In Vitro Studies of Polyhedral Oligo Silsesquioxanes: Evidence for Their Low Cytotoxicity
Abstract
:1. Introduction
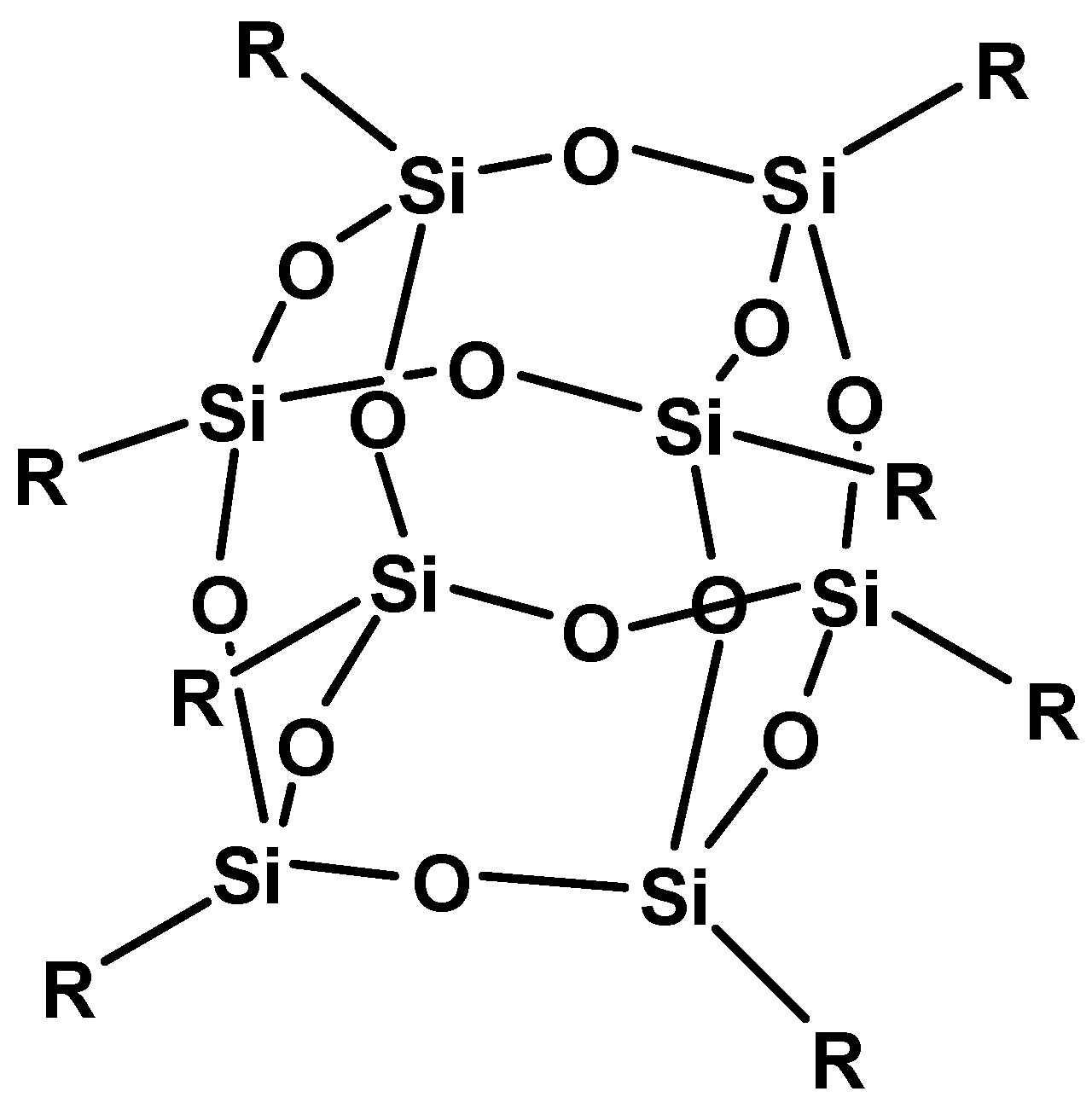
2. Results and Discussion
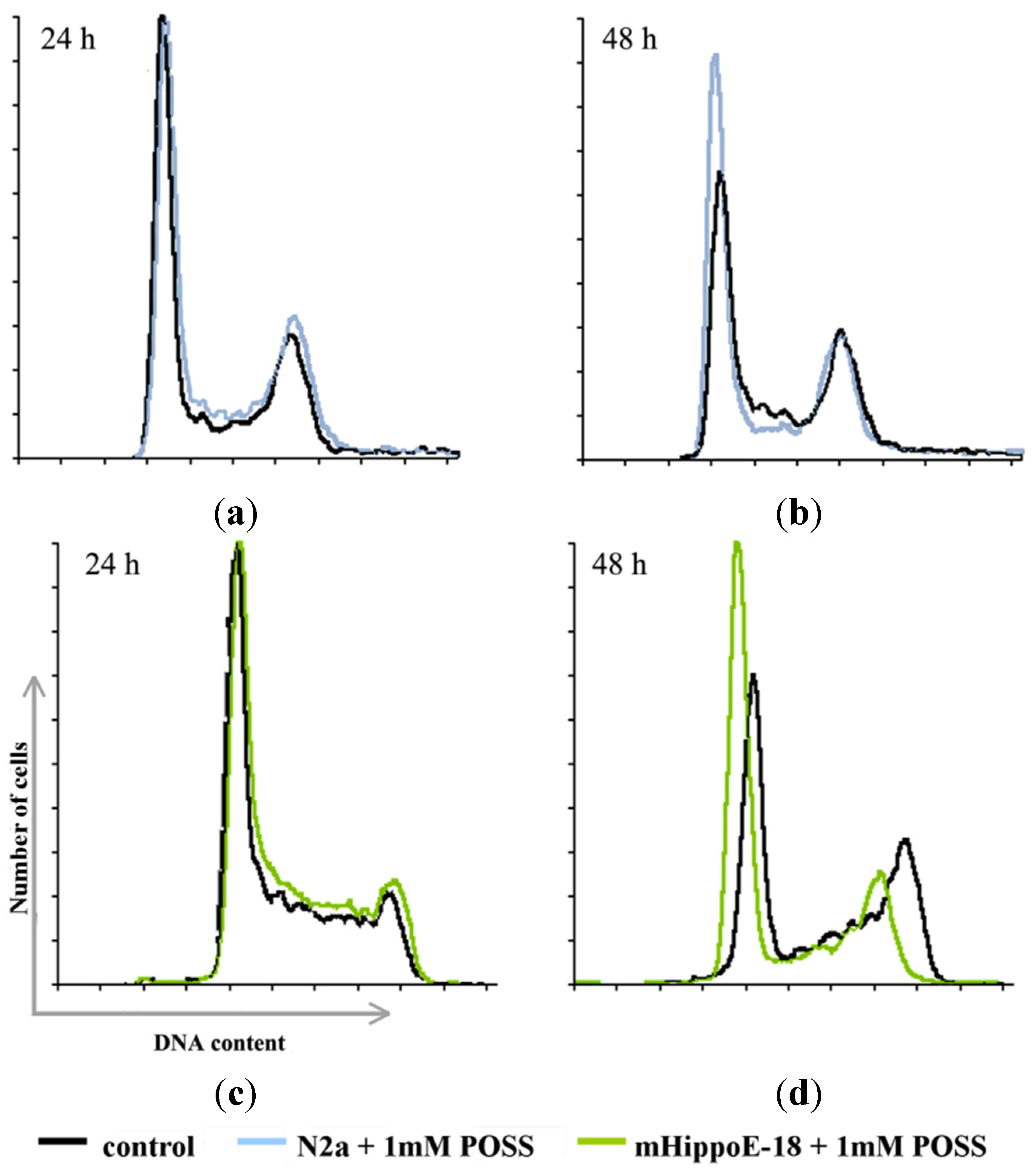
| N2a | G0/G1 | S | M/G2 | |
|---|---|---|---|---|
| 24 h | Control | 52.70 ± 0.52 | 13.23 ± 0.93 | 29.77 ± 0.80 |
| 1 mM POSS | 54.57 * ± 0.49 | 11.40 * ± 0.50 | 29.53 ± 0.61 | |
| 48 h | Control | 53.67 ± 0.65 | 13.57 ± 0.70 | 30.40 ± 1.36 |
| 1 mM POSS | 62.63 ** ± 0.91 | 11.57 ± 0.90 | 22.83 ** ± 0.17 | |
| mHippoE-18 | G0/G1 | S | M/G2 | |
| 24 h | Control | 54.17 ± 0.97 | 12.57 ± 0.68 | 29.13 ± 0.40 |
| 1 mM POSS | 52.83 ± 0.49 | 11.83 ± 0.95 | 30.77 ± 0.40 | |
| 48 h | Control | 48.50 ± 0.87 | 13.33 ± 0.15 | 28.93 ± 1.10 |
| 1 mM POSS | 57.17 ** ± 0.29 | 9.67 ** ± 0.55 | 26.23 ** ± 0.60 | |

3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Cell Cycle Analysis
3.4. In Vitro Cytotoxicity
3.5. Detection of Apoptotic and Necrotic Cells
3.6. Detection of Intracellular ROS Formation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hu, J.; Xiao, J.; Cheng, Y. Dendrimer and cancer: A patent review (2006–present). Expert Opin. Ther. Pat. 2013, 23, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Z.; Zhu, J.Y.; Wang, X.L.; Cheng, H.; Chen, G.; Zhao, Y.F.; Zeng, X.