Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(methyl methacrylate) Denture Base Material Doped with Inorganic Filler
Abstract
:1. Introduction
2. Results
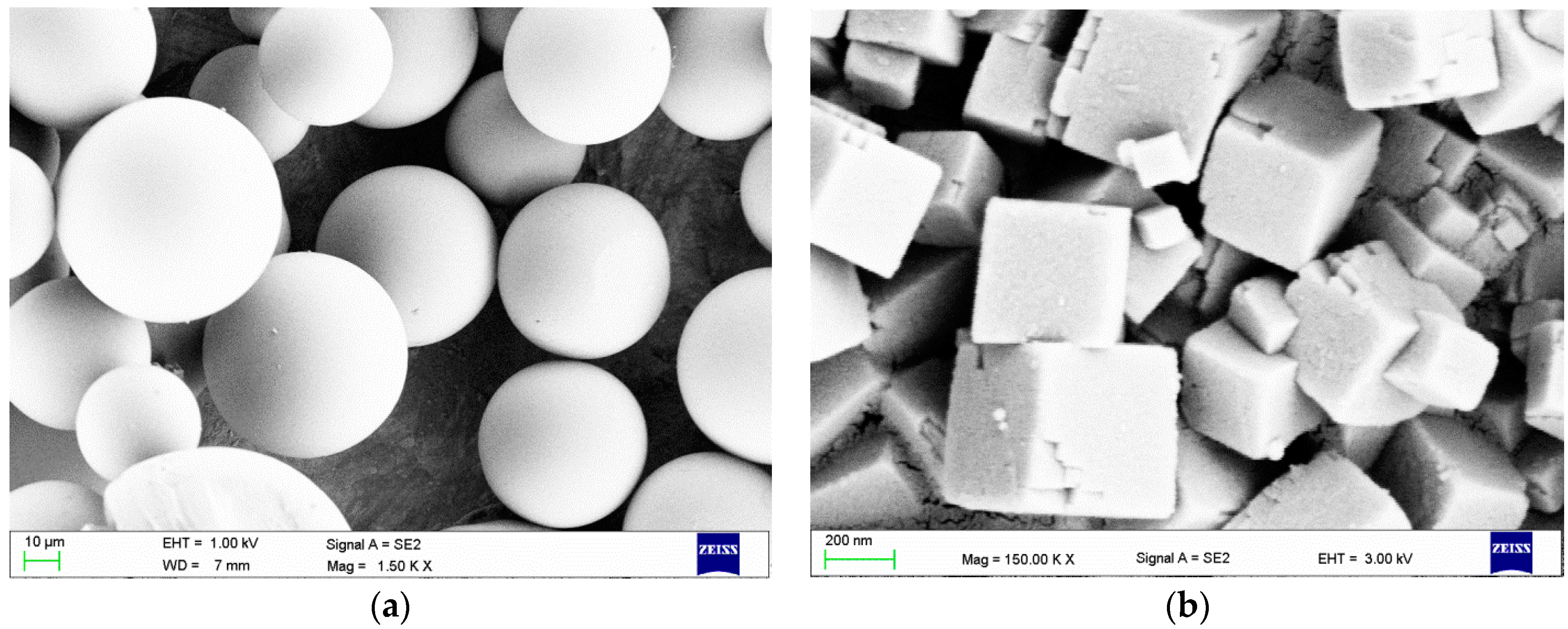
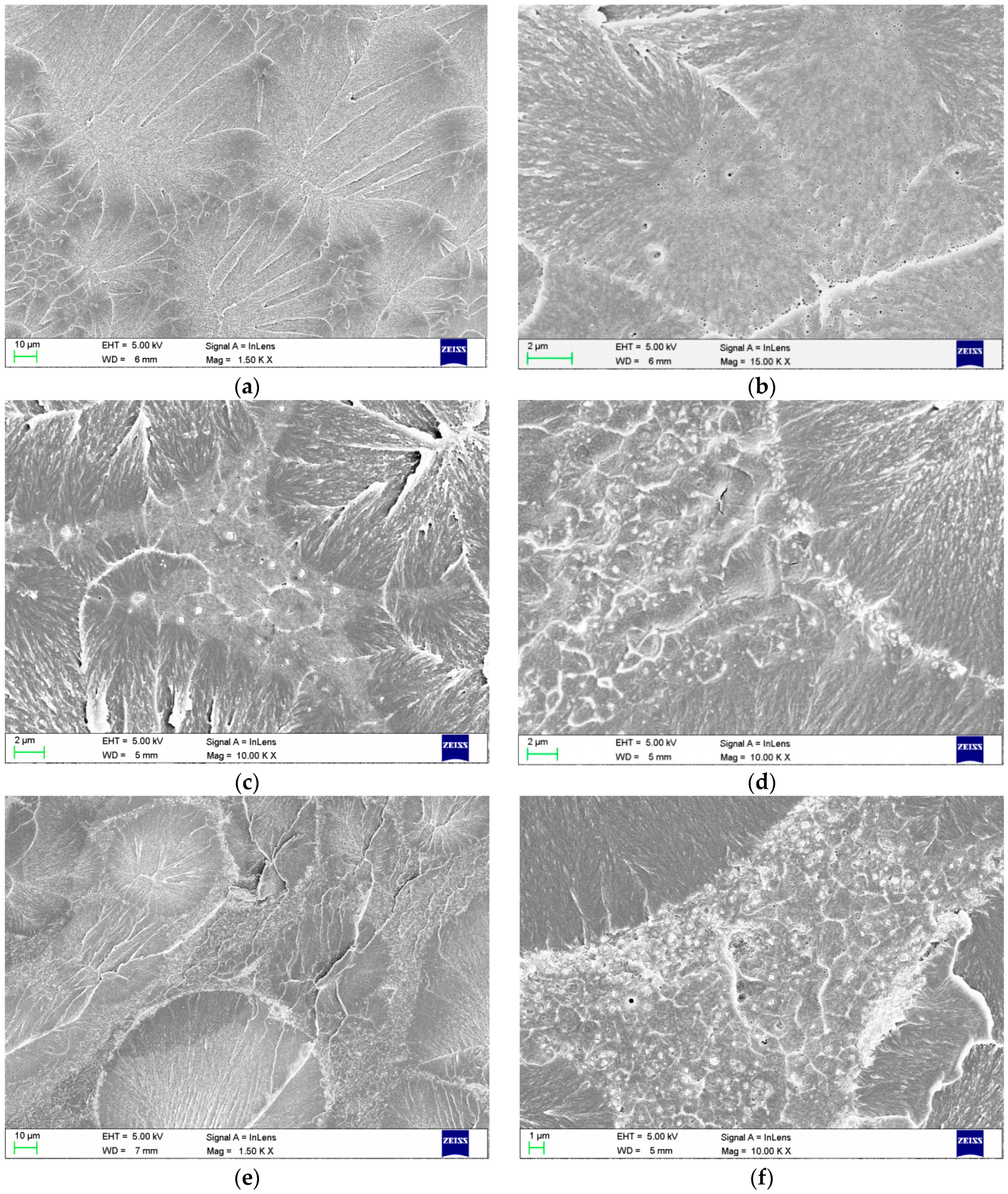
2.1. Scanning Electron Microscopy (SEM) Investigations
2.2. Microbiological Tests
3. Discussion
4. Materials and Methods
4.1. Material Preparation
4.2. Preparation of Polymerized Samples
4.3. SEM Investigations of Polymerized Samples
4.4. Microbiological Tests
4.5. Statistical Analysis
5. Conclusions
- The increased SRs with prolonged storage duration indicate that further storage would result in a loss of resistance against microorganisms. For materials modified with antimicrobial fillers, additional long-term storage tests should be conducted. For denture base materials (and other similarly used materials), it is important to consider that new dentures may not be colonized immediately and that the initial resistance against fungi and bacteria should only be used as a starting point.
