Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass
Abstract
:1. Introduction
2. Results
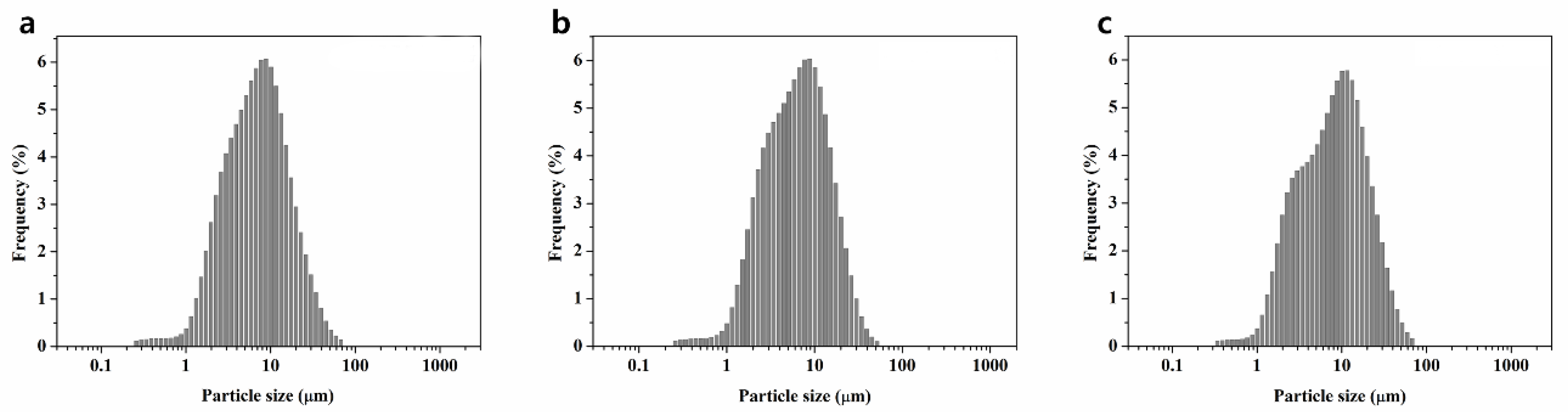
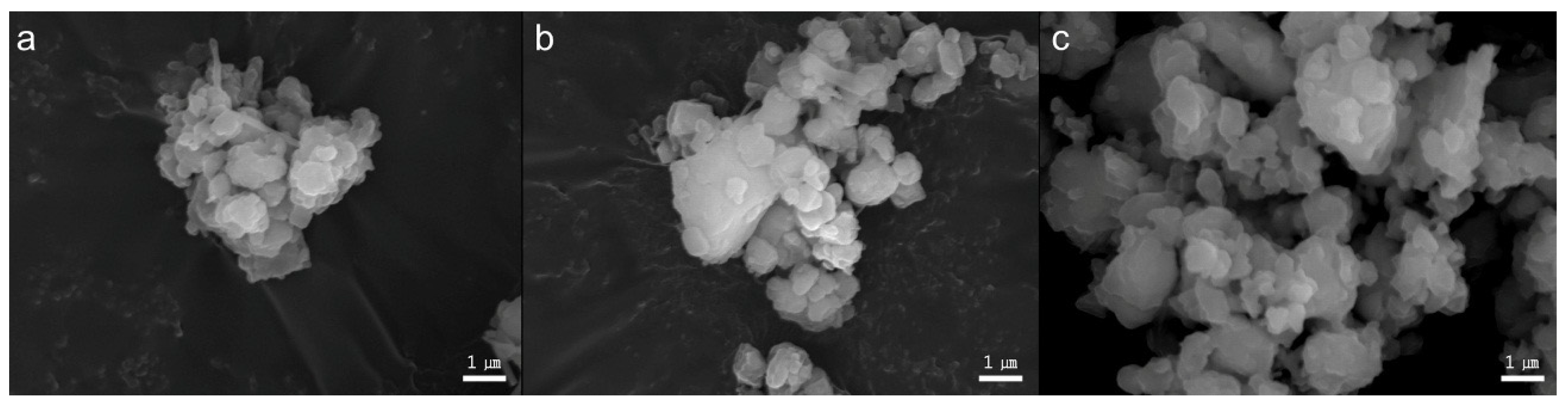
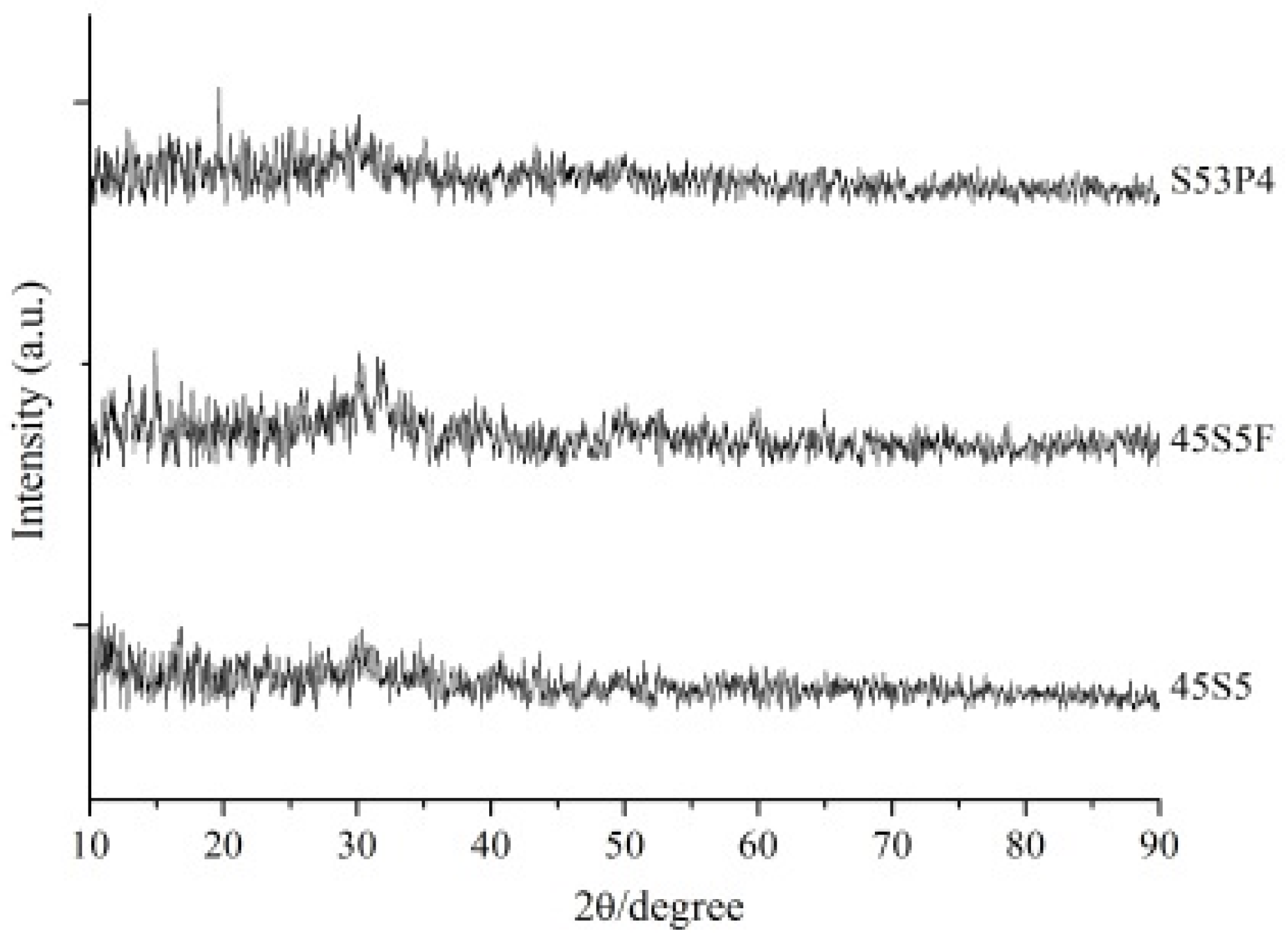
2.1. Powder Characterization of Three Types of BAG
2.2. Acid Neutralizing Ability
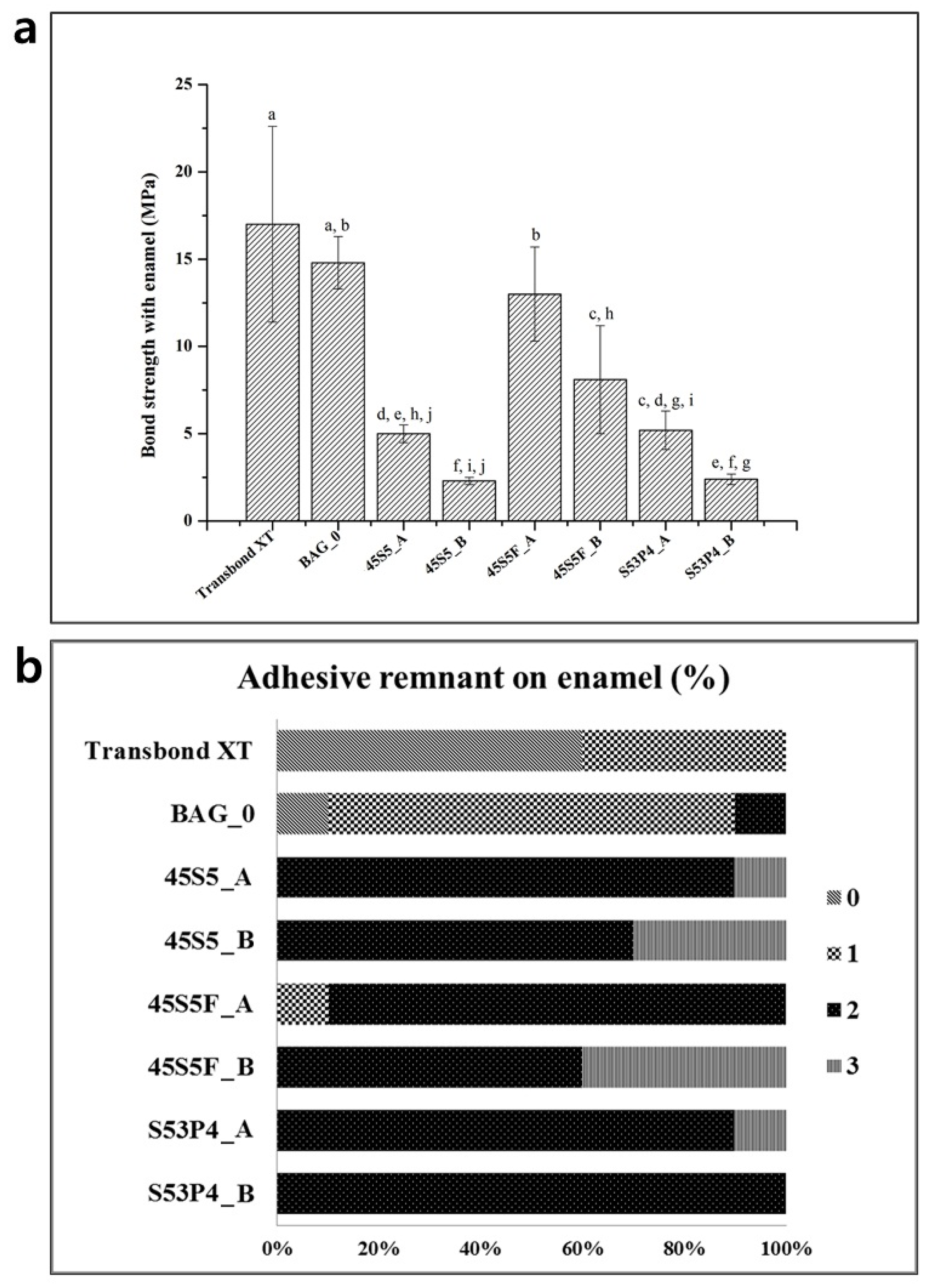
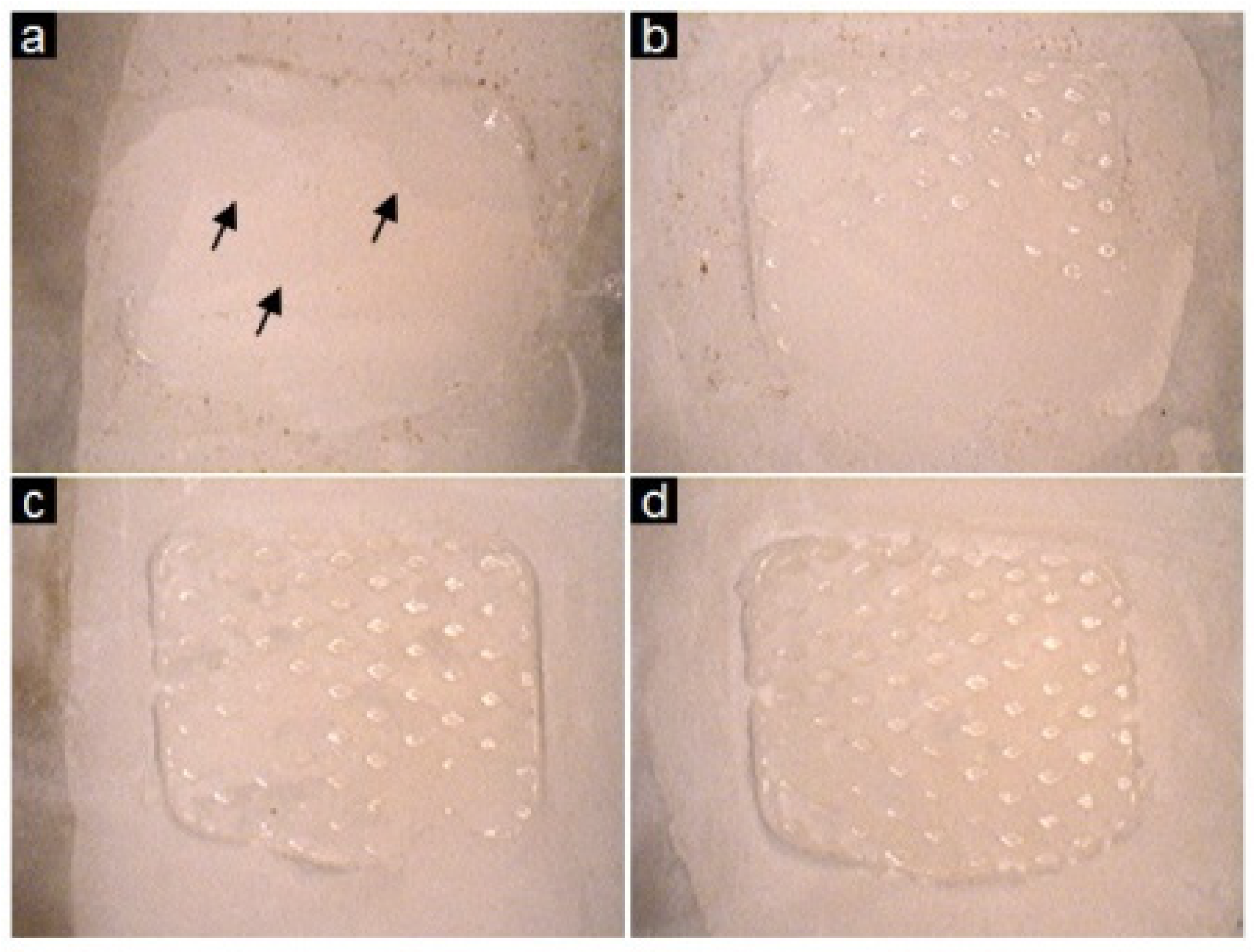
2.3. Shear Bond Testing and the Adhesive Remnant Index
3. Discussion
4. Experimental Section
4.1. Preparation of Orthodontic Adhesives Containing BAG
4.2. Acid Neutralizing Ability
4.3. Shear Bond Testing and the Adhesive Remnant Index
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tufekci, E.; Dixon, J.S.; Gunsolley, J.C.; Lindauer, S.J. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle. Orthod. 2011, 81, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Roberts, W.E.; Eckert, G.J.; Kula, K.S.; Gonzalez-Cabezas, C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofacial. Orthop. 2010, 138, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Lim, B.S.; Lee, S.J. Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. Am. J. Orthod. Dentofacial. Orthop. 2010, 137, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, W.; Gizani, S.; Nassika, M.; Kontou, E.; Nakou, M. Adhesion of Streptococcus mutans to different types of brackets. Angle. Orthod. 2007, 77, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Demling, A.; Elter, C.; Heidenblut, T.; Bach, F.W.; Hahn, A.; Schwestka-Polly, R.; Stiesch, M.; Heuer, W. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur. J. Orthod. 2010, 32, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L. Decalcification during orthodontic treatment with fixed appliances-an overview. Br. J. Orthod. 1992, 19, 199–205. [Google Scholar] [CrossRef] [PubMed]