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. A boronate-linked linear-hyperbranched polymeric nanovehicle for pH-dependent tumor-targeted drug delivery. Biomaterials 2014, 35, 5240–5249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pang, Y.; Zhu, Z.; Wang, D.; Li, C.; Huang, W.; Zhu, X.; Yan, D. Therapeutic nanocarriers with hydrogen peroxide-triggered drug release for cancer treatment. Biomacromolecules 2013, 14, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.; Carroll, J.B.; Rotello, V.M. Cationic polyhedral oligomeric silsesquioxane (POSS) units as carriers for drug delivery processes. Chem. Commun. 2005, 28, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Inafuku, K.; Naka, K.; Chujo, Y. Enhancement of entrapping ability of dendrimers by a cubic silsesquioxane core. Org. Biomol. Chem. 2008, 6, 3899–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Luo, K.; Lai, Y.; Pu, Y.; He, B.; Wang, G.; Wu, Y.; Gu, Z. A novel poly(l-glutamic acid) dendrimer based drug delivery system with both pH-sensitive and targeting functions. Mol. Pharm. 2010, 7, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Dickens, S.H.; Flaim, G.M. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent. Mater. 2005, 21, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.S.; Rafiei, Y.; Tzifa, A.; Seifalian, A.M. In situ endothelialization of intravascular stents from progenitor stem cells coated with nanocomposite and functionalized biomolecules. Biotechnol. Appl. Biochem. 