1. Introduction
Due to their industrial scale of production, fly ash and slag are now presented as low life cycle CO
2 emissions, environmentally-friendly and sustainable solid precursors for alkali-activated cement, mortar and concrete [
1,
2]. A wide variety of such industrial wastes is described in the literature with very variable composition [
3,
4] thus making their alkali-activation a complex issue. Apart from coal fly ash, blast furnace slag, calcined kaolins, and few others for which it can be said confidently that are suitable for EN 197 cements and can also be properly and successfully employed in the production of geopolymers or hybrid alkaline cements, few recent works have been aimed at valorization of poorly reactive/not-recycled waste materials [
5,
6]. In the present study two different steel slags were used with metakaolin and sand to generate an aluminosilicatic matrix via alkali activation. This aluminosilicatic matrix was designed to attain a good chemical stability taking into account the existing models on mixed C–S–H (CaO–SiO
2–H
2O) structure. In fact, the chemical and mechanical stability of alkali-activated materials is based on some equilibrium between the geopolymer gel phase and the reacted aggregate that constitutes the alkali activated matrix. The stability of silicates-based binders, including ordinary Portland cement, has been found to be improved by the presence of Al ions during the polycondensation reactions [
1,
7]. Aluminum cations incorporated in C–S–H gel were found to improve the resistance to carbonation. It was observed that all aluminum present in the silicate tetrahedra chains were five-fold coordinated species in interlayer and six-fold coordinated at the surface and theywere incorporated into the amorphous silica phase forming fully condensed tetrahedral Al(-SiO)
3 sites [
1,
7]. Myers
et al. [
1] identified in granulated blast furnace slag three distinct tetrahedral Al sites: Q
3(1Al), Q
4(3Al) and Q
4(4Al) which are indicative on the cross-linking degree in the calcium (alkali) aluminosilicate hydrate (C–(N–)A–S–H) gels and the presence of additional highly polymerized aluminosilicate products. For appropriate Si/Al ratio, the amount of non-bridging oxygen (NBO) is a function of SiO
2/Na
2O molar ratio. Low value of SiO
2/Na
2O is consistent with the increasing number of NBO sites where SiQ
2 and SiQ
1 structural units were preferentially formed from silicate chains, dimers and monomers. High SiO
2/Na
2O molar ratio generates a decreasing number of NBO sites with structures consisting principally of SiQ
3 and SiQ
4 structural units which form silicate 3D frameworks and sheets [
7]. When CaO is present, C–S–H is the principal hydration product and primary binding phase; it can be described as a single chain structure that faces polymerization decreasing with the increase of Ca content [
8].
While pure geopolymer (N–A–S–H or K–A–S–H) and ordinary Portland cement (C–S–H) phases are generally formed from pure metakaolin and kaolin-calcite respectively, the industrial and natural wastes (fly ash, slag, incinerator bottom ash, volcanic ash, rice husk ash,
etc.) are complex mixes of geopolymers and OPC forming elements. The high temperature of process, the level of vitrification and melting, the cooling rate and the initial bulk composition will result in the formation of amorphous or partially crystalline phases, as mullite, anorthite, plagioclase, pyroxene
etc. [
9,
10,
11]. The better crystallized phases will not dissolve even in high alkaline media, thus hindering the polycondensation. Therefore, the appropriate mode to design the alkali-activated materials should not be generalized to a simple activation of finely grounded powder with the alkaline solution, but a more complex mix-design approach is necessary.
Slag from ferrous metallurgy are generally calcium- and iron-rich (40 wt %–52 wt % CaO; 70 wt %–80 wt % FeO; 20 wt %–30 wt % Fe
2O
3 [
9]) with limited Si and Al content (10 wt %–19 wt % SiO
2; 1 wt %–3 wt % Al
2O
3 [
9]). The main minerals consist of C
3S, C
2S, C
4AF, C
12A
7, RO
ss (R = Ca, Fe, Mn, Mg solid solution) and free CaO [
9,
10,
11] embedded in a variable amount of amorphous phase depending on the slag cooling practice. Steel slag has a large amount of non-active compounds such as RO and Fe
3O
4. In the high alkaline context, like that of inorganic polymer cement, the non-active components as well as the others crystalline phases of the slag are dissolved in an incongruent manner essentially at their surfaces. With their fine particle size, they seem attractive for the mechanical reinforcement of the matrices through the filler effect and densification. Additionally, they can ideally act as nucleation sites to optimize the reaction of geopolymerization.
Starting from the considerations listed above, the approach used for this research was to consider the slag powder as binder precursor, finer and more amorphous particles, as well as fine aggregates, non-reactive particles. We also operated to obtain a geopolymeric paste with Si/Al close to 2.0–2.5 and Na/Al equal to 1, with the addition of a minimum amount of metakaolin. The larger unreacted slag phases remained embedded in such a matrix as aggregate in a mortar.
The two different steel slags from Electric Arc Furnace (EAF) steelmaking industry (Italy), used in this study, were prepared in an industrial process of production of manufactured aggregates. The presence of slag’s reactive phases, eventually metals, in alkaline environment were supposed to produced geopolymeric cements with a relevant porosity suitable for application as passive phase change humidity control material, which can moderate the indoor moisture. In fact our formulations were expected to be close to vesuvianite, sepiolite and zeolite which are all used as hygroscopic base materials in the manufacturing of moisture control materials for indoor wall coatings. Actually, it is generally observed that materials produced with cold setting processes as cement based mortars, concretes, etc. is capable to collect up to 8 wt %–10 wt % of humidity after complete curing and exposure to ambient environment. So for, aside the determination of the adsorption/desorption capacity and the permeable voids assessed with water absorption for these steel slag based inorganic polymer mortars, we also evaluated the morphology and the microstructure using several techniques, in particular X-ray diffraction (XRD), Fourier Infrared spectroscopy (FIT-IR), Mercury Intrusion Porosimeter (MIP), three-point flexural strength and Environmental Scanning Electronic Microscope (ESEM).