- James, J.W.; Miller, B.H.; English, J.D.; Tadlock, L.P.; Buschang, P.H. Effects of high-speed curing devices on shear bond strength and microleakage of orthodontic brackets. Am. J. Orthod. Dentofacial. Orthop. 2003, 123, 555–561. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta. Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.J.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Zhong, J.; Clark, A.E.; Du, M.Q. Anti-gingivitis effect of a dentifrice containing bioactive glass (NovaMin) particulate. J. Clin. Periodontol. 2006, 33, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Du Min, Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical Evaluation of a Dentifrice Containing Calcium Sodium Phosphosilicate (Novamin) for the Treatment of Dentin Hypersensitivity. Am. J. Dent. 2008, 21, 210–214. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18795515?dopt=Abstract (accessed on 1 August 2008). [Google Scholar] [PubMed]
- Zehnder, M.; Soderling, E.; Salonen, J.; Waltimo, T. Preliminary evaluation of bioactive glass S53P4 as an endodontic medication in vitro. J. Endod. 2004, 30, 220–224. [Google Scholar] [CrossRef]
- Khoroushi, M.; Mousavinasab, S.M.; Keshani, F.; Hashemi, S. Effect of resin-modified glass ionomer containing bioactive glass on the flexural strength and morphology of demineralized dentin. Oper. Dent. 2013, 38, E21–E30. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhao, J.; Weng, Y.; Park, J.G.; Jiang, H.; Platt, J.A. Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur. J. Oral. Sci. 2008, 116, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Piao, Y.Z.; Kim, S.M.; Lee, Y.K.; Kim, K.N.; Kim, K.M. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45S5 bioactive glass. Dent. Mater. 2013, 29, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.; Panzeri, H.; Soares, R.G.; Peitl, O.; Zanotto, E.D. A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz. Oral. Res. 2010, 24, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.L.; Davis, H.B.; Tufekci, E.; Crowe, J.J.; Covell, D.A.; Mitchell, J.C. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. Angle. Orthod. 2011, 81, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Manfred, L.; Covell, D.A.; Crowe, J.J.; Tufekci, E.; Mitchell, J.C. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle. Orthod. 2013, 83, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Greenspan, D.; Powers, K. Effect of pH and ionic strength on the reactivity of Bioglass 45S5. Biomaterials 2005, 26, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Brauer, D.S.; Al-Noaman, A.; Hill, R.G.; Doweidar, H. Density-structure correlations in fluoride-containing bioactive glasses. Mater. Chem. Phys. 2011, 130, 121–125. [Google Scholar] [CrossRef]
- Forsback, A.P.; Areva, S.; Salonen, J.I. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta. Odontol. Scand. 2004, 62, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Massera, J.; Fagerlund, S.; Hupa, L.; Hupa, M. Crystallization Mechanism of the Bioactive Glasses, 45S5 and S53P4. J. Am. Ceram. Soc. 2012, 95, 607–613. [Google Scholar] [CrossRef]