- The use of commercially available, two-component, PMMA denture base materials presents limitations because the pre-polymerized spheres are a major cause of inhomogeneity among the obtained composites. This inhomogeneity creates areas free of filler, which affects the properties of the materials. Therefore, additional research should be performed on the introduction of fillers to these spheres during suspension polymerization to obtain better distributions at lower filler concentrations and to improve the antimicrobial properties.
- Long-term studies of the migration of silver ions from the polymer matrix and the influence of different media on ion emission need to be performed. Future results should be coupled with the presented research as well as with cytotoxicity studies.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Arendorf, T.M.; Walker, D.M. The prevalence and intra-oral distribution of Candida albicans in man. Arch. Oral Biol. 1980, 25, 1–10. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Darwazeh, A.M.G.; Shukri, L.A.K. Isolation of Candida species from the oral cavity and fingertips of Complete and partial dentures wearers. J. Dent. Health Oral Disord. Ther. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Spiechowicz, E.; Mierzwińska-Nastalska, E. Fungal Infections of Oral Cavity, 1st ed.; Med Tour Press International: Warszawa, Poland, 1998; p. 34. [Google Scholar]
- Scully, C.; el-Kabir, M.; Samaranayake, L.P. Candida and oral candidosis: A review. Crit. Rev. Oral Biol. Med. 1994, 5, 125–157. [Google Scholar] [PubMed]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 2. Oral diseases caused by Candida species. Aust. Dent. J. 1998, 43, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgensen, E. Oral mucosal lesions associated with the wearing of removable dentures. J. Oral Pathol. Med. 1981, 10, 65–80. [Google Scholar] [CrossRef]
- Nikawa, H.; Yamamoto, T.; Hamada, T.; Sadamori, S.; Agrawal, S. Cleansing efficacy of commercial denture cleansers: Ability to reduce Candida albicans biofilm activity. Int. J. Prosthodont. 1995, 8, 527–534. [Google Scholar] [PubMed]
- Pinto, T.M.; Neves, A.C.; Leão, M.V.; Jorge, A.O. Vinegar as an antimicrobial agent for control of Candida spp. in complete denture wearers. J. Appl. Oral Sci. 2008, 16, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Scully, C. Oral and Maxillofacial Medicine: The Basis of Diagnosis and Treatment, 3rd ed.; Churchill Livingstone Elsevier: Edinburgh, UK, 2013; pp. 254–267. [Google Scholar]
- Van Reenen, J.F. Microbiologic studies on denture stomatitis. J. Prosthet. Dent. 1973, 30, 493–505. [Google Scholar] [PubMed]
- Nyquist, G. The influence of denture hygiene and the bacterial flora on the condition of the oral mucosa in full denture cases. Acta Odontol. Scand. 1953, 11, 24–60. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.C.; Andrade, I.M.; Peracini, A.; Souza-Gugelmin, M.C.; Silva-Lovato, C.H.; de Souza, R.F.; Paranhos Hde, F. The effectiveness of chemical denture cleansers and ultrasonic device in biofilm removal from complete dentures. J. Appl. Oral Sci. 2011, 19, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Harada, A.; Yamada, Y.; Odashima, Y.; Nakamura, K.; Inagaki, R.; Kanno, T.; Sasaki, K.; Niwano, Y. Influence of a new denture cleaning technique based on photolysis of H2O2 the mechanical properties and color change of acrylic denture base resin. Dent. Mater. 2013, 32, 529–536. [Google Scholar] [CrossRef]