2. Materials and Methods
2.1. Characterization of Ferricalsialic and Calsialic Slags
Two steel slags from EAF steelmaking process were provided by Acciaierie Bertoli Safau in Italy and identified as ABS black (BSS) and ABS white (WSS). The first slag is obtained by the primary process in production of steel (melting of scrap in EAF furnace), while the second is obtained by the secondary metallurgical process (final steel alloy in ladle furnace). Additionally, BSS can be classified as an oxidation slag, while WSS is a reduction slag. Both slags, also indicated as SS, coming out from the furnaces, after cooling process, constitute the raw materials for production of aggregates. The cooling of slags is realized in a two-step process: Firstly the hot material is placed underneath water sprinklers of in order to rapidly reduce its temperature, and second the material is stocked in a pile to conclude the cooling process by air. Especially for black slag, the cooling process is very important and necessary to reduce the amount of free lime and to determine a high mechanical resistance for material. The black slag is generally considered as not suitable for the use in production of cement, while it is considered a good material to produce aggregates for concrete, bituminous conglomerate and civil constructions. Due to its high content of free lime and poor mechanical resistance, the white slag is instead suitable for the production of cement and may substitute the lime itself in stabilization of soils. The steel slags used in this study were provided as manufactured aggregates, obtained by processing the raw materials with the following operations: ageing storage, removing of scrap particles by magnetic separation, reduction of dimension by crushing, calibration of different products by screening.
The chemical compositions of the slag were determined by X-ray fluorescence (ARL™ QUANT'X EDXRF, Thermo Fisher Scientific, Waltham, MA, USA) are presented in
Table 1. Both slags had negligible content of C. The S content is 0.3% for BSS and 1.6% for WSS. The sulphur content is reported as SO
3 according to XRF analysis. The chemical dissolution of white ABS slag performed using concentrated NaOH (8M) (extended description of the test method is reported in Ref. [
12]) allowed to determine 35.89 wt % of reactive species, while the remaining 64.11 wt % could be accounted as of aggregates. For the black slag, 72.96 wt %was reactive species while only 27.04 wt % was identified as aggregates.
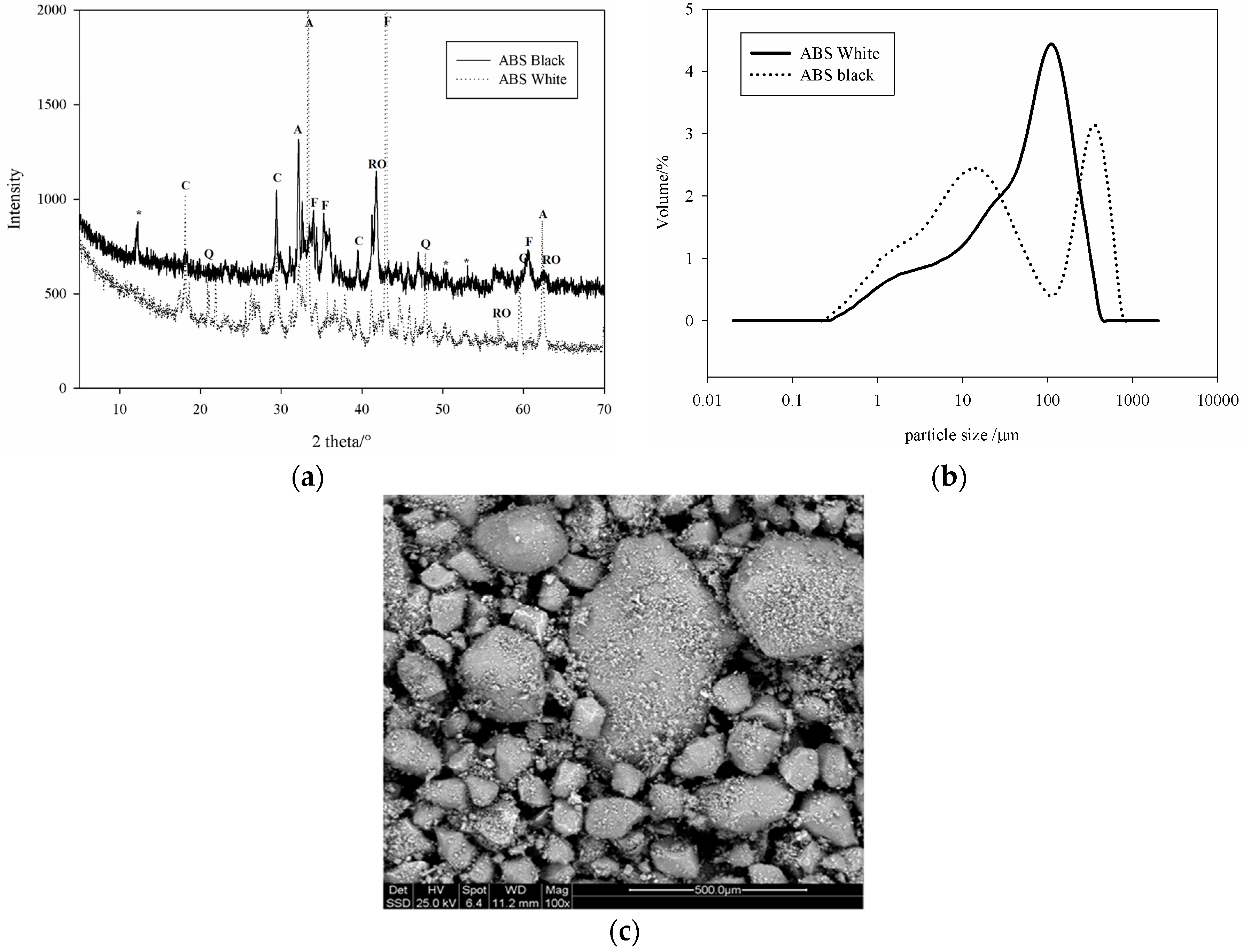
The mineralogical phases as determined by XRD analysis, using X-ray (X’Pert PRO—PANAlytical, Eindhoven, The Netherland) CuKα, Ni-filtered radiation (λ = 1.54184 Å) are given in
Figure 1a. Based on the chemical compositions in
Table 1, the SS can be classified as calsialic for the white and ferricalsialic for the black slag specimens. The particle size distributions which were measured by a laser particle size instrument (MASTER SIZER 2000, Malvern, UK) arepresented in
Figure 1b. The morphology of the grains as observed by ESEM is presented in
Figure 1c. It is evident that the black slag is finer than the white sample in the range of small particles, up to 32 µm (
Figure 1b). Between 32 and 283 µm the B specimen is finer and when the particle size was greater than 283 µm the black slag is coarser. The white slag with low iron content and highest CaO presents particles size similar to that of ordinary Portland cement [
2] while the particle size distribution of the black sample is typical of steel slag. The iron oxide and RO phases constitute the essential of the coarse particles.
