1. Introduction
Pervious concrete is a special type of concrete with an open structure that allows water to drain through. It is typically used in parking lots and other low volume pavements as a stormwater management tool by reducing runoff. Due to the porous nature of pervious concrete, its strength is generally lower than that of conventional concrete. The design of pervious concrete can be quite difficult as minor changes in the cement content, water-to-cement ratio, or aggregate gradation can have significant impact on strength and/or permeability.
Pervious concrete in cold weather presents additional challenges. Freezing and thawing cycles, in the presence of deicers, can cause severe damage to pervious concrete [
1]. While damage due to freezing and thawing cycles in the presence of salt, or salt scaling, has been studied for many years in conventional concrete [
2], there is a need for a standard test method to evaluate the resistance of pervious concrete to freezing and thawing cycles in the presence of deicing salts.
In conventional concrete, cycles of freezing and thawing in the presence of deicing salts results in the removal of chips from the surface of the concrete. As this phenomenon has been studied for many years, standard test methods, such as the ASTM C672, are used to evaluate the resistance of conventional concrete to this type of damage. In this test, a deicing solution is ponded on the top surface of a concrete slab. The slab then undergoes freezing and thawing cycles. The damage is determined visually and also by determining the mass of material removed from the surface.
While a number of mechanisms have been proposed over the years [
3,
4,
5] suggesting that a physical mechanism is behind the failure due to freezing and thawing in the presence of salts in conventional concrete, it is important to consider the effects of long term exposure to deicing solutions. The effects of different types of decing salts on conventional concrete have been studied by a number of researchers. In these studies, a number of deicers are often compared against each other by exposing the concrete to freeze-thaw cycles, wetting/drying cycles, or submerging samples in a deicing solution at various temperatures.
The damaging effects of calcium chloride (CaCl
2), magnesium chloride (MgCl
2), and CMA has been reported in a number of earlier studies [
6,
7,
8,
9]. It was found that through wetting/drying cycles, CaCl
2, MgCl
2, and CMA can cause significant damage in terms of mass loss and a reduction in stiffness, particularly at higher concentrations [
6]. In the same study, it was found that exposure to NaCl did not cause any significant negative effects on conventional concrete. The damaging effect of both CaCl
2 and MgCl
2 is often attributed to the formation of calcium oxychloride (3CaO·CaCl
2·15H
2O) [
7,
8]. While calcium oxychloride was reported to form quickest between 4 °C and 10 °C, damage still occurred through wetting/drying cycles at higher temperatures [
6]. Long term exposure to CMA at high concentrations was found to cause severe deterioration of the cement matrix but can be at least partially mitigated through the use of slag as partial replacement of Portland cement [
10]. The damage cause by CMA may be due to the formation of calcium acetate hydrate identified using X-ray diffraction analysis [
11].
Acetates are often used as an alternative to conventional deicing salts as the chloride-based deicers can cause corrosive damage to reinforcing steel. The effects of potassium acetate on salt scaling had been studied by Wang et al. [
12]. It was found that potassium acetate had caused only minor scaling at a 13.6% concentration. By contrast, NaCl (at 13.3%) and CaCl
2 (at 9.5%) exhibited more severe scaling under the same testing conditions. However, the use of acetate based deicers can accelerate alkali silica reactions (ASR) if reactive aggregates are used [
13]. Additionally, concretes made with aggregates with a high proportion of microfines suffered deterioration of the paste when exposed to potassium acetate [
14].
In terms of resistance of pervious concrete to the damaging effects of freezing and thawing cycles in the presence of salts, a number of researchers have attempted to carry out tests using various lengths of freezing/thawing cycles, range of temperatures, different deicers, and different exposures [
15,
16,
17,
18]. While numerous mechanisms have been suggested for salt scaling in conventional concrete [
3,
4,
19] as discussed above, there is no extensive published data for mechanisms of salt scaling in pervious concrete.
Mata [
18] used the freeze-thaw cycle described in ASTM C672 with a freezing period of 16–18 h at −18 °C and a thawing period of 6–8 h at 23 °C. Since a deicing solution cannot be ponded on the top surface as described in ASTM C672, the bottom half of the discs were submerged in a 1% by mass CaCl
2 solution. Both mixture types that did not contain any fine aggregate failed after eight cycles, even with high dosages of air entraining admixture. Damage was assessed through mass loss and change in dynamic modulus. The inclusion of fine aggregate and higher dosage of air entraining admixtures in the mix was found to enhance the durability.
Cutler et al. [
15] tested the resistance to freezing and thawing in the presence of deicers of pervious concrete under both saturated and drained conditions. In the saturated conditions, samples were completely submerged in solution during testing. In the drained test method, samples would be submerged initially, but the solution would be drained prior to freezing. In both cases, the temperature varied between 10 °C and −18 °C. The change in temperature from the high end to the low end and vice versa took 2 h each, without holding the temperature at either the maximum or minimum, resulting in a total cycle length of only 4 h. Three different deicers were tested at a concentration of 9%, along with distilled water with no deicers. The deicers tested were calcium chloride (CaCl
2), sodium chloride (NaCl), and calcium magnesium acetate (CMA). All samples used for this study were 100 mm cubes cured for 28 days in a humidity room prior to testing. The level of damage was assessed by measuring the mass loss and the compressive strength at regular intervals. The authors concluded that CaCl
2 caused the most damage, followed by NaCl, and CMA. They had also found that the saturated test caused more damage than the drained test.
Anderson et al. [
16] tested a longer cycle that ranged from 25 °C for 8 h in laboratory air to −20 °C for 16 h in a freezer . Samples were submerged in solution during the last hour of the thawing phase and drained prior to freezing. The authors claimed that this would be the case for a pervious concrete pavement under service conditions. NaCl was the only deicer that was studied at various concentrations (2%, 4%, 8%, and 12% by mass) as well as a set of control samples exposed to freezing and thawing in water without NaCl. All samples used were cylinders with a diameter of 100 mm by 200 mm height. The mass loss of each sample was measured at the end of every 10 cycles until the samples failed at 15% mass loss or when the sample reached 100 freeze-thaw cycles. The results indicated that a salt concentration of 4% and 8% caused the most salt scaling damage, with the damage resulting mainly from the scaling of the cement paste, with little damage to the aggregate. In conventional concrete, a “pessimum” concentration of 3% by mass, has been reported in literature [
20]. It was also shown that the damage originated at the bottom of the sample where the level of saturation would be highest due to the draining of the solution. The solution would move towards the bottom of the sample as it drains and would likely freeze before leaving the sample completely. At lower and higher concentrations, 2% and 12% NaCl, very little damage was observed. It is likely that at high concentrations, the solution may take longer to freeze completely resulting in a reduced amount of time in a frozen state [
16].
In this paper different test conditions were investigated to reach an optimized test that produces quantifiable and repeatable damage in pervious concrete samples due to freezing and thawing cycles in the presence of salts. The test involves exposing the pervious concrete samples to cycles of freezing and thawing in the presence of deicers. To optimize the test, different test parameters were tested for their effect on the rate and level of deterioration of the tested samples. The tested parameters included (1) sample shape and size; (2) conditions of the samples in terms of brine saturation; and (3) length of the freezing/thawing cycles. Finally, the optimized test was evaluated for its capacity to differentiate between different deicers.