- Peracini, A.; Davi, L.R.; de Queiroz Ribeiro, N.; de Souza, R.F.; Lovato da Silva, C.H.; de Freitas Oliveira Paranhos, H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J. Prosthodont. (Res.) 2010, 54, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, H.F.O.; Peracini, A.; Pisani, M.X.; Oliveira Vde, C.; de Souza, R.F.; Silva-Lovato, C.H. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, J.; Motaghi, M.; Panahi, J.; Havasian, M.R.; Delpisheh, A.; Azizian, M.; Pakzad, I. Anti-fungal resistance in candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation 2014, 10, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Araj, G.F.; Asmar, R.G.; Avedissian, A.Z. Candida profiles and antifungal resistance evolution over a decade in Lebanon. J. Infect. Dev. Ctries 2015, 9, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Nam, K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J. Adv. Prosthodont. 2014, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. J. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin—A pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Lee, C.H.; Lee, C.J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Salabat, A.; Mirhoseini, F. Applications of a new type of poly(methyl methacrylate)/TiO2 nanocomposite as an antibacterial agent and a reducing photocatalyst. Photochem. Photobiol. Sci. 2015, 14, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Anehosur, G.V.; Kulkarni, R.D.; Naik, M.G.; Nadiger, R.K. Synthesis and determination of antimicrobial activity of visible light activated TiO2 nanoparticles with polymethyl methacrylate denture base resin against Staphylococcus aureus. J. Gerontol. Geriat. Res. 2012, 1, 103–111. [Google Scholar]
- Sokołowski, J.; Szynkowska, M.I.; Kleczewska, J.; Kowalski, Z.; Sobczak-Kupiec, A.; Pawlaczyk, A.; Sokołowski, K.; Łukomska-Szymańska, M. Evaluation of resin composites modified with nanogold and nanosilver. Acta Bioeng. Biomech. 2014, 16, 51–61. [Google Scholar]
- Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Vilar-Pineda, J.; Martínez-Espinosa, J.C.; de la Fuente-Hernández, J.; Castaño, V.M. Toxicology of antimicrobial nanoparticles for prosthetic devices. J. Int. J. Nanomed. 2014, 9, 3999–4006. [Google Scholar]
- Nazirkar, G.; Bhanushali, S.; Singh, S.; Pattanaik, B.; Raj, N. Effect of anatase titanium dioxide nanoparticles on the flexural strength of heat cured poly methyl methacrylate resins: An in-Vitro Study. J. Indian Prosthodont. Soc. 2014, 14, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Bahador, A.; Khalil, S.; Shahroudi, A.S.; Kassaee, M.Z. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly(methyl methacrylate) acrylic resins. J. Prosthodont. (Res.) 2013, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, T.; Hamedi-rad, F. Effect of silver nano-particles on tensile strength of acrylic resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 9, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, A.M.; Goiato, M.C.; Moreno, A.; Nobrega, A.S.; Pesqueira, A.A.; dos Santos, D.M. Influence of nanoparticles on color stability, microhardness, and flexural strength of acrylic resins specific for ocular prosthesis. J. Int. J. Nanomed. 2014, 9, 5779–5787. [Google Scholar]
- Asar, N.V.; Albayrak, H.; Korkmaz, T.; Turkyilmaz, I. Influence of various metal oxides on mechanical and physical properties of heat-cured polymethyl methacrylate denture base resins. J. Adv. Prosthodont. 2013, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Silver Sodium Hydrogen Zirconium Phosphate. National Industrial Chemicals Notification and Assessment Scheme (Nicnas), Full Public Report, File No STD/1081, 11 March 2004. Available online: https://www.nicnas.gov.au/__data/assets/word_doc/0004/18337/STD1081FR.docx (accessed on 28 April 2016).