The specific surface area as determined using nitrogen sorption method, BET (Brunauer-Emmet-Teller, method by nitrogen absorption on a GEMINI 2360, Micromeritics Instrument Corp., Norcross, GA, USA) gave 3.39 m
2/g for the white ABS slag and 2.06 m
2/g for the black ones. Under thermal analysis (
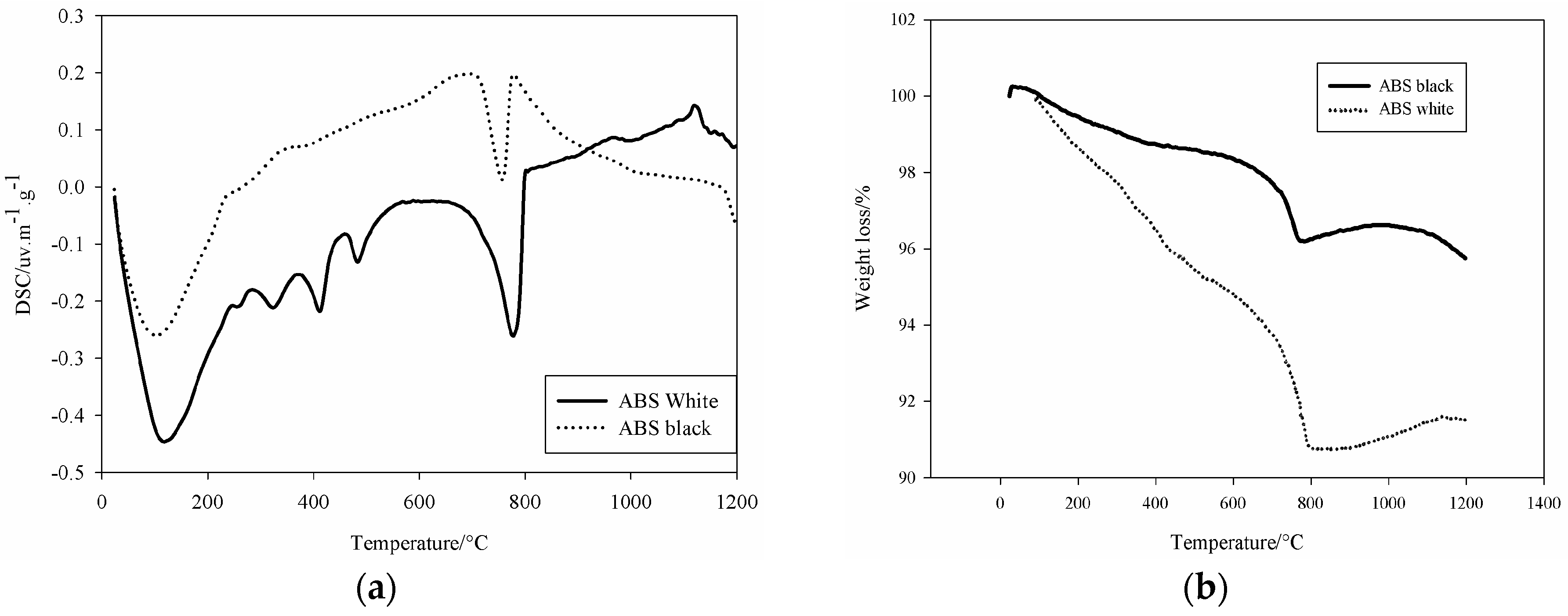
Figure 2), both slags presented endothermic peak around 106 °C related to the physico-absorbed water present within the samples. White slag showed peaks of decomposition at 256, 324, 413, and 487 °C. Peaks consecutive to transformation of hydrated for of Fe
2O
3. xH
2O, y-FeOH(OH) and Mg(OH)
2 [
4]. These compounds formed during the water granulation processes through the pouring into larger quantity of water to avoid excessive foaming. The peak situated at 760 °C corresponds to the decomposition of the Ca based phases of the slag. The peak at 1119 °C is related to the crystallization of aluminosilicate type mullite. The weight decrease for both steel slags (
Figure 2b) is indicative for the percentage of volatile materials into the samples (Ca(OH)
2, H
2O, CO
2,
etc.).
The metakaolin (MK) was obtained from a standard kaolin used for glaze formulation in the ceramic industry after calcinations at 700 °C for 4 hand ground finely down to 30 µm (D90). The oxide molar composition of the metakaolin, determined by XRF, gave a value of Si/Al = 1.3 in terms of the mass ratio [
13].
2.2. Mix-Design and Preparation of Inorganic Polymer Mortars
2.2.1. Mix-Design
The white and black ABS slags presented 40.31 wt % and 36.52 wt % of CaO but the SiO
2 contents of both materials were 11.33 wt % and 12.78 wt %, respectively. These contents result in Ca/Si molar ratios of 5.45 for the WSS and 4.38 for BSS. The main oxide content,
i.e., SiO
2 + Al
2O3 + Fe
2O
3, are less than 40 wt % for the white ABS and <50 wt % for the black sample, far from the ~80 wt % proposed by Davidovidts [
14] as requirement for the formulation of polysialates.
We proposed to adjust the bulk chemical composition with addition of metakaolin, MK, in a mix with semi-crystalline particles of silica sand. The white and black ABS slags, SS, were used with metakaolin in the following proportions: 1 SS:1 MK(B60 and W60), 2 SS:1 MK (B90 and W90) and 3 SS:1 MK (B120 and W120). The obtained solid precursors were finely ground down to 60 µm and used to form inorganic polymer mortars with sand representing two times the mass of slag-metakaolin, SS-MK, powder. The sand was also ground to reduce the particles size below 400 µm for research sake, but to improve the economy of the process the natural sand can be replaced with fine gravel.