4. Discussion
4.1. Sample Geometry
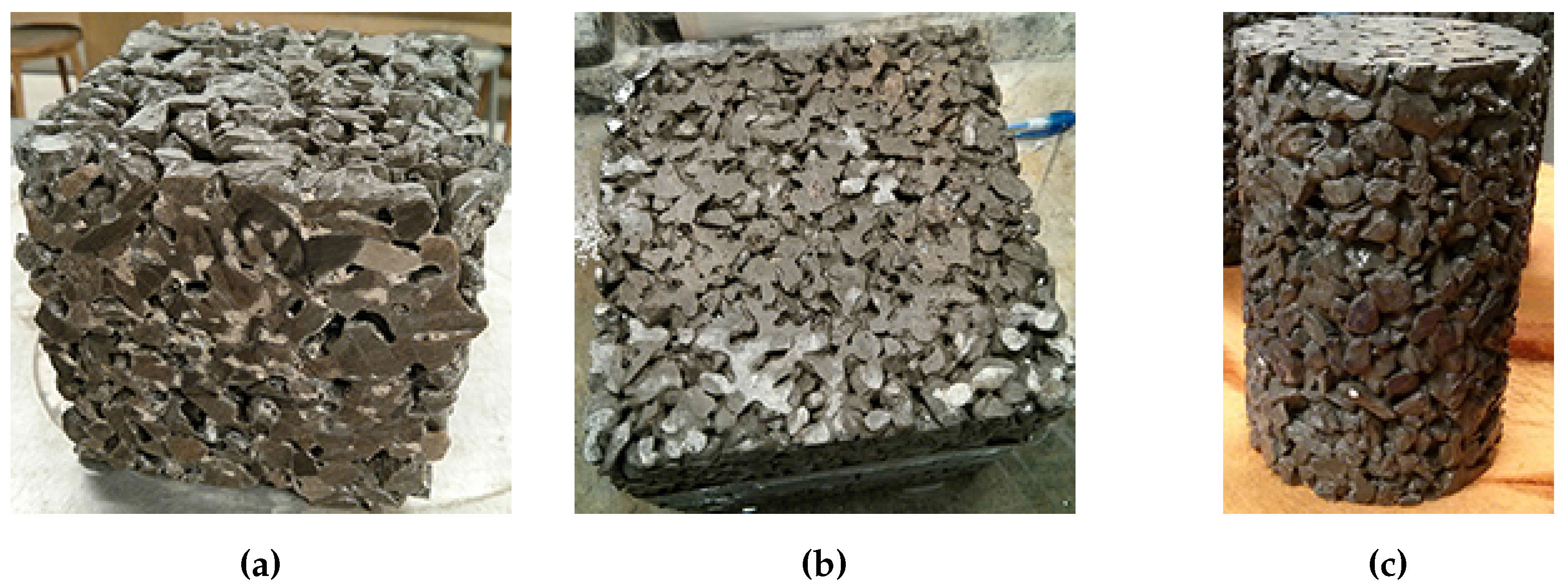
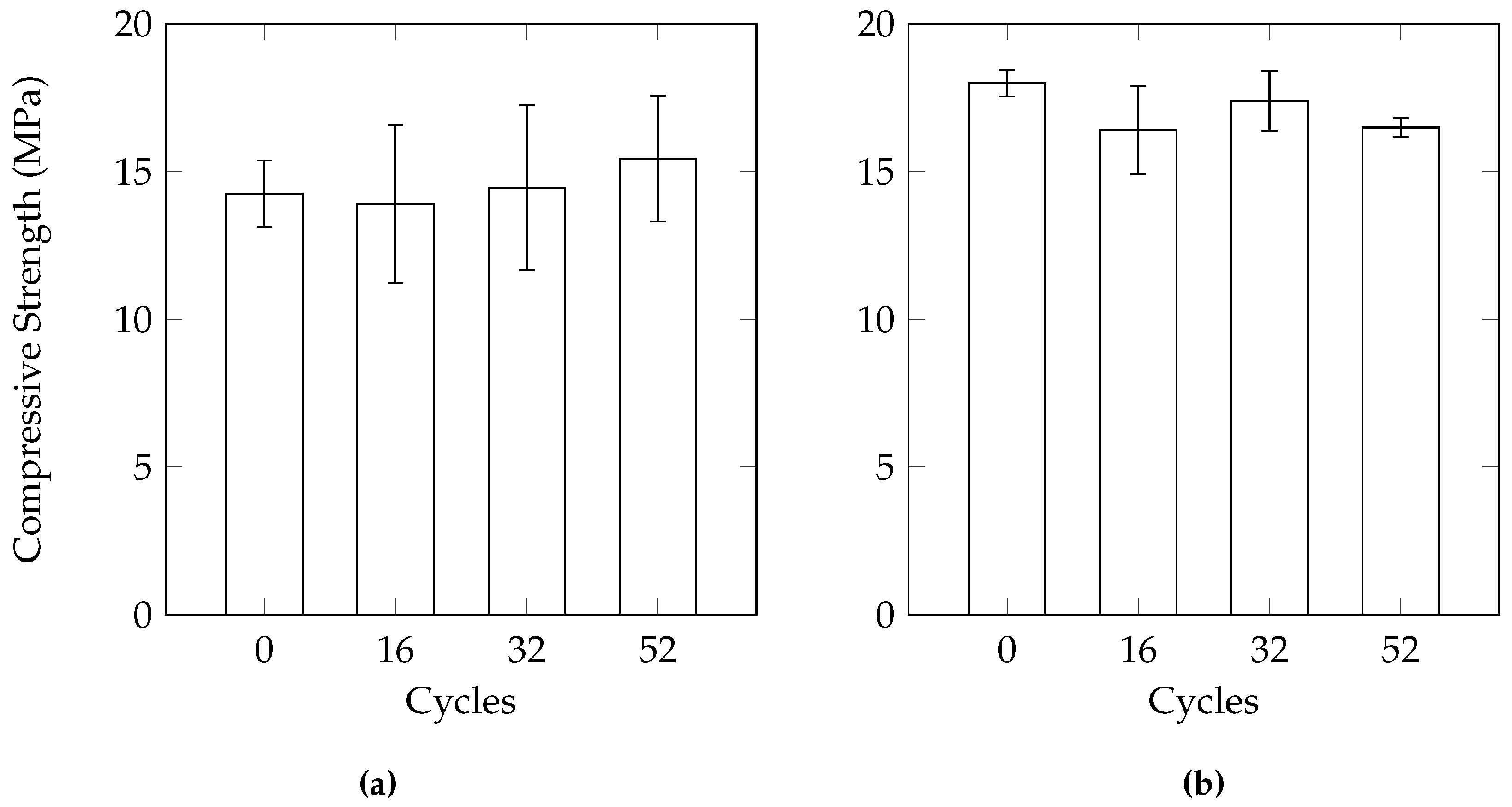
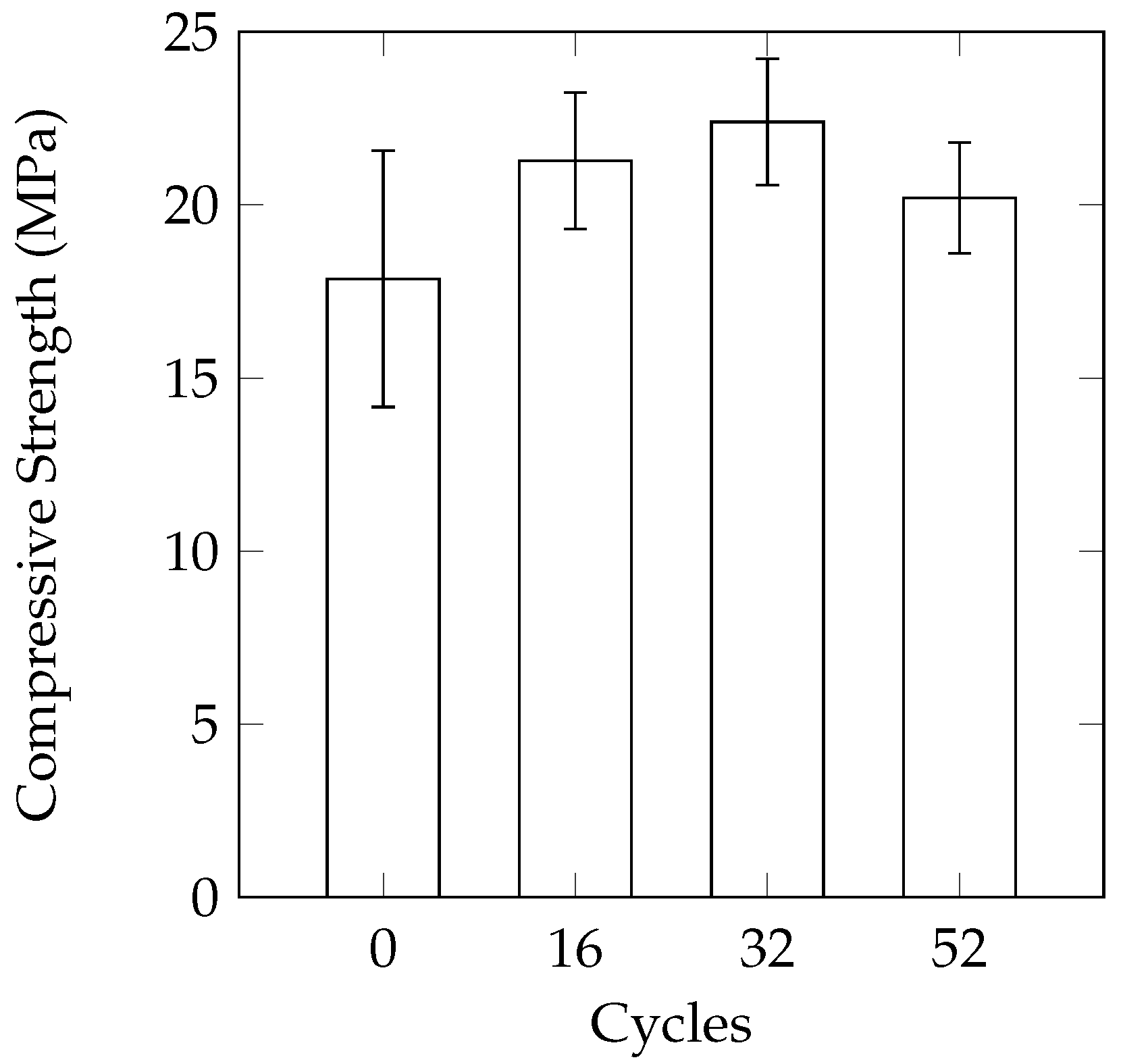
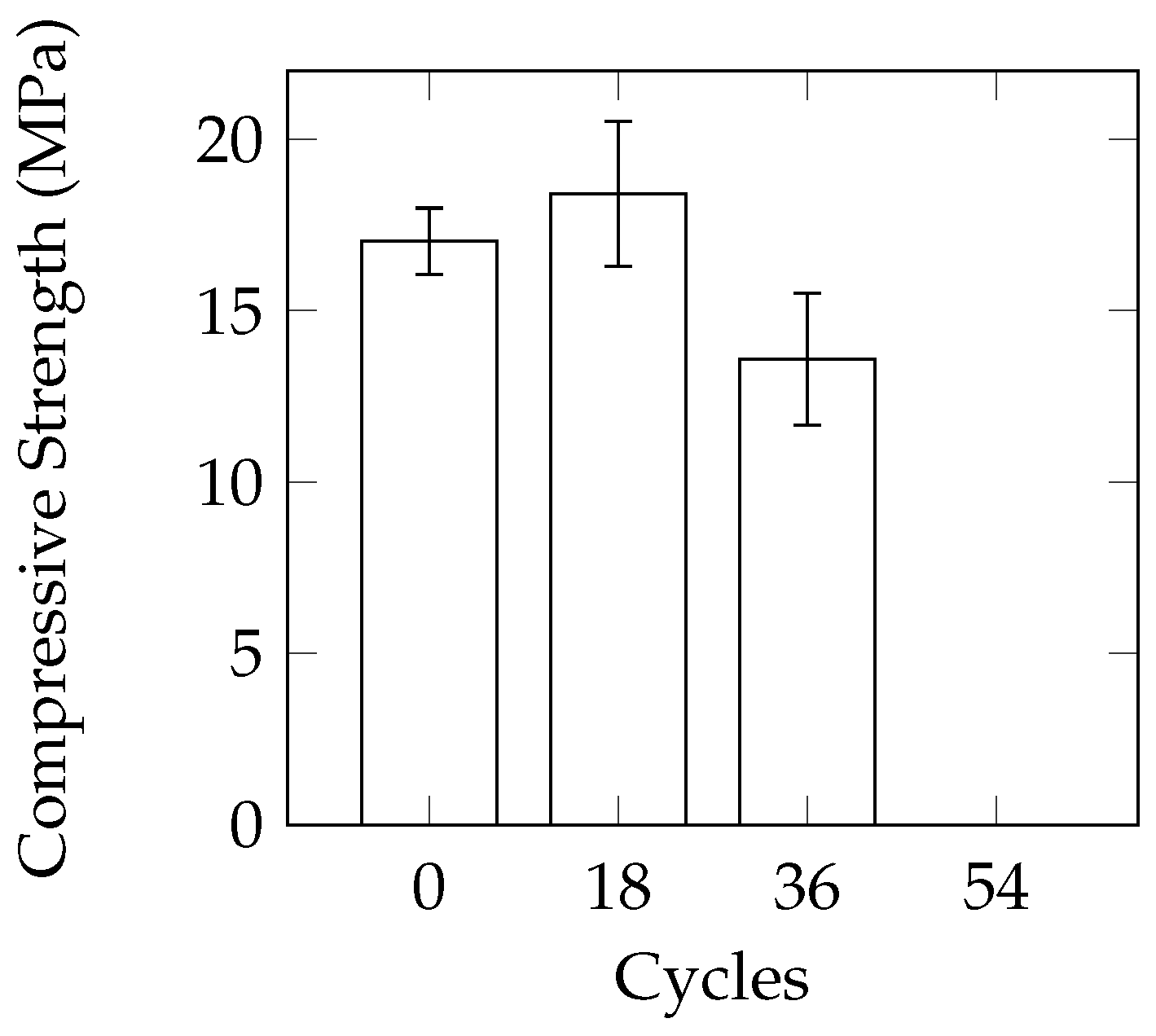
A comparison of the three different sample geometries (100 mm cube, 150 mm cube, 100 mm diameter by 150 mm height cylinders) does not reveal a large difference between variability of samples in terms of mass loss. The consistency of the compressive strength results of the cylinders was better than the 100 mm cubes. In the study by Cutler et al. [
15], the use of 100 mm cubes also resulted in fairly inconsistent compressive strengths. The use of 150 mm cube samples in the current work did not provide a significant improvement over the cylinders in terms of consistency. As freezer space can be a limiting factor in conducting laboratory experiments, the smaller size of the cylinders will allow more samples to be tested at the same time. The reduced size also allows for easier casting and handling of the samples.
