Synthesis of Ternary Borocarbonitrides by High Temperature Pyrolysis of Ethane 1,2-Diamineborane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of EDAB Precursor
2.2. High Temperature Thermolysis of EDAB Investigated by Combined Thermoanalytical Methods

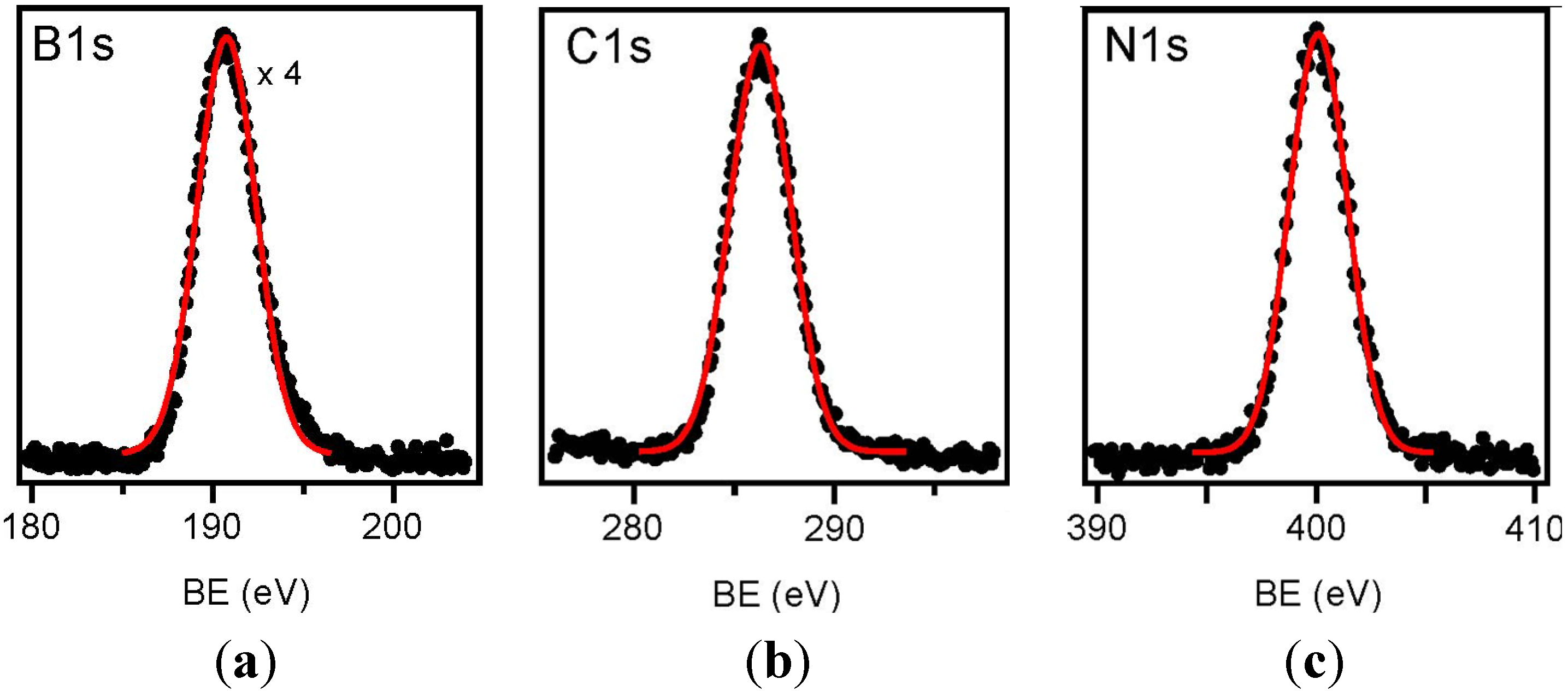
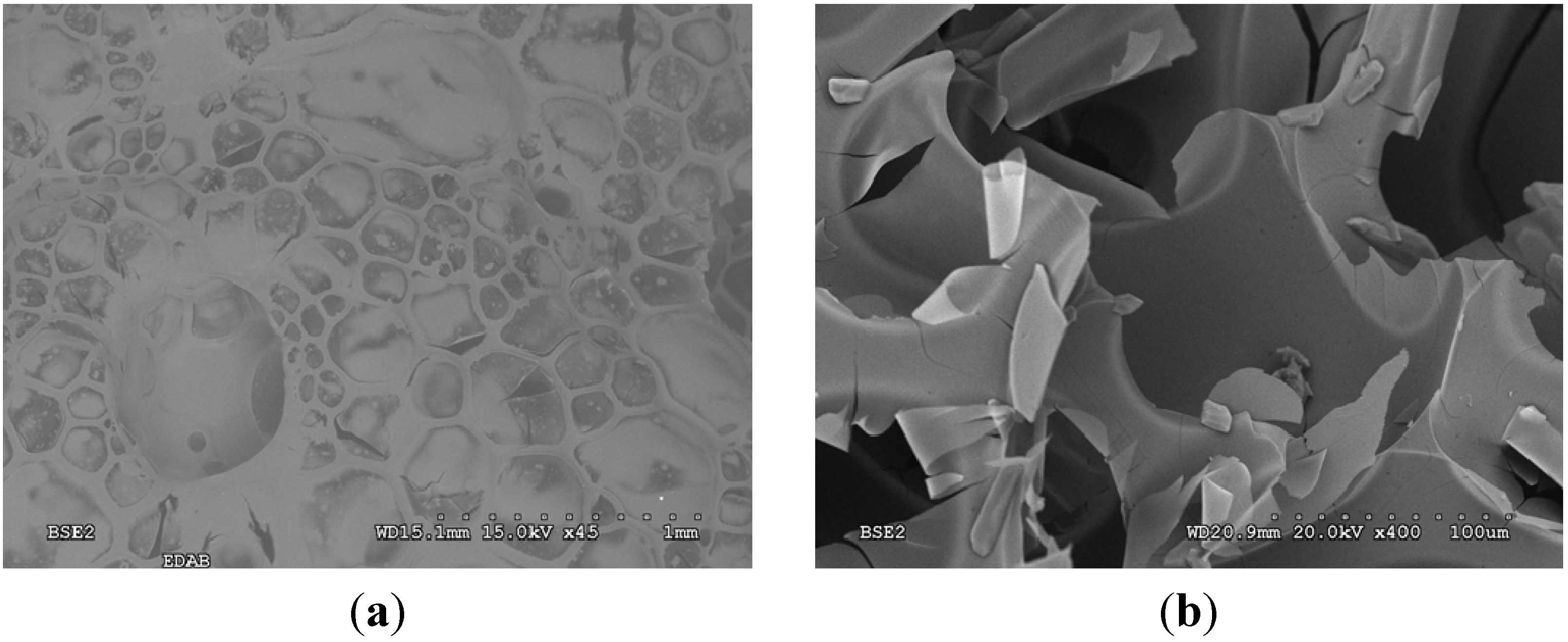
2.3. Characterization of the Thermolysis Product



3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Autrey, T. Boron-nitrogen-hydrogen (BNH) compounds: Recent developments in hydrogen storage, applications in hydrogenation and catalysis, and new syntheses. Energy Environ. Sci. 2012, 5, 9257–9268. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R. Boron–nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Baitalow, F.; Baumann, J.; Wolf, G.; Jaenicke-Rößler, K.; Leitner, G. Thermal decomposition of B–N–H compounds investigated by using combined thermoanalytical methods. Thermochim. Acta 2002, 391, 159–168. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King’ondu, C.; Suib, S.L. Pyrolytic decomposition of ammonia borane to boron nitride. Inorg. Chem. 2011, 50, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Miele, P. Nanostructured and architectured boron nitride from boron, nitrogen and hydrogen-containing molecular and polymeric precursors. Mater. Today 2014, 17, 443–450. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z. Graphene-analogous low-dimensional materials. Prog. Mater. Sci. 2013, 58, 1244–1315. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Ramakrishna Matte, H.S.S.; Maitra, U. Graphene analogues of inorganic layered materials. Angew. Chem. Int. Ed. 2013, 52, 13162–13185. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, L.; Zhao, S.; Huang, J.; Ma, L.; Zhang, J.; Lou, J.; Ajayan, P.M. Direct growth of graphene/hexagonal boron nitride stacked layers. Nano Lett. 2011, 11, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Hofmann, M.; Nezich, D.; Rodriguez-Nieva, J.F.; Dresselhaus, M.; Palacios, T.; et al. Synthesis of monolayer hexagonal boron nitride on Cu foil using chemical vapor deposition. Nano Lett. 2012, 12, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.C.; Edwards, J.O. Ethane 1,2-diamineborane. J. Am. Chem. Soc. 1960, 82, 4842–4846. [Google Scholar] [CrossRef]