- Yip, K.; Smales, R. Oral diagnosis and treatment planning: part 2. Dental caries and assessment of risk. Br. Dent. J. 2012, 213, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Lee, S.J.; Lee, J.W.; Ahn, S.J. Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am. J. Orthod. Dentofacial. Orthop. 2008, 133, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Soderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Brauer, D.S.; Karpukhina, N.; O’Donnell, M.D.; Law, R.V.; Hill, R.G. Fluoride-containing bioactive glasses: effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010, 6, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Bertinetti, L.; Cerrato, G.; Cerruti, M.; Lusvardi, G.; Malavasi, G.; Morterra, C.; Tacconi, L.; Menabue, L. On the dissolution/reaction of small-grain Bioglass® 45S5 and F-modified bioactive glasses in artificial saliva (AS). Appl. Surf. Sci. 2011, 257, 4185–4195. [Google Scholar] [CrossRef]
- Brauer, D.S.; Mneimne, M.; Hill, R.G. Fluoride-containing bioactive glasses: Fluoride loss during melting and ion release in tris buffer solution. J. Non-Cryst. Solids. 2011, 357, 3328–3333. [Google Scholar] [CrossRef]
- Shah, F.A.; Brauer, D.S.; Desai, N.; Hill, R.G.; Hing, K.A. Fluoride-containing bioactive glasses and Bioglass® 45S5 form apatite in low pH cell culture medium. Mater. Lett. 2014, 119, 96–99. [Google Scholar] [CrossRef]