4.2. Depth of Submersion
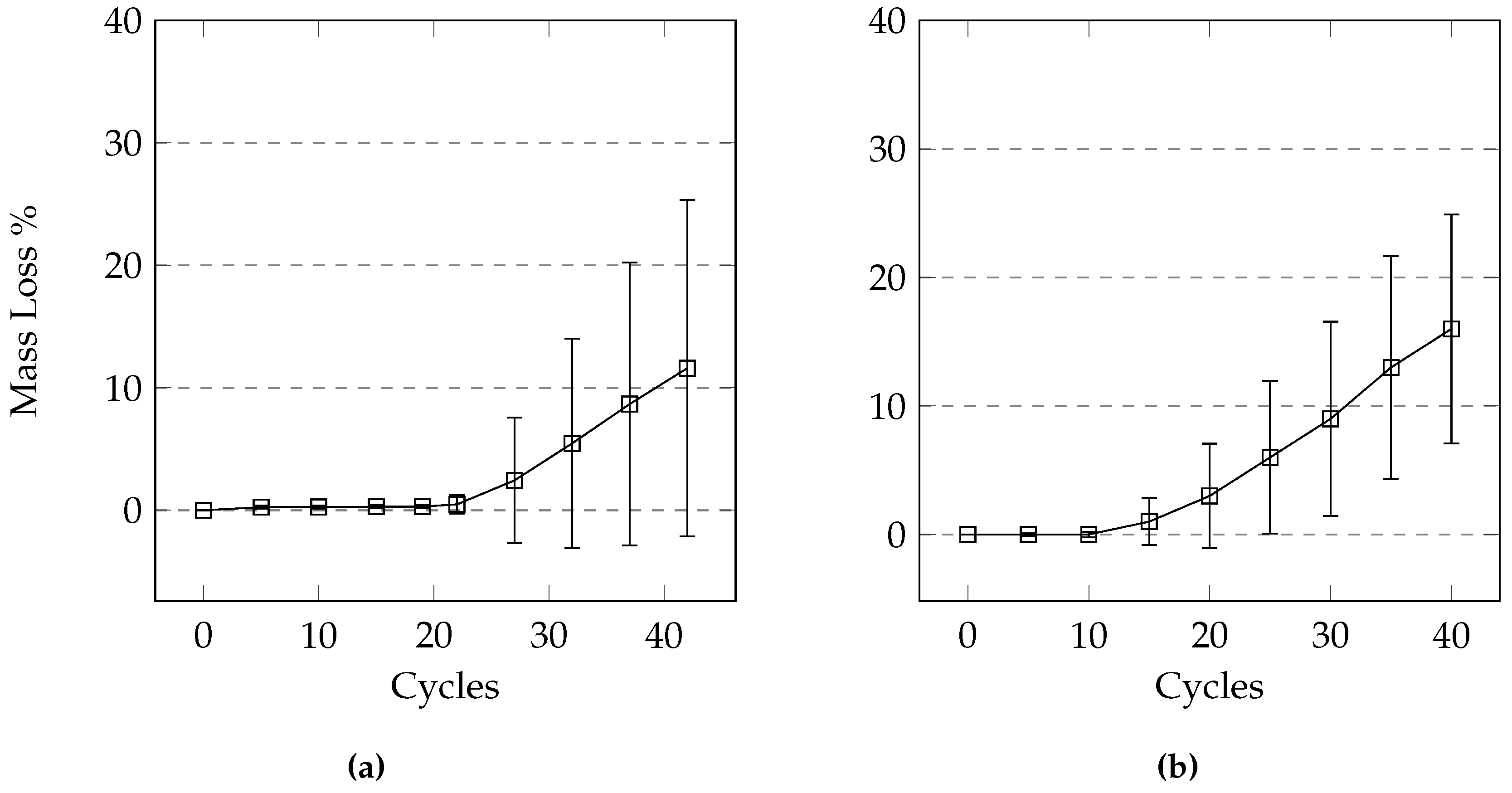
For samples that were partially submerged, the damage appeared to be concentrated near the top of the solution; the scaled off cement flakes were lost mainly at this height in the sample. A large part of this mass loss would not necessarily be due to salt scaling damage as much of the cement had not been scaled off. This sudden failure, where large pieces of concrete break off, also occurred in samples in the drained condition. While the damage in the drained condition appeared to be slow and minimal at first, the damage was concentrated at a certain height of the sample, leading to the sudden significant increase in mass loss. It should be noted that the samples were drained of excess liquids, but were not completely dried when frozen. There is a possibility that the failure occurs at the level where the paste was in a saturated surface dry condition. A submersion level of 10 mm seems appropriate for pervious concrete samples made with 14 mm limestone coarse aggregate since the tests show that the damage occurs mainly at the height of the solution. It is possible that pervious concrete made with larger aggregates will not suffer as much damage in this test as the level of solution might be in the mid-height of the stone and would not cause much scaling. Using 10 mm of solution is recommended as the damage occurs gradually rather than in significant jumps.
In conventional concrete the pond of solution on the concrete surface is necessary for salt scaling to occur [
2]. Damage still occurred in the drained test in this study, as well as the study by Anderson and Dewoolkar [
1] where mass loss exceeded 15% in some cases. It is possible that the solution adhering to the cement paste in pervious concrete is sufficient to act as a pond of solution given the thin layer of paste surrounding the aggregate. It is likely that the same mechanism responsible for salt scaling in conventional concrete is also responsible for salt scaling in pervious concrete. It should be noted that the mechanism of damage is not the main objective of this study.