- Goubeau, J.; Schneider, H. Borin-anlagerungsverbindungen des athylendiamins. Chem. Ber. 1961, 94, 816–821. [Google Scholar] [CrossRef]
- Kelly, H.C.; Edwards, J.O. Evidence for the open chain structure of ethane 1,2-diamineborane. Inorg. Chem. 1963, 2, 226–227. [Google Scholar] [CrossRef]
- Neiner, D.; Karkamkar, A.; Bowden, M.; Choi, Y.J.; Luedtke, A.; Holladay, J.; Fisher, A.; Szymczak, N.; Autrey, T. Kinetic and thermodynamic investigation of hydrogen release from ethane 1,2-di-amineborane. Energy Environ. Sci. 2011, 4, 4187–4193. [Google Scholar] [CrossRef]
- Leardini, F.; Valero-Pedraza, M.J.; Perez-Mayoral, E.; Cantelli, R.; Bañares, M.A. Thermolytic decomposition of ethane 1,2-diamineborane investigated by thermoanalytical methods and in situ vibrational spectroscopy. J. Chem. Phys. C 2014, 118, 17221–17230. [Google Scholar] [CrossRef]
- Kumar, N.; Moses, K.; Pramoda, K.; Shirodkar, S.N.; Mishra, A.K.; Waghmare, U.V.; Sundaresan, A.; Rao, C.N.R. Borocarbonitrides. J. Mater. Chem. A 2013, 1, 5806–5821. [Google Scholar]
- Ting, H.Y.; Watson, W.H.; Kelly, H.C. The Molecular and crystal structure of ethylenediamine-bisborane, C2H14B2N2. Inorg. Chem. 1972, 11, 374–377. [Google Scholar] [CrossRef]
- Jackson, S.T.; Nuzzo, R.G. Determining hybridization differences for amorphous carbon from the XPS C 1s envelope. Appl. Surf. Sci. 1995, 90, 195–203. [Google Scholar] [CrossRef]
- Filik, J.; May, P.W.; Pearce, S.R.J.; Wild, R.K.; Hallam, K.R. XPS and laser Raman analysis of hydrogenated amorphous carbon films. Diam. Relat. Mater. 2003, 12, 974–978. [Google Scholar] [CrossRef]
- Ci, L.; Song, L.; Jin, C.; Jariwala, D.; Wu, D.; Li, Y.; Srivastava, A.; Wang, Z.F.; Storr, K.; Balicas, L.; et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Kumar, N.; Raidongia, K.; Mishra, A.K.; Waghmare, U.V.; Sundaresan, A.; Rao, C.N.R. Synthetic approaches to borocarbonitrides, BCxN (x = 1–2). J. Solid State Chem. 2011, 184, 2902–2908. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Lisi, S.; Lizzit, S.; Baraldi, A.; Larciprete, R.; Mariani, C.; Betti, M.G. Graphene-induced substrate decoupling and ideal doping of a self-assembled iron-phthalocyanine single layer. J. Phys. Chem. C 2013, 117, 3019–3027. [Google Scholar] [CrossRef]
