Investigation of Coral-Like Cu2O Nano/Microstructures as Counter Electrodes for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Cu2O Counter Electrode (CE) Fabrication
2.2. Cu2O Counter Electrode (CE) Characterization
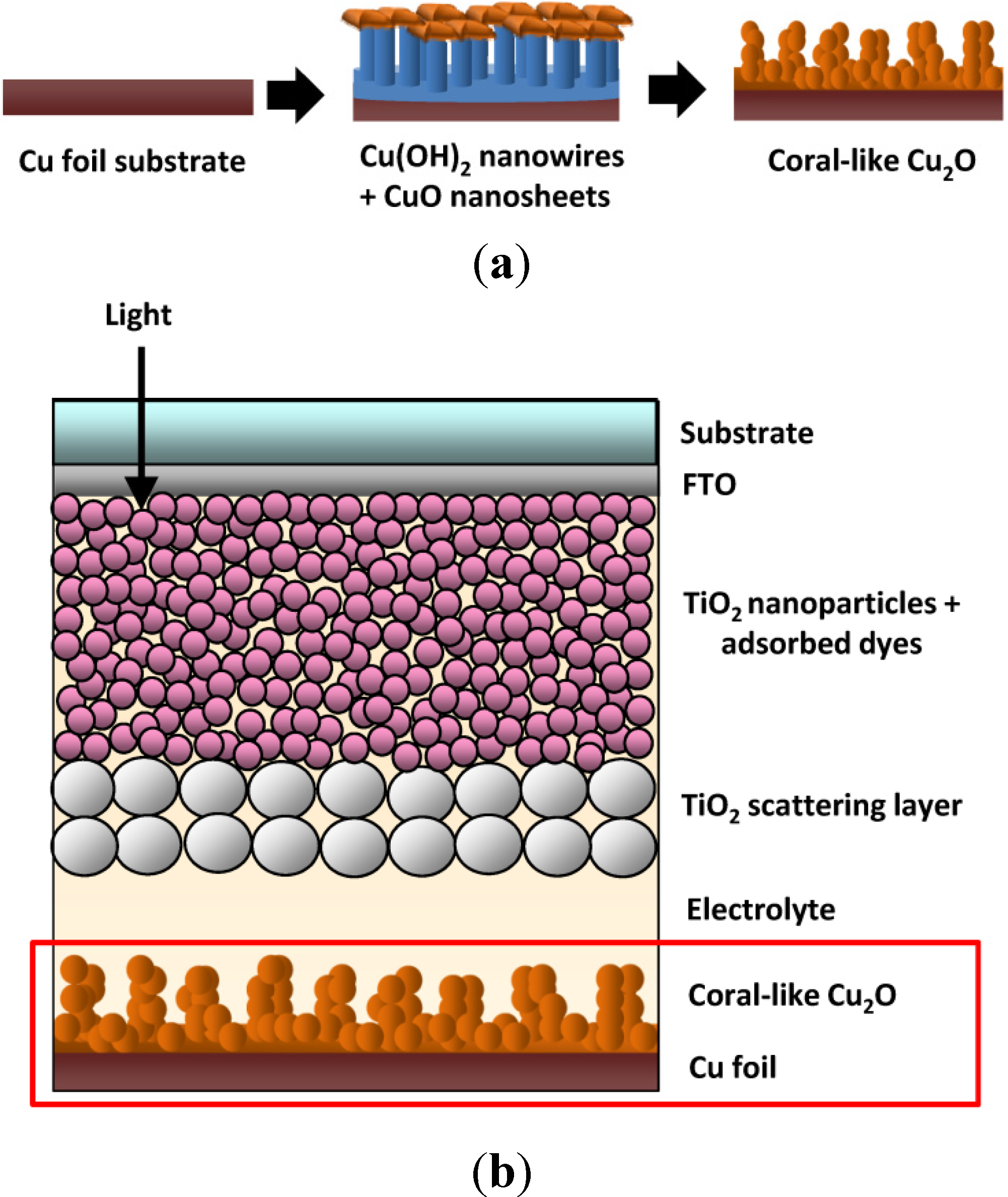
2.3. Dye-Sensitized Solar Cells (DSSC) Fabrication
2.4. Dye-Sensitized Solar Cells (DSSC) Characterization
3. Results and Discussion
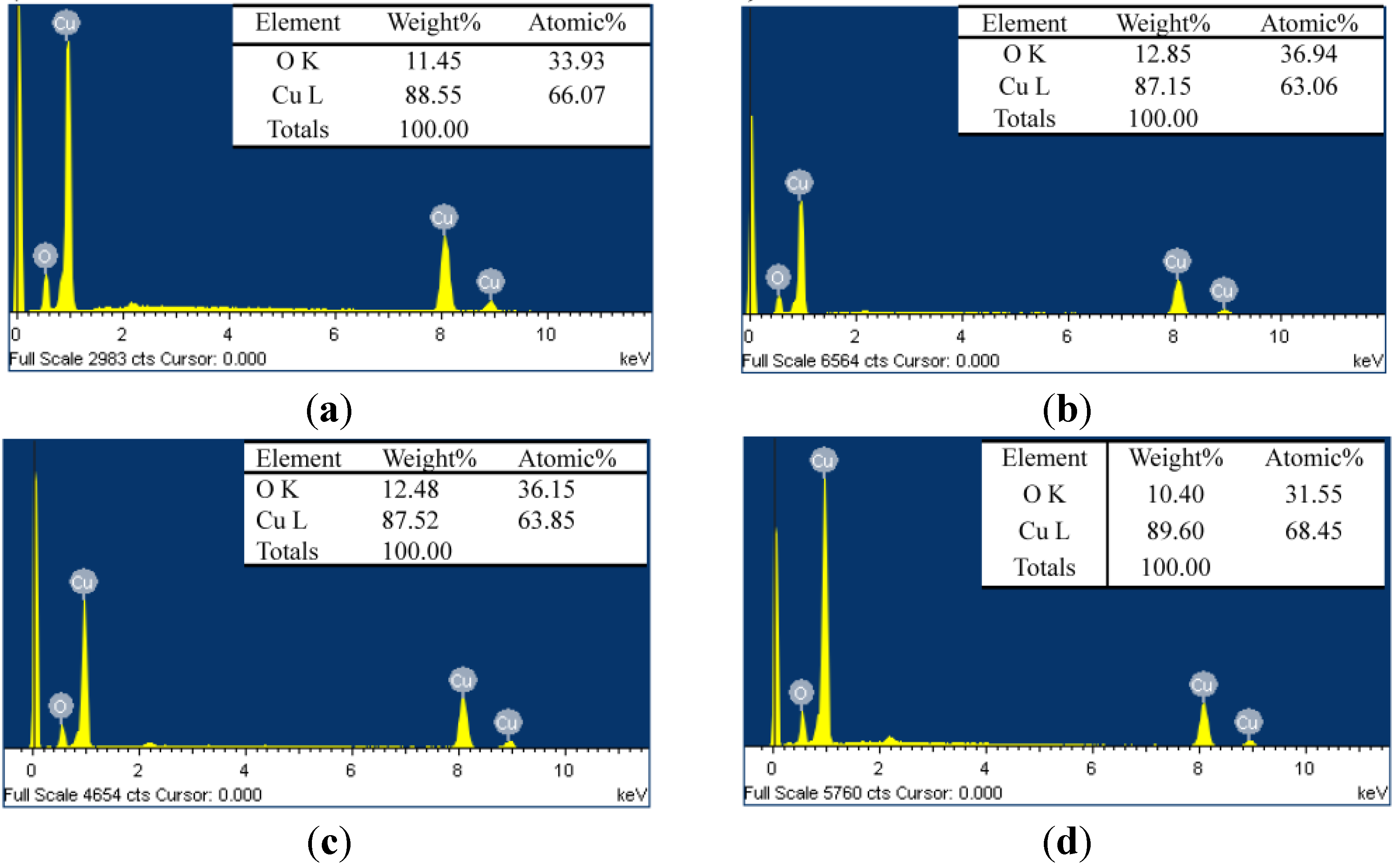
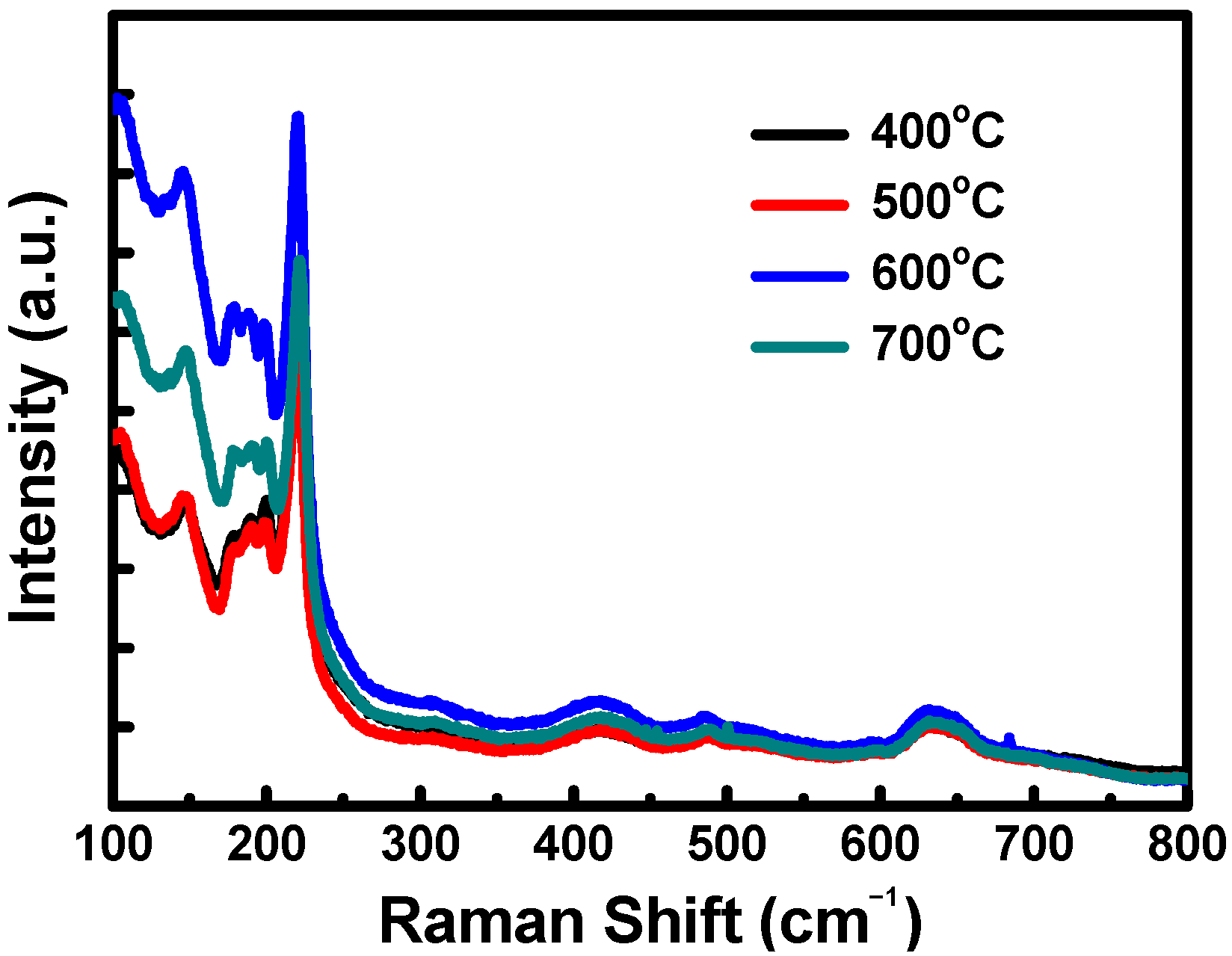
3.1. Cu2O Counter Electrodes (CE) Characterization



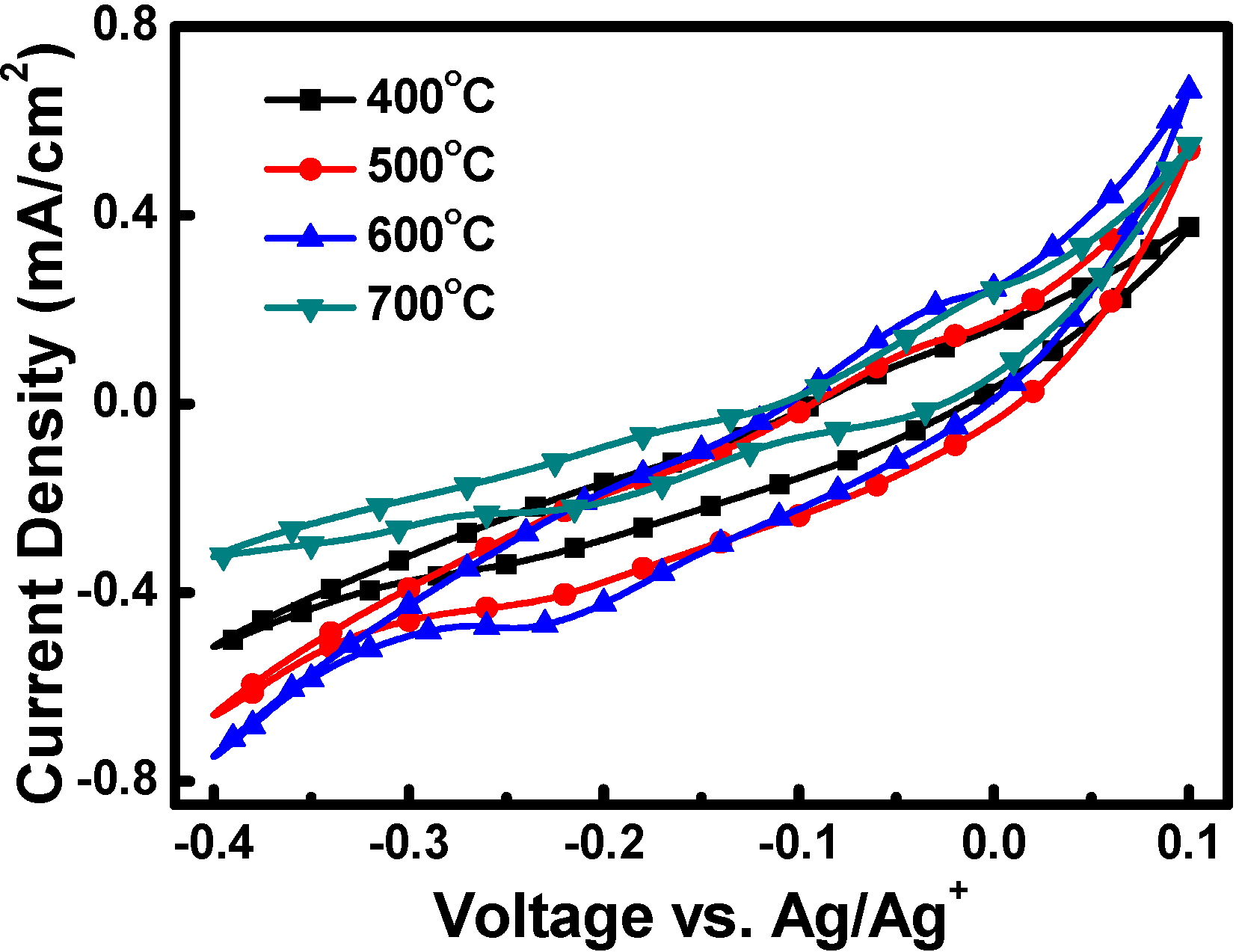
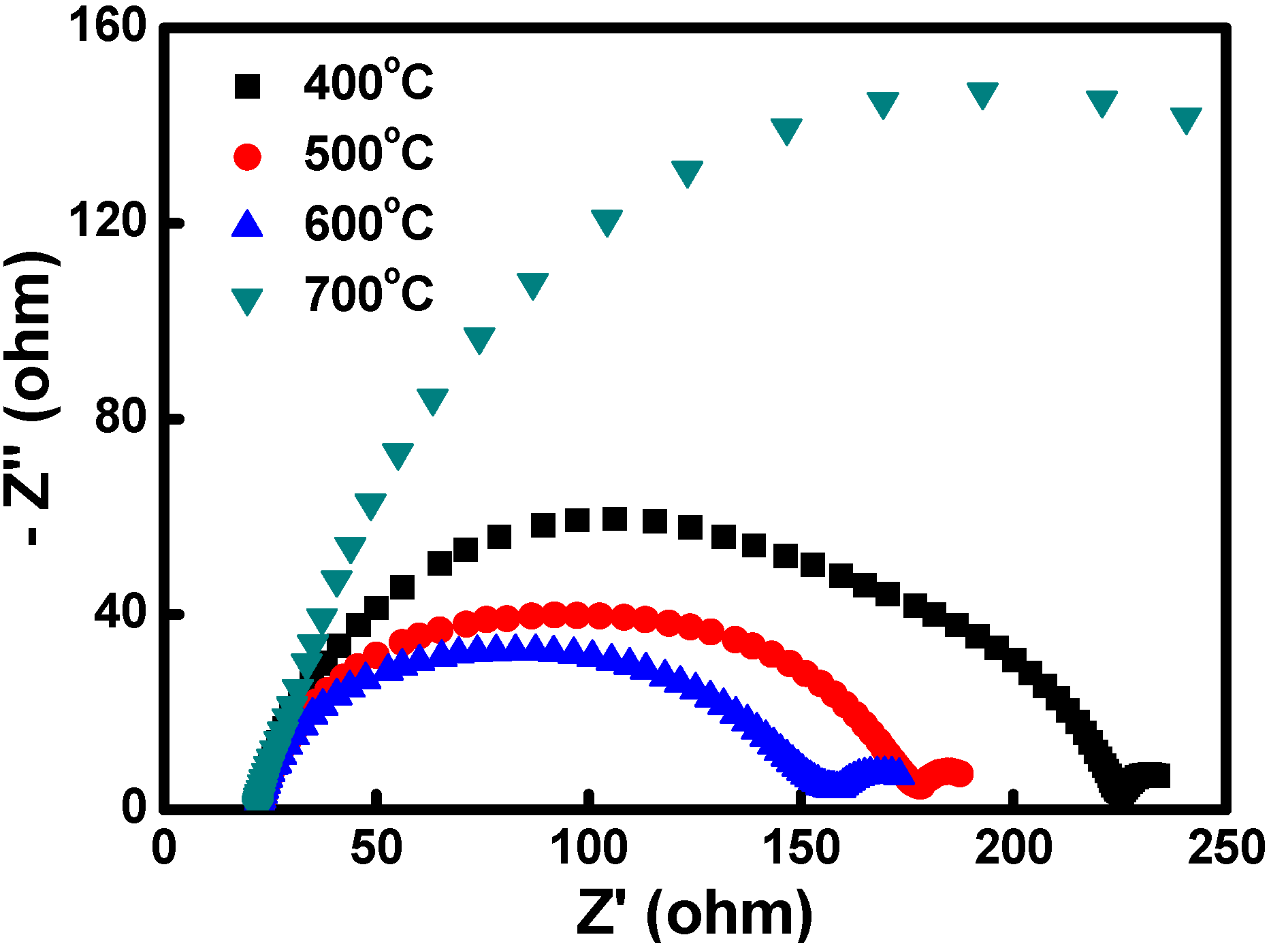
3.2. Dye-Sensitized Solar Cells (DSSC) Characterization

| Cu2O Counter Electrode (°C) | Jsc (mA/cm2) | Voc (V) | Fill Factor | Efficiency (%) |
|---|---|---|---|---|
| 400 | 6.61 ± 0.24 | 0.19 ± 0.02 | 0.34 ± 0.03 | 0.43 ± 0.10 |
| 500 | 8.64 ± 0.22 | 0.68 ± 0.01 | 0.53 ± 0.02 | 3.11 ± 0.25 |
| 600 | 11.35 ± 0.25 | 0.68 ± 0.01 | 0.47 ± 0.02 | 3.62 ± 0.31 |
| 700 | 3.94 ± 0.28 | 0.63 ± 0.02 | 0.35 ± 0.03 | 0.88 ± 0.16 |
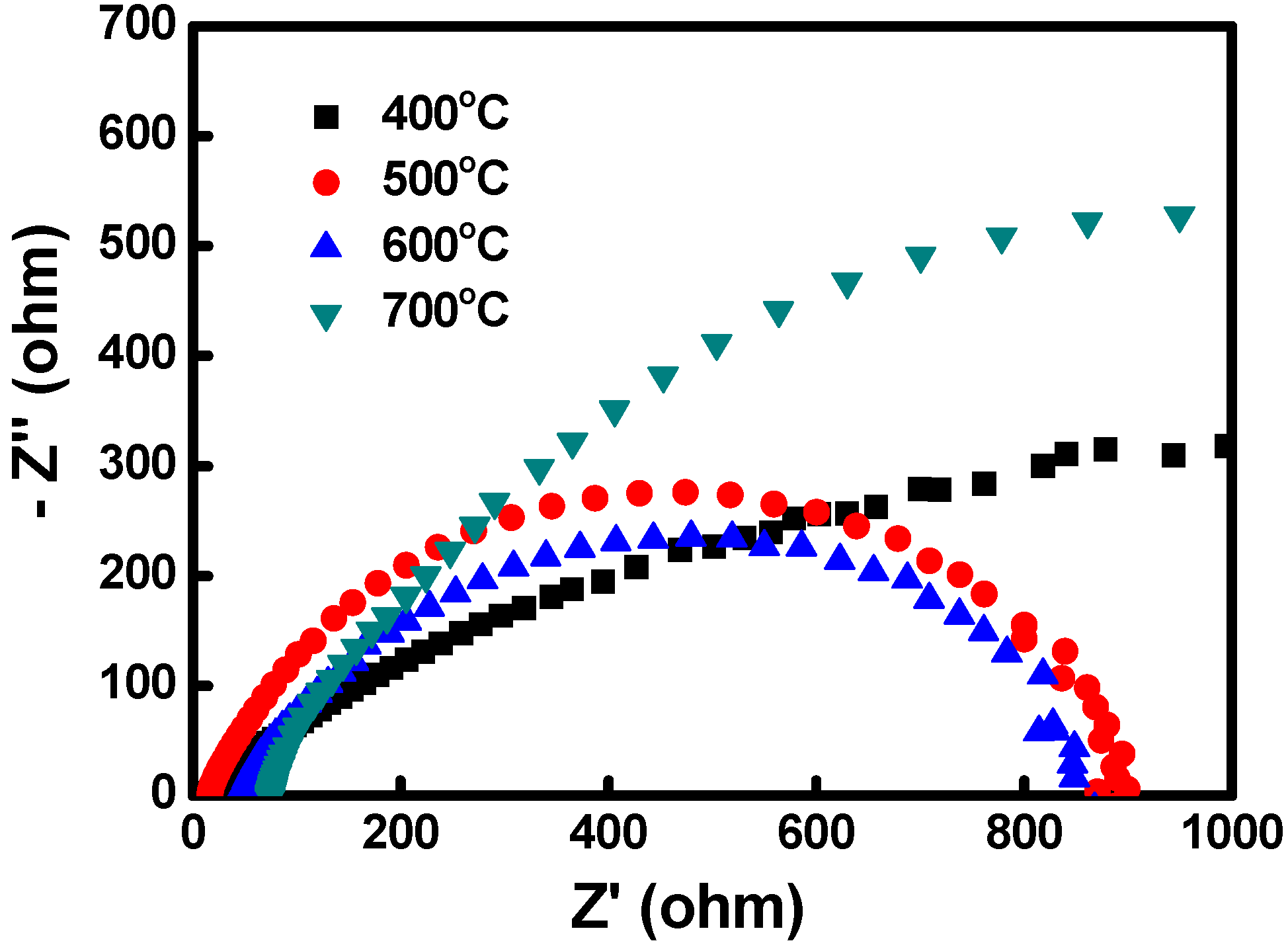