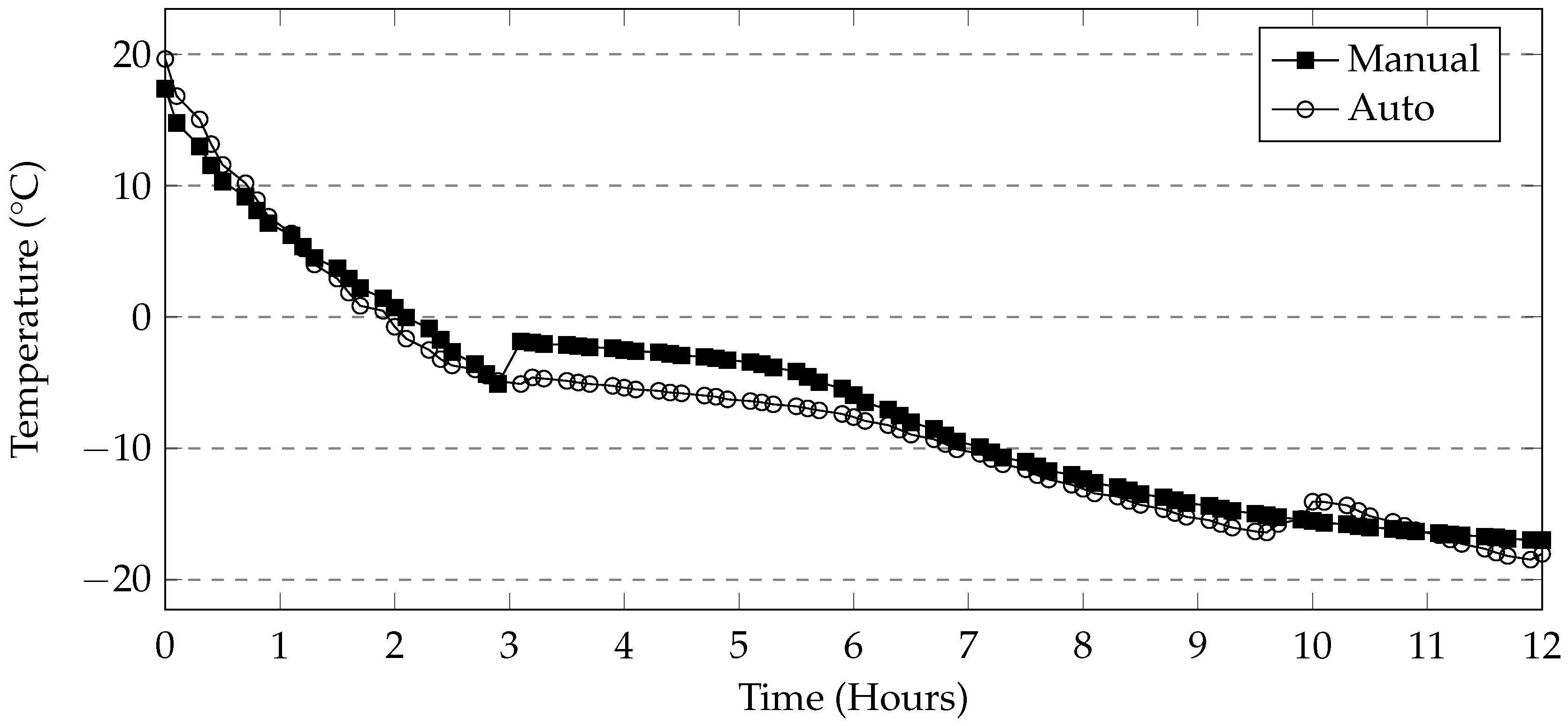
4.3. Automatic and Manual Cycles
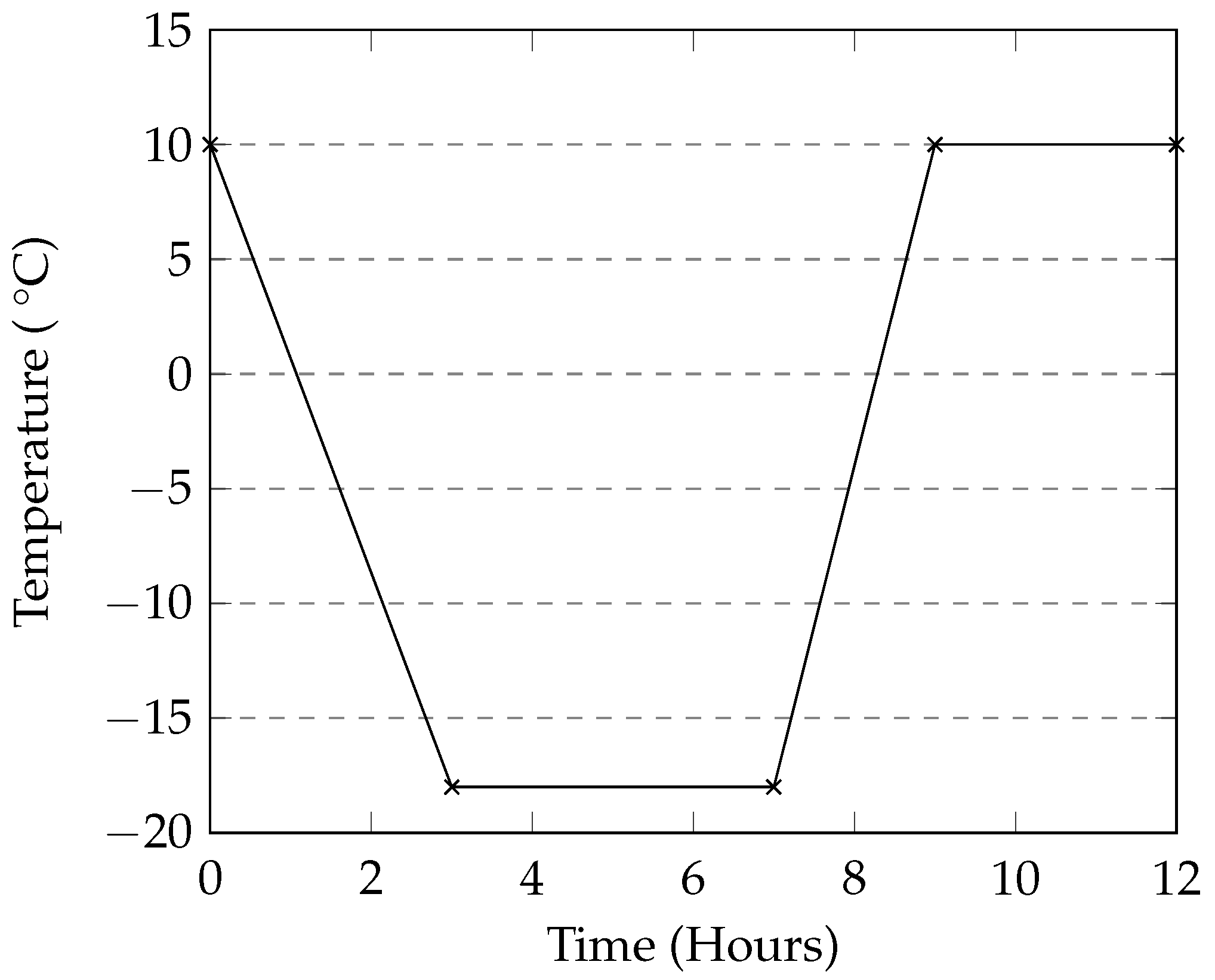
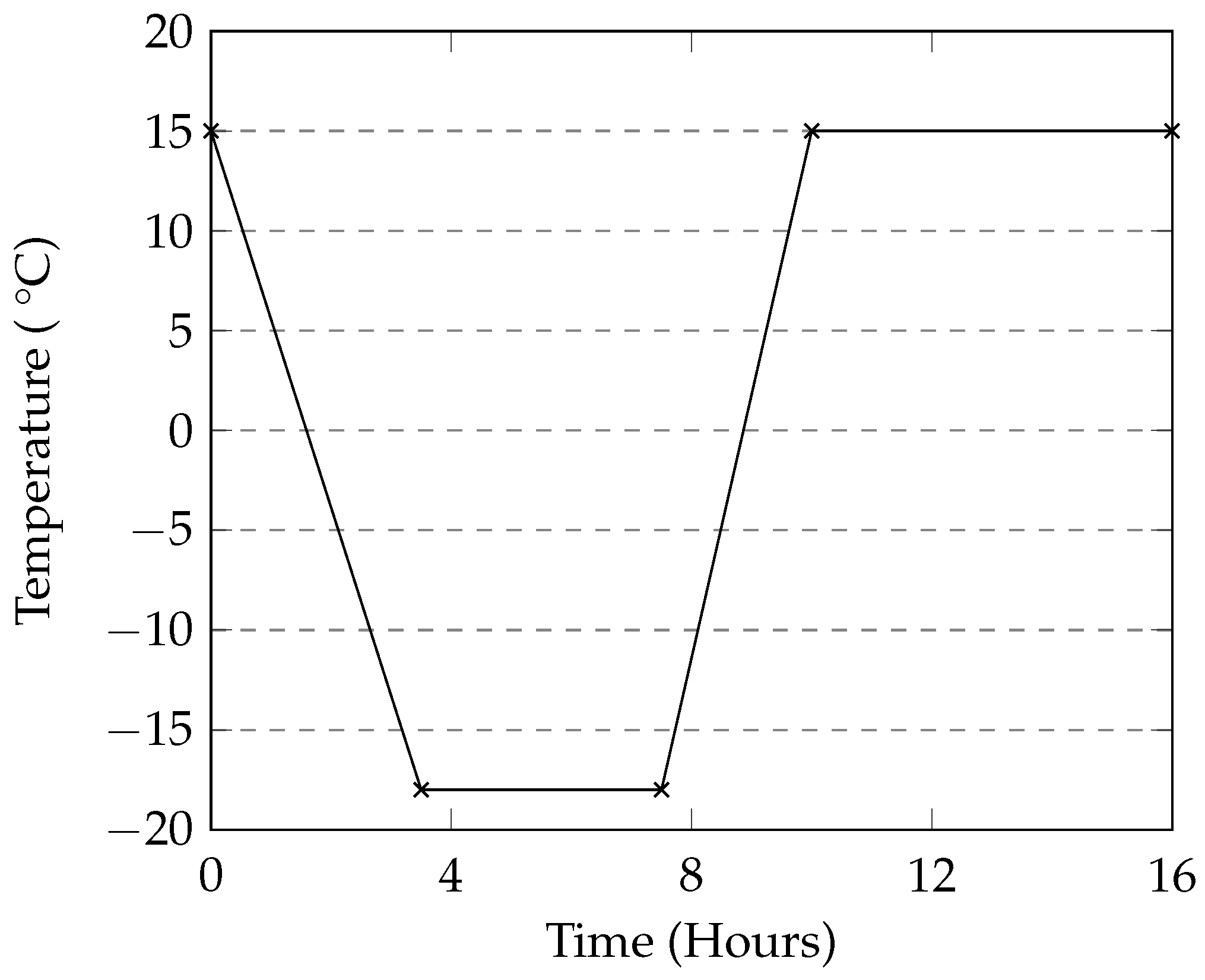
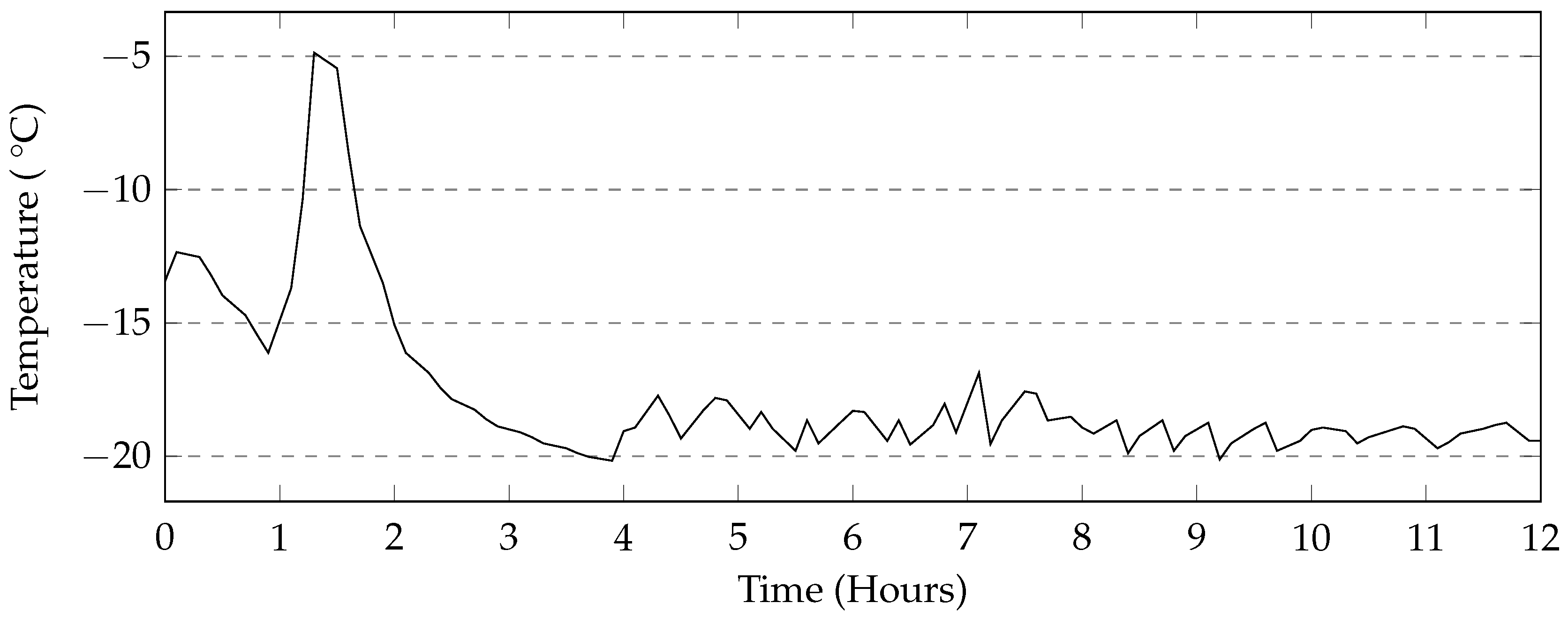
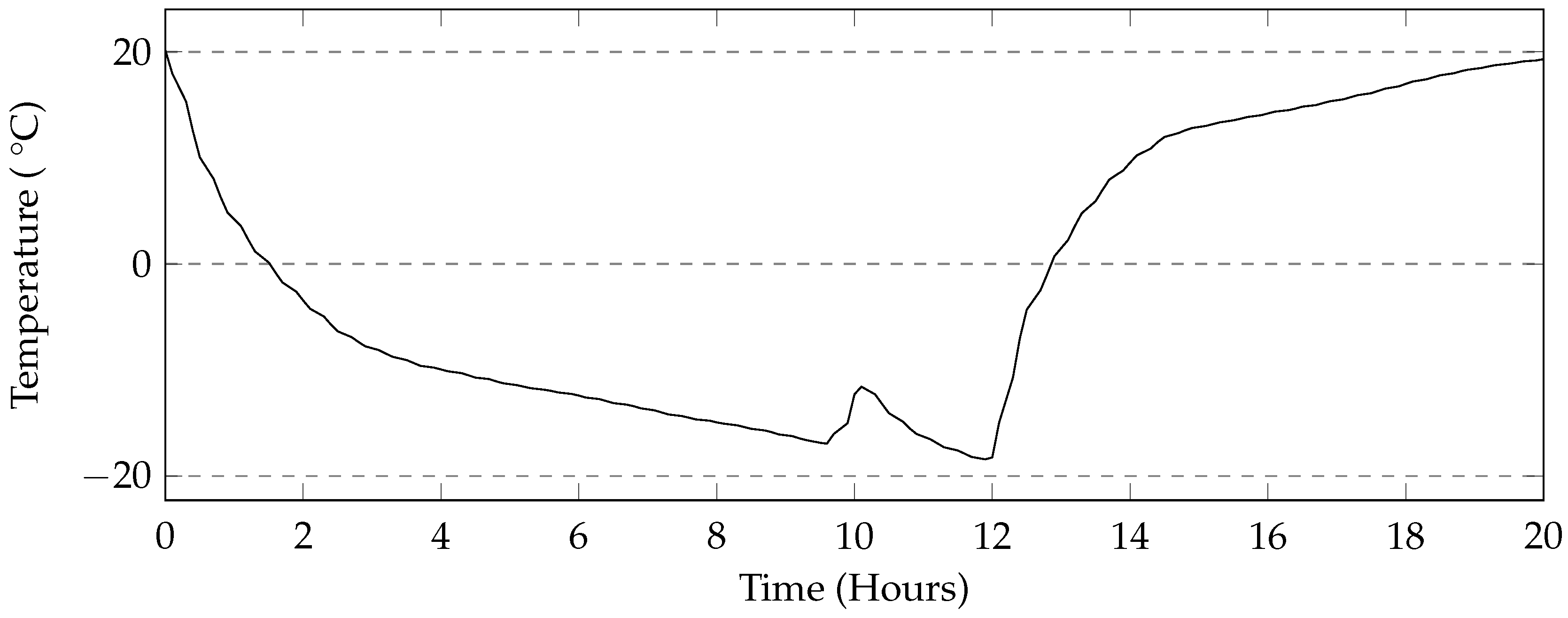
The programmable environmental chamber used in this study provided a rate of cooling of the deicing solution that matched that of the industrial freezer used in this study (
Figure 19). This makes it likely that the rapid mass loss seen in the manual methods is attributed to moving the samples to and from the freezer. It is likely that the movement of the samples will cause the specimens to fall over. The subsequent repositioning of the specimen in the solution will allow more damage to occur. Additionally, the samples in the manual method are washed once every 5 cycles, whereas in the automatic cycle, they are washed once every 7 cycles. This is because the use of the automatic or programmable environmental chamber enables testing of the samples over the weekend. Washing would cause much of the material still adhering to the sample to dislodge resulting in exposing new parts of the specimen to the salt solution. The sets of samples tested using MgCl