- Yue, Z.R.; Jiang, W.; Wang, L.; Gardner, S.D.; Pittman, C.U. Surface characterization of electrochemically oxidized carbon fibers. Carbon 1999, 37, 1785–1796. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An advanced Lithium-ion battery based on a graphene anode and a lithium iron phosphate cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.Y.; et al. Synthesis of few layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Polzonetti, G.; Alnot, P.; Brundle, C.R. The adsorption and reactions of NO2 on the Ag(111) surface: I. XPS/UPS and annealing studies between 90 and 300 K. Surf. Sci. 1990, 238, 226–236. [Google Scholar] [CrossRef]
- Moddeman, W.E.; Burke, A.R.; Bowlin, W.C.; Foose, D.S. Surface oxides of boron and B12O2 as determined by XPS. Surf. Interface Anal. 1989, 14, 224–232. [Google Scholar] [CrossRef]
- Cattelan, M.; Agnoli, S.; Favaro, M.; Garoli, D.; Romanato, F.; Meneghetti, M.; Barinov, A.; Dudin, P.; Granozzi, G. Microscopic view on a chemical vapor deposition route to boron doped graphene nanostructures. Chem. Mater. 2013, 25, 1490–1495. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces. Surf. Interface Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Arab, P.; Verlander, A.; El-Kaderi, H.M. Synthesis of a highly porous bis(imino)pyridine-linked polymer and its postsynthetic modification with inorganic fluorinated ions for selective CO2 capture. J. Phys. Chem. C 2015, 119, 8174–8182. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leardini, F.; Massimi, L.; Flores-Cuevas, E.; Fernández, J.F.; Ares, J.R.; Betti, M.G.; Mariani, C. Synthesis of Ternary Borocarbonitrides by High Temperature Pyrolysis of Ethane 1,2-Diamineborane. Materials 2015, 8, 5974-5985. https://doi.org/10.3390/ma8095285
Leardini F, Massimi L, Flores-Cuevas E, Fernández JF, Ares JR, Betti MG, Mariani C. Synthesis of Ternary Borocarbonitrides by High Temperature Pyrolysis of Ethane 1,2-Diamineborane. Materials. 2015; 8(9):5974-5985. https://doi.org/10.3390/ma8095285
Chicago/Turabian StyleLeardini, Fabrice, Lorenzo Massimi, Eduardo Flores-Cuevas, Jose Francisco Fernández, Jose Ramon Ares, Maria Grazia Betti, and Carlo Mariani. 2015. "Synthesis of Ternary Borocarbonitrides by High Temperature Pyrolysis of Ethane 1,2-Diamineborane" Materials 8, no. 9: 5974-5985. https://doi.org/10.3390/ma8095285