2.2.2. Sample Preparation
NaOH (laboratory grade of pellets from Sigma Aldrich, Milan, Italy) was dissolved in deionized water to reach a concentrated 8M solution. This solution, after having been stored for a minimum of 48 h, was mixed with sodium silicate (SiO
2/Na
2O, molar ratio = 2.98; industrial grade from Ingessil, Verona, Italy) in volume proportion of 2:3. The final mix was used to prepare the inorganic polymer pastes with proportions reported in
Table 2. The variation in the liquid: solid weight ratio (liquid = soda plus Na-silicate solution; solid = SS plus MK plus sand) as indicated into the
Table 2 as well as the NaOH:Na
2SiO
3 volume ratio were chosen considering the behavior of the steel slag into the alkaline solution and the objective to draw pastes with similar final viscosity. The difference between the series B and W was dependant on the difference between the potential reactive fraction of each steel slag. The pastes for the six formulations were mixed in a bench mixer for approximately 10 min and poured in 1 × 1 × 14 cm
3 polyurethane molds. Soon after pouring, the specimens were isolated from air into closed plastic films for the first 72 h. The curing continued in ambient conditions, room temperature around 21 ± 2 °C and 54% ± 1% of humidity (usual indoor environment) for up to 365 days. This curing procedure was considered optimal for the samples prepared in the laboratory with no defects. The mechanical characterization was performed on the specimens cured for 28 and 365 days.
2.3. Characterization of the Cured Steel Slag-BasedAlkali-Activated Mortars
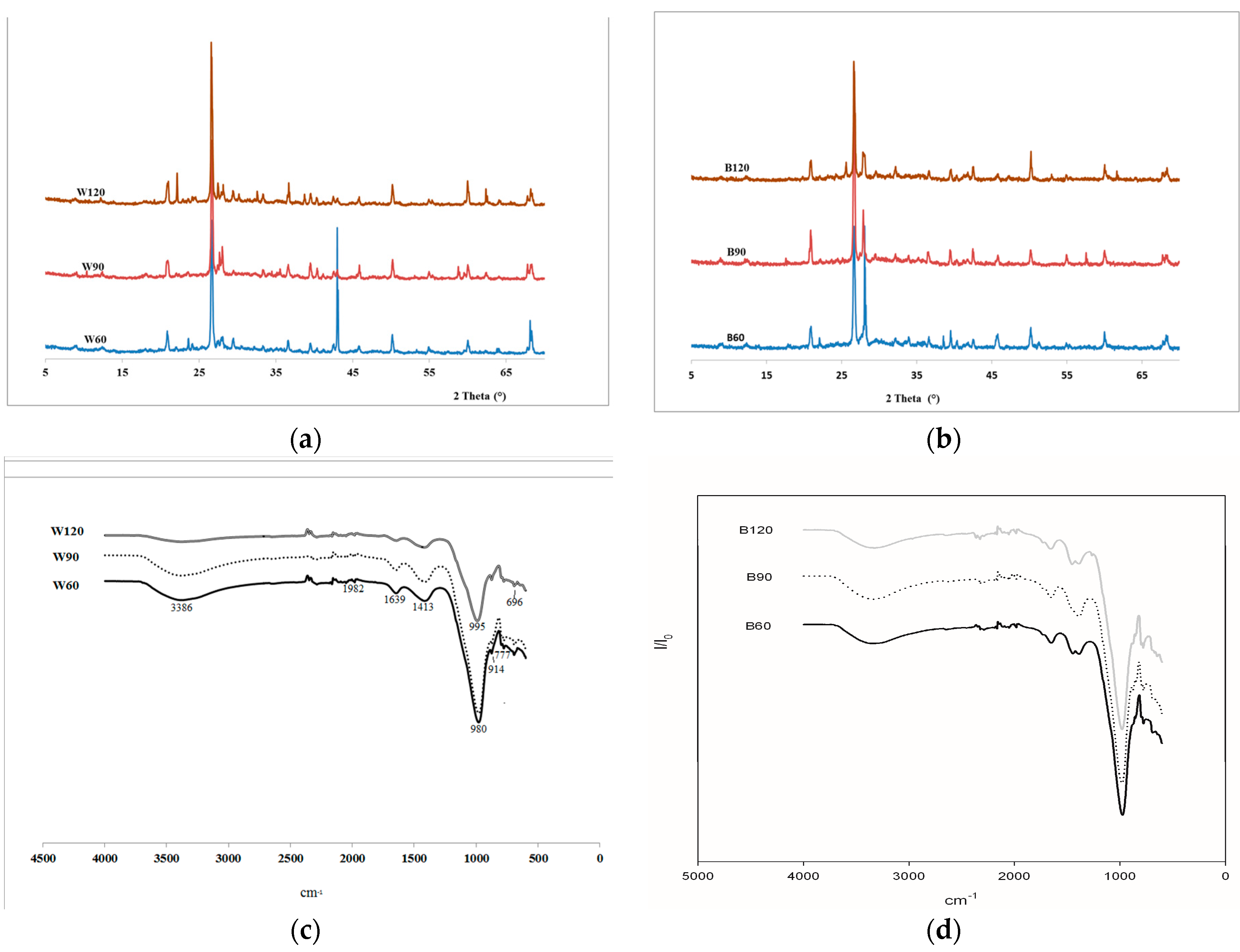
The mineralogical analysis of the alkali-activated steel slag, AAS, mortars were carried out with an X-ray powder diffractometer, XRD (see above) from 5° to 70°, 2theta steps and integrated at the rate of 2 s per step.
Fourier transformed infrared spectroscopy, FT-IR, (Avatar 330 FTIR, Thermo Nicolet, Waltham, MA, USA) was performed on each sample analyzing fine powders of ground specimens (ф < 80 µm) collected from samples used for the mechanical test after curing for 28 days. A minimum of 32 scans between 4000 and 500 cm−1 were averaged for each spectrum at intervals of 1 cm−1.