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Humphry-Baker, R.; Liska, P.; Grätzel, M. Investigation of sensitizer adsorption and the influence of protons on current and voltage of a dye-sensitized nanocrystalline TiO2 solar cell. J. Phys. Chem. B 2003, 107, 8981–8987. [Google Scholar] [CrossRef]
- Jiu, J.; Isoda, S.; Wang, F.; Adachi, M. Dye-sensitized solar cells based on a single-crystalline TiO2 nanorod film. J. Phys. Chem. B 2006, 110, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Grätzel, M. Molecular photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, 638–640. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.-H.; Grätzel, M. Acid−base equilibria of (2,2‘-Bipyridyl-4,4‘-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg. Chem. 1999, 38, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.Y.; Diau, E.W.G.; Grätzel, M. Highly efficient mesoscopic dye-sensitized solar cells based on donor–acceptor-substituted porphyrins. Angew. Chem. Int. Ed. 2010, 49, 6646–6649. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.C.; Tsai, C.H.; Chen, C.H.; Lin, L.Y.; Chou, S.H.; Wong, K.T.; Huang, T.W.; Wu, C.C. A novel amine-free dianchoring organic dye for efficient dye-sensitized solar cells. Org. Lett. 2012, 14, 6338–6341. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Teng, H.; Lee, Y.L. In situ gelation of electrolytes for highly efficient gel-state dye-sensitized solar cells. Adv. Mater. 2011, 23, 4199–4204. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Hsu, S.Y.; Lu, C.Y.; Tsai, Y.T.; Huang, T.W.; Chen, Y.F.; Jhang, Y.H.; Wu, C.C. Influences of textures in Pt counter electrode on characteristics of dye-sensitized solar cells. Org. Electron. 2012, 13, 199–205. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, M.; Li, J.Y.; Pootrakulchote, N.; Alibabaei, L.; Ngoc-le, C.; Decoppet, J.; Tsai, J.; Grätzel, C.; Wu, C.G.; et al. Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 2009, 3, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Hauch, A.; Georg, A. Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim. Acta 2001, 46, 3457–3466. [Google Scholar] [CrossRef]
