials. 2016; 9(6):414. https://doi.org/10.3390/ma9060414
Chicago/Turabian StyleMei, May Lei, Quan-Li Li, and Chun Hung Chu. 2016. "Inhibition of Cariogenic Plaque Formation on Root Surface with Polydopamine-Induced-Polyethylene Glycol Coating" Materials 9, no. 6: 414. https://doi.org/10.3390/ma9060414
APA StyleMei, M. L., Li, Q.-L., & Chu, C. H. (2016). Inhibition of Cariogenic Plaque Formation on Root Surface with Polydopamine-Induced-Polyethylene Glycol Coating. Materials, 9(6), 414. https://doi.org/10.3390/ma9060414