- Rankine, C.A.; Moreno, E.C.; Vogel, G.L.; Margolis, H.C. Micro-analytical determination of pH, calcium, and phosphate in plaque fluid. J. Dent. Res. 1985, 64, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Weir, M.D.; Sun, L. Nanocomposites with Ca and PO4 release: Effects of reinforcement, dicalcium phosphate particle size and silanization. Dent. Mater. 2007, 23, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Muguruma, T.; Brantley, W.A.; Yuasa, T.; Uechi, J.; Mizoguchi, I. Effect of mechanical properties of fillers on the grindability of composite resin adhesives. J. Orthod. Dentofacial. Orthop. 2010, 138, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Combe, E.C.; Speidel, T.M. Shear bond strength of stainless steel orthodontic brackets with a moisture-insensitive primer. Am. J. Orthod. Dentofacial. Orthop. 2001, 119, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Gordan, V.V.; VonWald, L.; Olson, M.E. Effect of an acidic primer on shear bond strength of orthodontic brackets. Am. J. Orthod. Dentofacial. Orthop. 1998, 114, 243–247. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kwon, J.S.; Kim, K.N.; Kim, K.M. Enamel surface with pit and fissure sealant containing 45S5 Bioactive glass. J. Dent. Res. 2016. Online publishing. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Morais, W.A.; Passos, V.F.; Lima, J.P.; Rodrigues, L.K. Fluoride releasing and enamel demineralization around orthodontic brackets by fluoride-releasing composite containing nanoparticles. Clin. Oral. Investig. 2014, 18, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Wu, J.; Weir, M.D.; Xu, H.H. Novel antibacterial orthodontic cement containing quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2014, 42, 1193–1201. [Google Scholar] [CrossRef] [PubMed]