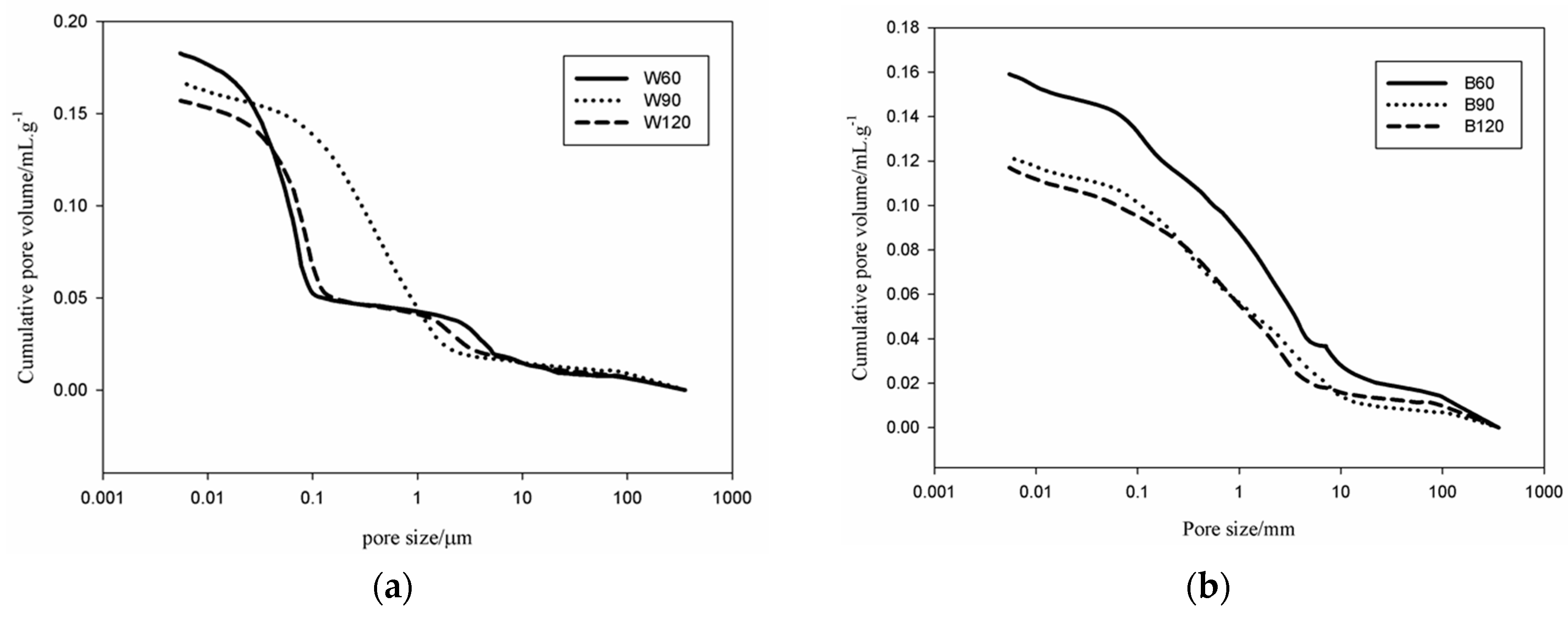
An Autopore IV 9500, Micromeritics, 33000 psi (228 MPa) Mercury Intrusion Porosimeter (MIP, Manchester, UK) covering the pore diameter range from approximately 360 to 0.005 μm having two low-pressure ports and one high-pressure chamber was used for the pores analysis. Pieces were prepared from the bulk of each sample with specimens of ~1 cm3 of volume for the MIP. Apart from the pore volume and size, the equipment software also automatically evaluated the tortuosity as the ratio of the length of the path described by the pore space to the length of the shortest path across a porous mass.
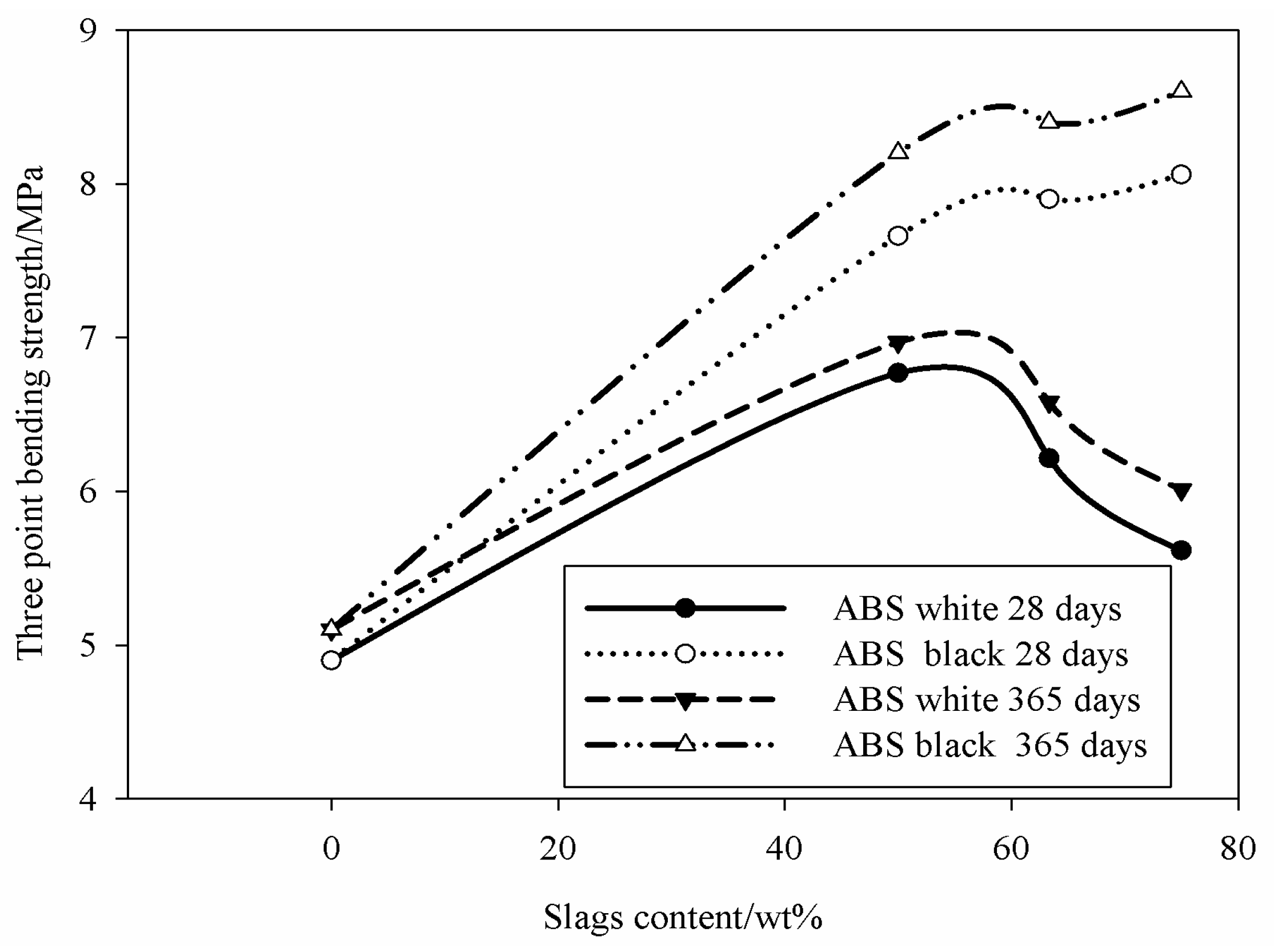
Specimens (10 for each composition to reduce the error within 20%) with nominal size of 10 ± 0.10 mm in width, 10 ± 0.10 mm thick and a length of 140 ± 0.10 mm were used for the mechanical tests by using a three-point bending configuration with span equal to 40 mm according to ASTM C1161-02c standard. The specimens were loaded by a universal testing machine, type MTS 810, (MTS Systems Corporation, Eden Prairie, MN, USA) with cross-head speed of 5 mm/min.
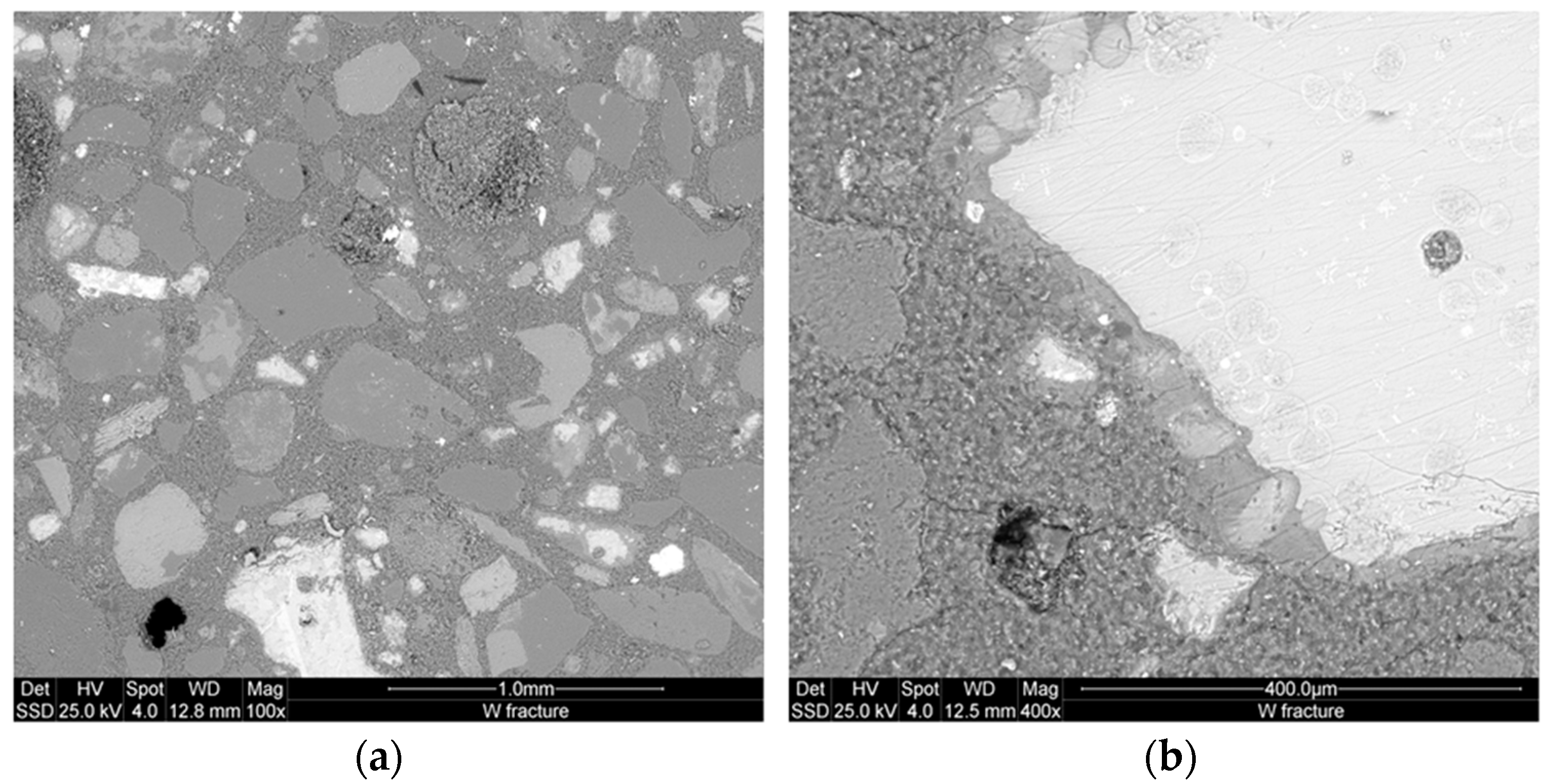
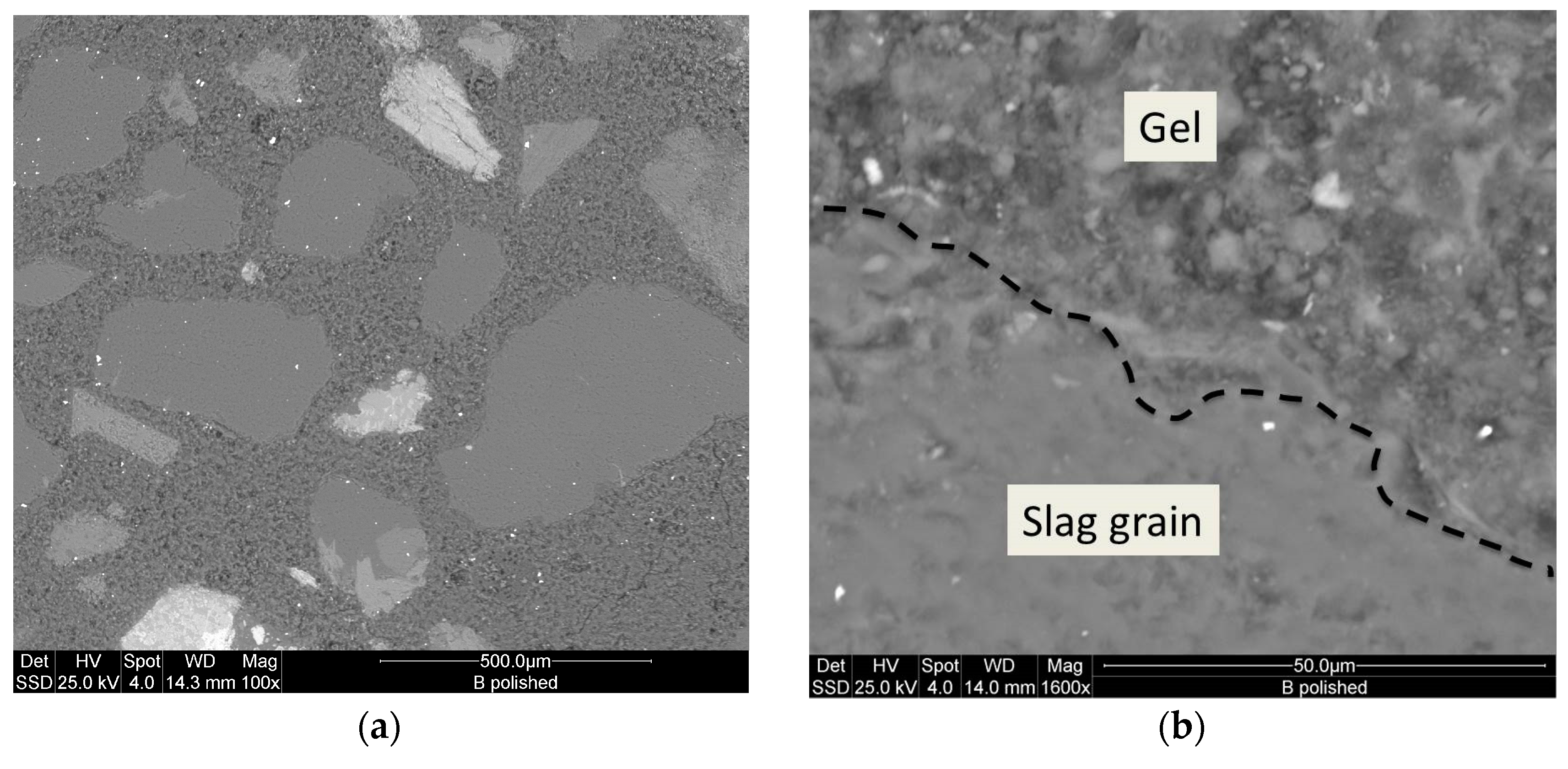
The microstructure of the inorganic polymer cement specimens was studied using an Environmental Scanning Electron Microscope (ESEM, Model Quanta 200, FEI, Hillsboro, OR, USA) at low vacuum. Both fractured pieces from mechanical testing and etched polished specimens were used. The specimens were preliminarily coated with 10 nm thick gold layer. The ESEM was equipped with an Oxford Instruments energy dispersive spectrometer (EDS) for the microanalysis thus facilitating the investigation of phase distribution in the matrix.