2 and CMA in this method also show low levels of damage when compared to the manual method. The general trends in mass loss caused by the different deicers appears to be the same in both cases. A programmable environmental chamber would likely result in better consistency and repeatability.
4.4. Cycle Length
Extending the freezing period appears to cause more damage. In the case where samples were partially saturated, the extension of the freezing period allowed the solution to reach a lower temperature. The 12-h freezing period of the 24-h cycles was sufficient to bring the temperature of the solution down to the minimum temperature of −18 °C. The long 12-h thawing period was required to allow both thawing and washing of the samples. As the entire washing and weighing procedure can take up to four hours, the solution requires enough time to ensure complete thawing by the time the washing procedure begins. Based on this, the 24-h cycle is recommended for testing previous concrete.
4.5. Different Deicers
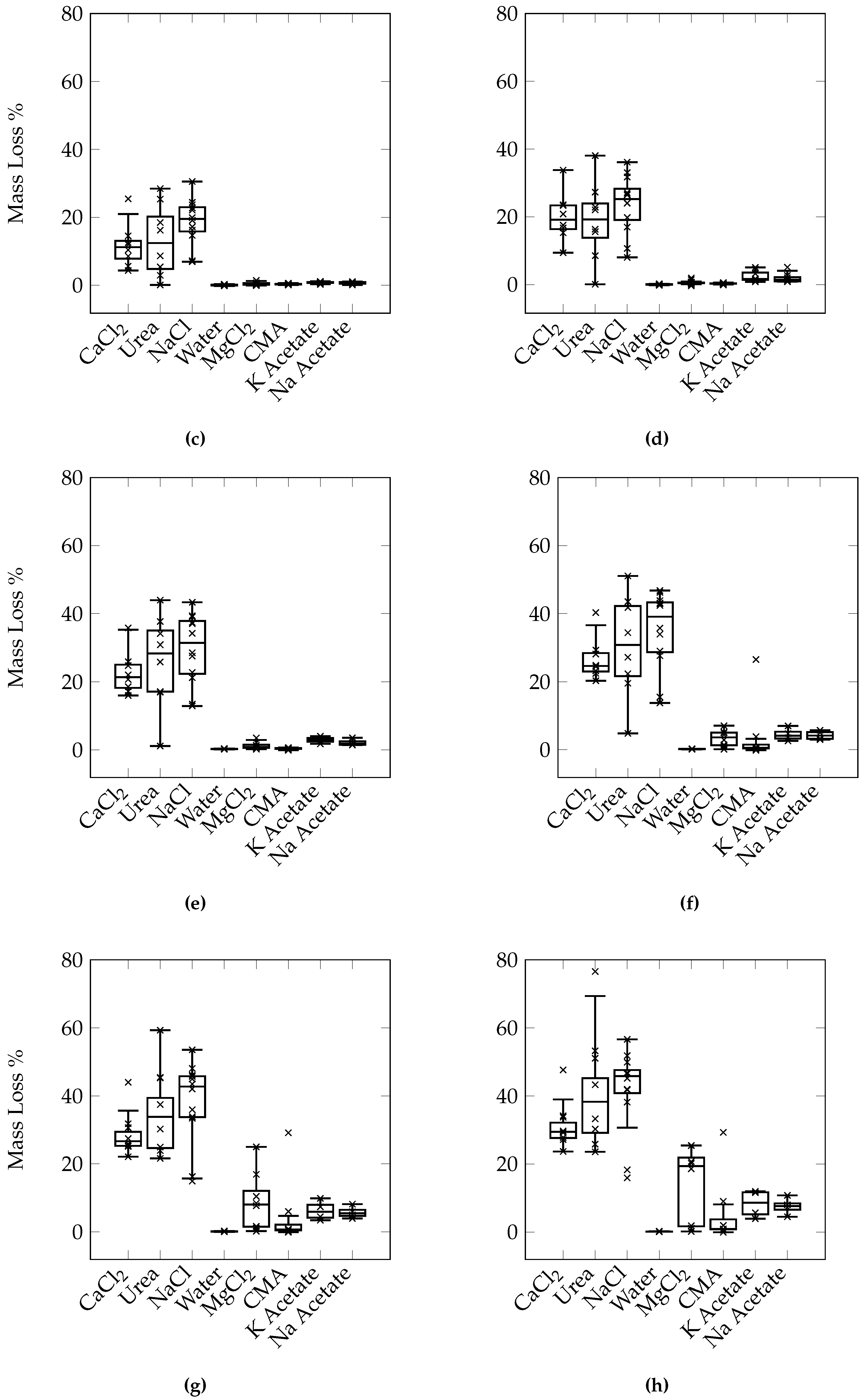
While a conclusive order of the relative levels of damage of each deicer cannot be concluded from the results of this study, a general trend can be formed. CaCl2, NaCl, and urea appear to be the most damaging, MgCl2, K acetate, Na acetate, and CMA are less damaging, but all deicers cause more damage than freezing/thawing in water alone. The difference between the various deciers can be due to a number of reasons. It should first be noted that although all the salts are at the same concentration by mass percent, the molality of each is different. As molality is calculated by dividing the number of moles of the solute by the mass of the solvent, an equal mass of solute would result in a higher molality for a solute with a lower molecular weight since there would be more moles of that solute at an equal mass. Additionally, the resulting freezing point depression is also different for the different deicers. The molality of both the NaCl and MgCl2 solutions is higher than CaCl2, while the freezing point of both is lower. However, the results indicate that while NaCl potentially causes more damage than CaCl2, MgCl2 does not. If either of these factors alone correlate with the damage, NaCl and MgCl2 should both cause either more damage or less damage than CaCl2.