- Acosta-Torres, L.S.; López-Marín, L.M.; Nunez-Anita, R.E.; Hernández-Padrón, G.; Castaño, V.M. Biocompatible metal-oxide nanoparticles: Nanotechnology improvement of conventional prosthetic acrylic resins. J. Nanomater. 2011, 20, 12–17. [Google Scholar] [CrossRef]
- Łukomska-Szymańska, M.M.; Kleczewska, J.; Bielinski, D.M.; Jakubowski, W.; Sokołowski, J. Bactericidal properties of experimental dental composites based on dimethacrylate resins reinforced by nanoparticles. Eur. J. Chem. 2014, 5, 419–423. [Google Scholar] [CrossRef]
- Kvitek, L.; Panacek, A.; Prucek, R.; Soukupova, J.; Vanickova, M.; Kolar, M.; Zboril, R. Antibacterial activity and toxicity of silver—Nanosilver versus ionic silver. J. Phys. Conf. Ser. 2011, 304, 12–29. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Shinohara, Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem. Biophys. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Alternat. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Dorairaj, J. Nanosilver weds acrylic resin: A fit or misfit? A review. J. Adv. Oral Res. 2015, 6, 11–15. [Google Scholar]
- Pereira-Cenci, T.; Fernandes, F.S.; Skupien, J.A.; Mesko, M.E.; Straioto, F.G.; Del Bel Cury, A.A. Can new dentures decrease Candida levels? Int. J. Prosthodont. 2013, 26, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Gondim, J.O.; Duque, C.; Hebling, J.; Giro, E.M. Influence of human dentine on the antibacterial activity of self-etching adhesive systems against cariogenic bacteria. J. Dent. 2008, 36, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; dos Santos, P.H.; Filho, J.N.; Spolidorio, D.M. Colonisation of soft lining materials by micro-organisms. Gerodontology 2010, 27, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gratzl, G.; Christian, P.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Farah, S.; Aviv, O.; Laout, N.; Ratner, S.; Beyth, N.; Domb, A.J. Quaternary ammonium poly(diethylaminoethyl methacrylate) possessing antimicrobial activity. Colloids Surf. B Biointerfaces 2015, 128, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2016, 59, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, R.; Palanisamy, S.; Chen, S.M.; Chelladurai, K.; Padmavathy, S.; Saravanan, M.; Prakash, P.; Ali, A.M.; Al-Hemaid, F.M. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater. Sci. Eng. C 2015, 56, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, K.; Szynkowska, M.I.; Pawlaczyk, A.; Łukomska-Szymańska, M.; Sokołowski, J. The impact of nanosilver addition on element ions release form light-cured dental composite and compomer into 0.9% NaCl. Acta Biochim. Pol. 2014, 61, 317–323. [Google Scholar] [PubMed]
- Kampmann, Y.; De Clerck, E.; Kohn, S.; Patchala, D.K.; Langerock, R.; Kreyenschmidt, J. Study on the antimicrobial effect of silver—Containing inner liners in refrigerators. J. Appl Microbiol. 2008, 104, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhu, C.; Chen, J.; Zhong, J. Preparation and characterization of silver containing chitosan fibers. J. Appl. Polym. Sci. 2007, 104, 3622–3627. [Google Scholar] [CrossRef]
- Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.H.; Tessier, C.A.; Youngs, W.J. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Taylor, P.L.; Ussher, A.L.; Burrell, R.E. Impact of heat on nanocrystalline silver dressings. Part I: Chemical and biological properties. Biomaterials 2005, 26, 7221–7229. [Google Scholar] [CrossRef] [PubMed]