- Calandra, P.; Calogero, G.; Sinopoli, A.; Gucciardi, P.G. Metal nanoparticles and carbon-based nanostructures as advanced materials for cathode application in dye-sensitized solar cells. Int. J. Photoenergy 2010, 2010. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, C.H.; Hsiao, Y.C.; Chuang, P.Y. Investigation of graphene nanosheets as counter electrodes for efficient dye-sensitized solar cells. Org. Electron. 2015, 17, 57–65. [Google Scholar] [CrossRef]
- Wang, M.; Lin, Y.; Zhou, X.; Xiao, X.; Yang, L.; Feng, S.; Li, X. Solidification of liquid electrolyte with imidazole polymers for quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 107, 61–66. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Maier, W.F.; Grätzel, M. An iodine/triodide reduction electrocatalyst for aqueous and organic media. J. Electrochem. Soc. 1997, 144, 876–884. [Google Scholar] [CrossRef]
- Fang, X.; Ma, T.; Guan, G.; Akiyama, M.; Kida, T.; Abe, E. Effect of the thickness of the Pt film coated on a counter electrode on the performance of a dye-sensitized solar cell. J. Electroanal. Chem. 2004, 570, 257–263. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, M.; Kumagai, M.; Yanagida, S. Application of carbon nanotubes to counter electrodes of dye-sensitized solar cells. Chem. Lett. 2003, 32, 28–29. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Li, K.; Li, D.; Luo, Y.; Li, H.; Song, W.; Chen, L.; Meng, Q. Application of carbon materials as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2007, 9, 596–598. [Google Scholar] [CrossRef]
- Saito, Y.; Kitamura, T.; Wada, Y.; Yanagida, S. Application of poly(3,4-ethylenedioxythiophene) to counter electrode in dye-sensitized solar cells. Chem. Lett. 2002, 31, 1060–1061. [Google Scholar] [CrossRef]
- Saito, Y.; Kubo, W.; Kitamura, T.; Wada, Y.; Yanagida, S. I−/I3− redox reaction behavior on poly(3,4-ethylenedioxythiophene) counter electrode in dye-sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 153–157. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Li, G.R.; Xiao, X.P. Highly ordered TiN nanotube arrays as counter electrodes for dye-sensitized solar cells. Chem. Commun. 2009, 41, 6720–6722. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.K.; Anghel, A.M.; Marsan, B.; Ha, N.