From the reported literature, CaCl
2, MgCl
2, and CMA often cause more severe damage than NaCl through chemical deterioration after long term exposure [
6]. For a number of deicers in this test, the damage was apparent as early as ten cycles.
While the results of the study by Cutler et al. [
15], showed that CaCl
2 caused more damage than NaCl, the concentration of each deicer was at 9%. Therefore it cannot be said that the results of the present study contradicts the results presented by Cutler et al. [
15]. In conventional concrete, at the pessimum concentration, it has been shown that NaCl causes more damage than CaCl
2 [
2]. While at higher concentrations, Wang et al. [
12] found that CaCl
2 causes more salt scaling damage than NaCl, similar to Cutler et al. [
15].
It should be reemphasized that the use of different salts in this research was mainly to evaluate the possibility of the optimized test method with different salts. The long term effects of different salts on pervious concrete under different temperatures was not investigated here.
4.6. Failure Criteria
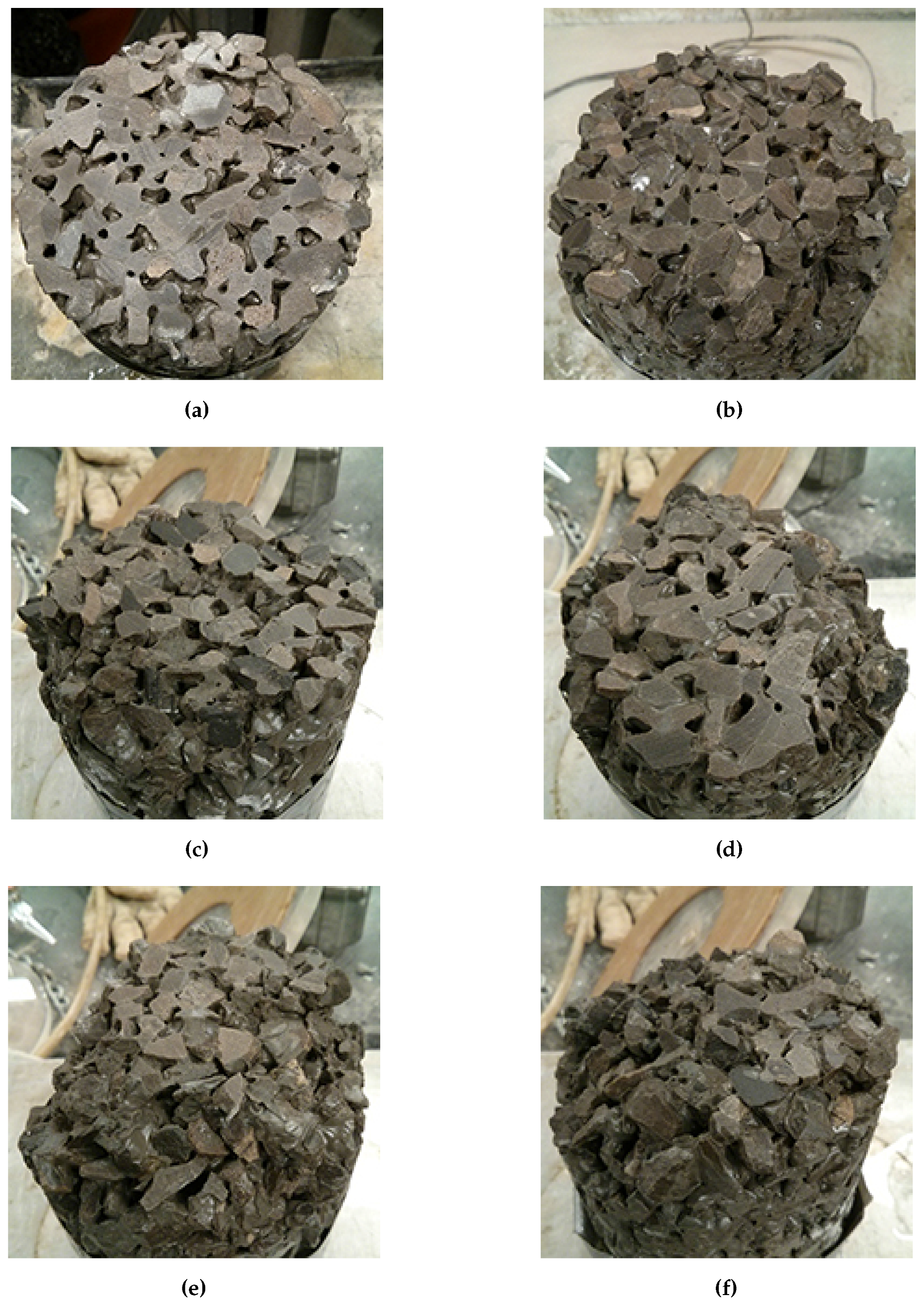
Based on a visual inspection of the remaining flat surface area of each sample compared to its mass loss, a cutoff point can be determined. For a cylinder sample with a diameter of 100 mm and a height of 150 mm, a mass loss in the range of 3%–5% is suggested based the remaining area of the initial cut surface (
Figure 20). Most samples are still able to stand upright in this range, however, it is apparent that severe damage has occurred. The ability of the samples to stand up can be taken as an indication of adequate contact or load transfer between the pervious concrete and the underlying granular material, assuming that the damage occurs at the bottom of the concrete layer. If scaling occurs at the top of the pervious concrete, 3%–5% mass loss represents a pervious concrete layer that is still reasonably intact.
Using this criteria, CaCl2, urea, and NaCl exceeded 3%–5% mass loss within 30 cycles. The exact number of cycles is difficult to determine as the mass was taken only once every 5 cycles. Additionally, this occurs at different times for different specimens of the same set. It is suggested that the average or the median mass loss within each set of samples be used to determine the number of cycles to failure. Due to the variability present, the use of a median mass loss may be more appropriate. Using this, samples exposed to solutions of CaCl2, urea, and NaCl failed after 20, 25, and 15 cycles respectively. Samples exposed to solutions of MgCl2, K acetate, and Na acetate would all be considered to have failed at either 40 or 45 cycles, depending on whether the upper or lower limit of the criterion is selected. Samples exposed only to water did not show any signs of damage, confirming that the damage is mainly due to the use of salts. Although the use of the median value indicates that CMA will not cause sample failure even after 50 cycles, a number of individual samples did suffer particularly high levels of mass loss. It is possible that continued long term use of CMA will eventually lead to severe deterioration. On the other hand, the ability of the pervious concrete to drain water coupled with the exposure of pavements to rain without salts in the summer may help to flush the system and reduce the accumulation of deicers within the concrete and its associated chemical attack. Field trials should be conducted to better correlate the test results to in field performance.