| Compositions | SiO2 | CaCO3 | Na2CO3 | P2O5 | CaF2 |
|---|---|---|---|---|---|
| 45S5 | 45.0 | 24.5 | 24.5 | 6.0 | – |
| 45S5F | 45.0 | 12.5 | 24.25 | 6.0 | 12.25 |
| S53P4 | 53.0 | 20.0 | 23.0 | 4.0 | – |
| Experimental Group | Resin Matrix | Silanized Dental Glass Filler | BAG filler |
|---|---|---|---|
| Transbond XT * | 20 to 30 | 70 to 80 | – |
| BAG_0 | 30.0 | 70.0 | – |
| 45S5_A | 30.0 | 17.50 | 52.50 |
| 45S5_B | 30.0 | 8.75 | 61.25 |
| 45S5F_A | 30.0 | 17.50 | 52.50 |
| 45S5F_B | 30.0 | 8.75 | 61.25 |
| S53P4_A | 30.0 | 17.50 | 52.50 |
| S53P4_B | 30.0 | 8.75 | 61.25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-Y.; Kim, S.-H.; Choi, S.-Y.; Kim, K.-M. Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass. Materials 2016, 9, 125. https://doi.org/10.3390/ma9030125
Yang S-Y, Kim S-H, Choi S-Y, Kim K-M. Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass. Materials. 2016; 9(3):125. https://doi.org/10.3390/ma9030125
Chicago/Turabian StyleYang, Song-Yi, Seong-Hwan Kim, Se-Young Choi, and Kwang-Mahn Kim. 2016. "Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass" Materials 9, no. 3: 125. https://doi.org/10.3390/ma9030125
APA StyleYang, S.-Y., Kim, S.-H., Choi, S.-Y., & Kim, K.-M. (2016). Acid Neutralizing Ability and Shear Bond Strength Using Orthodontic Adhesives Containing Three Different Types of Bioactive Glass. Materials, 9(3), 125. https://doi.org/10.3390/ma9030125