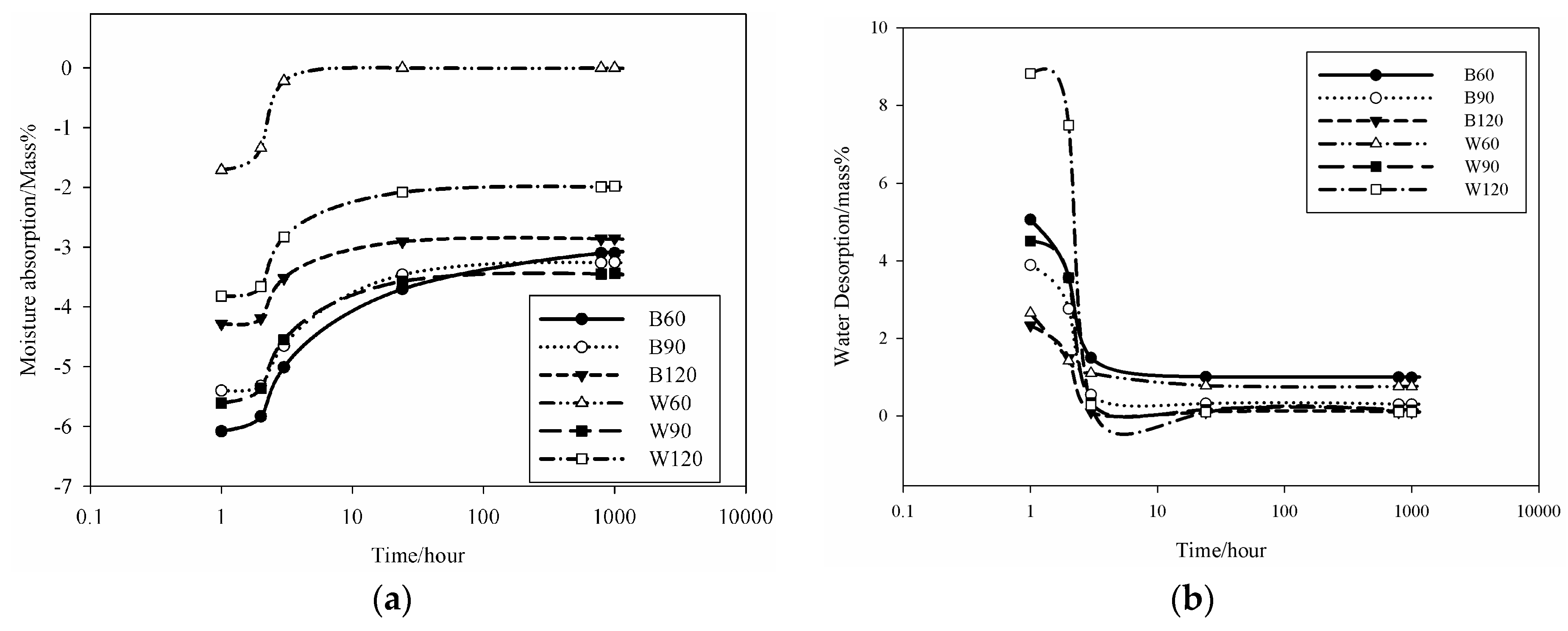
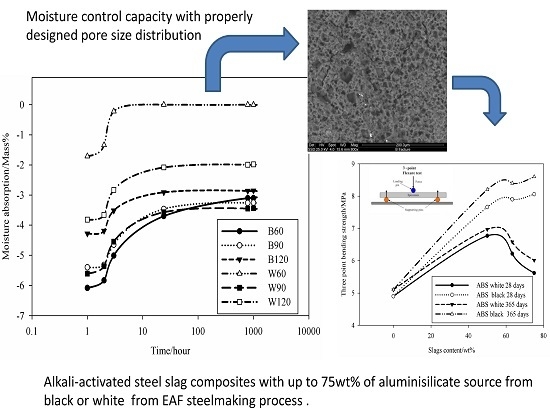
To evaluate the capability to help in the control of indoor humidity in buildings, the adsorption/desorption behavior of the AAS mortars was evaluated on two specimens aged 28 days per each formulation dried at room temperature for 24 h and then soaked in deionizer water for 24 h accordingly to previous studies [
15]. Specimens of prismatic shape as those used for mechanical characterization were weighted in their saturated condition and the time of the first measurement was considered as
t0. After each hour—for the first 10 h—the measurement was repeated, then weight measurement of the sample continued every 24 h for 1000 h. This measurement was repeated four times (four cycles, also indicated as water cycles) in the laboratory conditions (T = 21 ± 2 °C, R.H. (Room Humidity) 54% ± 1%). Finally, water absorption was determined as ratio of the water saturated specimen with respect to the dry one; variation of the water release after saturation is expressed as rate of weight change and the humidity content coincides with the water retained by the sample after the 1000 h test time. Additionally, the shrinkage and expansion were monitored, however, changes in dimensions were not identified.
4. Discussion and Conclusions
Astutingsih and Liu [
30] indicated that in alkali-activated slag, alkali ions (K
+, Na
+,
etc.) are located at a non-bridging oxygen site [
31,
32,