-L.C.; Pootrakulchote, N.; Zakeeruddin, S.M.; Grätzel, M. CoS supersedes Pt as efficient electrocatalyst for triiodide reduction in dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 15976–15977. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chen, P.Y.; Hsu, C.Y.; Huang, J.H.; Ho, W.H.; Chen, H.C.; Ho, K.C. A high-performance counter electrode based on poly(3,4-alkylenedioxythiophene) for dye-sensitized solar cells. J. Power Sources 2009, 188, 313–318. [Google Scholar] [CrossRef]
- Chen, J.K.; Li, K.X.; Luo, Y.H.; Guo, X.Z.; Li, D.M.; Deng, M.H.; Huang, S.Q.; Meng, Q.B. A flexible carbon counter electrode for dye-sensitized solar cells. Carbon 2009, 47, 2704–2708. [Google Scholar] [CrossRef]
- Wan, L.; Wang, S.M.; Wang, X.B.; Dong, B.H.; Xu, Z.X.; Zhang, X.H.; Yang, B.; Peng, S.M.; Wang, J.C.; Xu, C.H. Room-temperature fabrication of graphene films on variable substrates and its use as counter electrodes for dye-sensitized solar cells. Solid State Sci. 2011, 13, 468–475. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, Y.J.; Kim, B.S.; Lee, J.S. Enhancement of the efficiency of dye-sensitized solar cell by utilizing carbon nanotube counter electrode. Scripta Mater. 