2011, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kitamura, N.; Takahashi, Y.; Chujo, Y. Reversible signal regulation system of 19F NMR by redox reactions using a metal complex as a switching module. Bioorg. Med. Chem. 2009, 17, 3818–3823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, J.H.; Jan, M.S.; Lin, Y.L. Interactions between U-937 human macrophages and poly(propyleneimine) dendrimers. J. Control. Release 2007, 120, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Van Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 6, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Byrne, H.J. Generation of intracellular reactive oxygen species and genotoxicity effect to exposure of nanosized polyamidoamine (PAMAM) dendrimers in PLHC-1 cells in vitro. Aquat. Toxicol. 2013, 15, 132–133. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janaszewska, A.; Gradzinska, K.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Stanczyk, W.A. In Vitro Studies of Polyhedral Oligo Silsesquioxanes: Evidence for Their Low Cytotoxicity. Materials 2015, 8, 6062-6070. https://doi.org/10.3390/ma8095291
Janaszewska A, Gradzinska K, Marcinkowska M, Klajnert-Maculewicz B, Stanczyk WA. In Vitro Studies of Polyhedral Oligo Silsesquioxanes: Evidence for Their Low Cytotoxicity. Materials. 2015; 8(9):6062-6070. https://doi.org/10.3390/ma8095291
Chicago/Turabian StyleJanaszewska, Anna, Kinga Gradzinska, Monika Marcinkowska, Barbara Klajnert-Maculewicz, and Wlodzimierz A. Stanczyk. 2015. "In Vitro Studies of Polyhedral Oligo Silsesquioxanes: Evidence for Their Low Cytotoxicity" Materials 8, no. 9: 6062-6070. https://doi.org/10.3390/ma8095291