| Filler Concentration % | Survival Rate (SR) of Candida albicans ATCC 10231 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Stored (p = 0.048) ‡ | 7 Days (p = 0.003) ‡ | 30 Days (p = 0.004) ‡ | 60 Days (p = 0.004) ‡ | 90 Days (p = 0.002) ‡ | ||||||
| Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | |
| 0 (p = 0.217) † | 107.24 | 92.35/123.29 | 96.75 | 87.11/101.96 | 100 | * | 100 | * | 119.59 | 99.18/132.78 |
| 0.25 (p = 0.223) † | 57.2 | 48.89/89.35 | 100.03 | 66.06/112.48 | 100 | * | 100 | * | 88.14 | 85.11/139.3 |
| 0.5 (p = 0.004) † | 5.25 | 0.92/11.77 | 24.5 | 17.07/34.38 | 100 | * | 100 | * | 99.65 | 64.18/141.4 |
| 1 (p = 0.042) † | 0 | 0/7.04 | 0.06 | 0/0.12 | <0.01 | <0.01/0.02 | <0.01 | # | 57.21 | 45.93/68.49 |
| 2 (p = 0.01) † | 0 | 0/1.84 | 0 | * | <0.01 | 0/<0.01 | <0.01 | # | 10.29 | 6.86/30.11 |
| 4 (p = 0.007) † | 0.52 | 0/1.84 | 0 | * | <0.01 | 0/<0.01 | <0.01 | 0/<0.01 | 18.43 | 8.72/45.69 |
| 8 (p = 0.035) † | 0.23 | 0/0.92 | 0 | * | <0.01 | 0/<0.01 | <0.01 | 0/<0.01 | 0.12 | 0.12/0.34 |
| Filler Concentration % | Survival Rate (SR) of Escherichia coli ATCC 25922 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Stored (p < 0.001) ‡ | 7 Days (p < 0.001) ‡ | 30 Days (p < 0.001) ‡ | 60 Days (p < 0.001) ‡ | 90 Days (p < 0.001) ‡ | ||||||
| Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | |
| 0 (p = 1) † | 100 | * | 100 | * | 100 | * | 100 | * | 100 | * |
| 0.25 (p < 0.001) † | <0.01 | # | 100 | * | 100 | * | 100 | * | 100 | * |
| 0.5 (p < 0.001) † | 0 | * | 100 | * | 100 | * | 100 | * | 100 | * |
| 1 (p = 0.002) † | 0 | * | 0 | * | 0 | * | <0.01 | # | <0.01 | # |
| 2 (p = 0.406) † | 0 | * | 0 | * | 0 | * | 0 | * | 0 | 0/<0.01 |
| 4 (p = 1) † | 0 | * | 0 | * | 0 | * | 0 | * | 0 | * |
| 8 (p = 1) † | 0 | * | 0 | * | 0 | * | 0 | * | 0 | * |
| Filler Concentration % | Survival Rate (SR) of Staphylococcus aureus ATCC 25923 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Stored (p = 0.005) ‡ | 7 Days (p = 0.001) ‡ | 30 Days (p = 0.001) ‡ | 60 Days (p = 0.001) ‡ | 90 Days (p = 0.001) ‡ | ||||||
| Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | Median | Min/Max | |
| 0 (p < 0.001) † | <0.01 | # | 100 | * | 107.69 | * | 100.0 | * | 106.25 | * |
| 0.25 (p < 0.001) † | <0.01 | # | 100 | * | 107.69 | * | 100.0 | * | 106.25 | * |
| 0.5 (p = 0.001) † | <0.01 | 0/<0.01 | <0.01 | # | 107.69 | * | 100.0 | * | 106.25 | * |
| 1 (p = 0.003) † | <0.01 | # | <0.01 | # | <0.01 | # | <0.01 | # | <0.01 | # |
| 2 (p = 0.02) † | <0.01 | 0/<0.01 | <0.01 | # | <0.01 | # | <0.01 | # | <0.01 | # |
| 4 (p = 0.013) † | <0.01 | 0/<0.01 | <0.01 | # | <0.01 | # | <0.01 | # | <0.01 | # |
| 8 (p = 0.637) † | 0 | 0/<0.01 | <0.01 | 0/<0.01 | <0.01 | 0/< 0.01 | <0.01 | 0/<0.01 | <0.01 | 0/<0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Basa, K.; Mertas, A.; Pakieła, W.; Żmudzki, J.; Bobela, E.; Król, W. Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(methyl methacrylate) Denture Base Material Doped with Inorganic Filler. Materials 2016, 9, 328. https://doi.org/10.3390/ma9050328
Chladek G, Basa K, Mertas A, Pakieła W, Żmudzki J, Bobela E, Król W. Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(methyl methacrylate) Denture Base Material Doped with Inorganic Filler. Materials. 2016; 9(5):328. https://doi.org/10.3390/ma9050328
Chicago/Turabian StyleChladek, Grzegorz, Katarzyna Basa, Anna Mertas, Wojciech Pakieła, Jarosław Żmudzki, Elżbieta Bobela, and Wojciech Król. 2016. "Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(methyl methacrylate) Denture Base Material Doped with Inorganic Filler" Materials 9, no. 5: 328. https://doi.org/10.3390/ma9050328