33]. The use of amorphous alumina and silica from metakaolin and semi-crystalline silica sand generates structural matrices with high flexural strength as the result of the cracks stabilization, reduction of the cumulative pore volume and improvement of the microstructure. The strengthening mechanism is focused on the existence of both N–A–S–H and C–N–(A)–H suitable adhesive bonds to embed the various aggregates in the matrix. The metallic particles content of the various mix acts at first through its corrosion in alkaline environment to enhance the reactions heat thus densifying the inter-pores partitions although the porosity between 1 and 10 µm results from the iron activities. Fe
3+ and Fe
2+ from the corrosive reactions and the dissolution of some RO minerals react with the alkaline solution to form new ferric sites which may be located at amorphous gel-like phase [
23].
Kotama
et al. [
34] correlated the moisture control capacity with pore size distribution makethe difference between the gel pores and the capillary pores. They designed ideal construction materials with pores distributed between those with size <0.1 µm, considered as pores responsible for the absorption, and capillary pores (0.1 µm < ф < 10 µm) responsible for desorption. The detailed analysis of the pores in the AAS mortars show that the BSS series present 15.6 vol % for B60 and 17 vol % for B90 and B120 of pores with size 0.1 µm. For WSS series, apart from W90, the specimens present pores having 60% with size <0.1 µm. The analysis of moisture absorption–desorption performance indicates the potential absorption up to ~6% moisture in the context of maximum humidity. In general, increasing the gel pores content (ф < 0.1 µm) will increase moisture absorption. Pores with size between 0.1 and 10 µm contribute to enhance the moisture desorption capacity. In fact, the moisture absorbed was found to be desorbed almost totally within ~8 h. Less than 2.5 vol % moisture remains in B60 and W60. The moisture percentage decreases below 0.5 vol % for B90, B120, W90 and W120. It can be concluded that the presence of the steel slag improves the moisture control capacity leading to the production of pore network that enable regulation of absorption and desorption within a short range of time (
Figure 6). The above described pore network allows: (i) no accumulation of moisture within the bulk structure of the mortars and by the way high exchange capacity; and (ii) by using the geopolymer process technology, the surface thickness described by Kotama
et al. [
34] can be achieved with the molding design in relation to the viscosity of the alkali-activated pastes making the surface thick enough to avoid the ingress of aggressive agents while improving the exchange rate between the environment and the bulk.
The above-described formulations based on the steel slag with particular class of capillary porosity have shown that alkali activation accompanied by an appropriate mix design is a procedure capable of reusing a high amount of end-of-waste materials or byproducts as steel slag. Apart from the low embodied energy, since the activation of slag and its consolidation required very low amount of energy with respect to the energy required for conventional fired bricks and cement clinker, these AAS mortars also showed an interesting adsorption/desorption behavior that suggested their use as coating material to maintain the stability of indoor relative humidity.
All of these characteristics are in line with the challenges of the EU Framework Programme Horizon 2020 [
35] for the eco-efficient construction and building materials research.