2010, 62, 148–150. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Yu, C.H.; Chen, Y.C.; Lin, Y.G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grazel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.M.; Kang, J.H.; Moon, J.H. Fabrication of 3D copper oxide structure by holographic lithography for photoelectrochemical electrodes. ACS Appl. Mater. Inter. 2010, 2, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, V.; Ristov, M. Electrodeposited cuprous oxide on indium tin oxide for solar applications. Sol. Energ. Mat. Sol. C. 2002, 73, 67–73. [Google Scholar] [CrossRef]
- Jeong, S.S.; Mittiga, A.; Salza, E.; Masci, A.; Passerini, S. Electrodeposited ZnO/Cu2O heterojunction solar cells. Electrochim. Acta 2008, 53, 2226–2231. [Google Scholar] [CrossRef]
- Mittiga, A.; Salza, E.; Sarto, F.; Tucci, M.; Vasanthi, R. Heterojunction solar cell with 2% efficiency based on a Cu2O substrate. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Hsu, C.L.; Chang, S.J.; Guo, P.W.; Hsieh, J.H.; Chen, I. Cu2O/n-ZnO nanowire solar cells on ZnO: Ga/glass templates. Scripta Mater. 2007, 57, 53–56. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Skromme, B.J.; Akimoto, K.S.; Pachuta, J. Properties of the shallow O-related acceptor level in ZnSe. J. Appl. Phys. 1995, 78. [Google Scholar] [CrossRef]
- Grätzel, M. Perspectives for dye-sensitized nanocrystalline solar cells. Prog. Photovolt. Res. Appl. 2000, 8, 171–185. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Fei, P.-H.; Chen, C.-H. Investigation of Coral-Like Cu2O Nano/Microstructures as Counter Electrodes for Dye-Sensitized Solar Cells. Materials 2015, 8, 5715-5729. https://doi.org/10.3390/ma8095274
Tsai C-H, Fei P-H, Chen C-H. Investigation of Coral-Like Cu2O Nano/Microstructures as Counter Electrodes for Dye-Sensitized Solar Cells. Materials. 2015; 8(9):5715-5729. https://doi.org/10.3390/ma8095274
Chicago/Turabian StyleTsai, Chih-Hung, Po-Hsi Fei, and Chih-Han Chen. 2015. "Investigation of Coral-Like Cu2O Nano/Microstructures as Counter Electrodes for Dye-Sensitized Solar Cells" Materials 8, no. 9: 5715-5729. https://doi.org/10.3390/ma8095274





