1. Introduction
Polymeric hybrid nanofibers are gaining popularity among biomaterial researchers and industries because of their outstanding functional properties [
1,
2]. These hybrid nanofibers exhibit excellent mechanical and biological properties, which make them very attractive for various biomedical applications, such as tissue engineering scaffolds, wound dressings devices, drug delivery materials, medical implants, biosensors and filtration devices [
1]. Nanofibrous structures give rise to a high surface area-to-volume ratio similar to that of proteoglycans (glycosaminoglycan) and fibrous proteins, which comprise the ECM of living tissue [
3,
4]. Several methods have been used to fabricate nanofibers, such as drawing, self-assembly and phase separation. However, in most cases, nanofibers are typically created using the electrospinning technique, which is cost-effective and reliable [
5]. This process is one of the well-established techniques to fabricate a 3D porous nanofiber-based matrix that can be easily scaled up from laboratory to commercial production. A high voltage is applied to a continuous dripping of polymeric solution, which makes the polymer surface uniformly charged, creating electrostatic repulsion forces. When the surface tension overcomes the electrostatic force, a jet is ejected as a nanofiber from the polymer droplet and is collected in the collector.
The choice of polymer material for electrospinning is guided by the desirable properties, such as biocompatibility, limited toxicity, biodegradability and sufficient mechanical/structural integrity in the corresponding fibers for various biomedical applications [
6]. Several natural and synthetic polymers have been used to generate nanofibers suitable for broad applications in biomedical engineering. The synthetic polymers include polylactic acid (PLA), polyglycolic acid (PGA)-based linear aliphatic polyesters and their copolymer, poly(ε-caprolactone) (PCL),
etc., and have been widely used [
7,
8]. Natural polymers, including collagen, gelatin, hyaluronan, silk, chitosan, alginate, keratin, fibrinogen and elastin, are the most commonly-used natural polymers in tissue engineering [
7,
9,
10]. Many of these natural polymers have cell-binding sites and biomolecular signatures that can mimic natural tissue [
11]. Synthetic materials usually have good mechanical properties compared to the natural polymers. Even though there are some advantages of using synthetic materials, they have limited cell affinity, primarily due to their hydrophobicity and lack of surface cell recognition sites [
7,
12,
13]. Natural polymers when used alone do not produce nanofibers with sufficient strength. Therefore, natural polymers are blended into synthetic polymers in which the favorable biological functionality is contributed by natural polymers, whereas the mechanical stiffness is provided by their synthetic counterparts. This type of hybrid system is expected to significantly improve material properties, while providing a stable, nurturing environment for a broad array of biomedical applications [
14].
In this study, we fabricated and characterized PCL-based composite nanofibers. Electrospun nanofibers were prepared from the solutions of PCL/magnesium oxide (PCL/MgO) and poly(ε-caprolactone)-keratin/magnesium oxide (PCL-K/MgO). PCL is popular in the area of biomaterials and tissue engineering [
15] due to its biodegradability, biocompatibility and superior mechanical properties [
16,
17]. Keratin, found largely in hair and fingernails [
18], has been used as the natural polymer. Keratin has intrinsic biological activity and cell binding motifs that can support cellular attachment [
19]. Keratins are the main subgroup of intermediate filament proteins and are the most abundant proteins in epithelial cells [
20] that contribute to the mechanical integrity of the cell and also function as regulatory protein. Keratin has also been used in several applications, such as in wound healing, drug delivery and tissue engineering. Additionally, there has been an increasing interest in magnesium and its alloys as potential biodegradable implant materials, in biomedical applications, such as for cardiovascular and orthopedic devices [
21]. Magnesium possesses excellent biocompatibility, biodegrades into soluble Mg ions (Mg
2+), which are non-toxic by-products, and has proven use as an excellent nutrient for human metabolism [
22,
23]. Soluble magnesium plays an important role in various cellular processes, such as cellular respiration, protein synthesis, membrane integrity, ATPase function and oxidative phosphorylation [
24,
25,
26]. MgO is an inorganic metal oxide and is a source of magnesium supplement that is biodegradable and provides soluble Mg
2+ ions [
27]. In this study, MgO was introduced in the electrospun nanofiber, and various physical, chemical and mechanical properties were analyzed. Nanofiber morphology was analyzed by using SEM; chemical composition and surface chemistry was determined by FTIR; and the mechanical properties of nanofibers were evaluated for tensile loading. Additionally, a release study was carried out at different time points in order to quantify the amount of magnesium released from the PCL-based composite nanofibers using inductively-coupled plasma optical emission spectroscopy (ICP-OES).
2. Materials and Method
2.1. Materials
Human hair was obtained from a local barbershop in Greensboro, NC. Peracetic acid solution and Trizma® base (Product Number T1503) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid (A144C-212 Lot 093601) (HCl) was purchased from Fisher Scientific (Pittsburgh, PA, USA). PCL (Mn70–90 kDa) and MgO (nanopowder, <50-nm particle size) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2,2,2-Trifluoroethanol (99+%) (TFE) was obtained from Alfa Aesar (Ward Hill, MA, USA). A 21-gauge, stainless steel dispensing needle, 1.5 inches long (product number: 75165A757) and with a 1/8 inch inner diameter, fluorinated ethylene propylene tubing and Luer lock syringe needle fittings were obtained from McMaster-Carr (Atlanta, GA, USA). Five-milliliter Luer lock syringes (Catalog Number 14-829-45) were obtained from Fisher Scientific (Pittsburgh, PA, USA).
2.3. PCL/MgO Solution Preparation
PCL was dissolved in TFE at a concentration of 10% (w/v) overnight to obtain a homogeneous mixture. Afterwards, PCL/MgO solutions were created by mixing PCL with MgO in ratios of 1:0, 1:0.05, 1:0.10, 1:0.20, 1:0.50, 1:1, 1:1.5, 1:2 and 1:3 respectively. Each solution was prepared separately by mixing in a vortex for 20 min.
2.4. PCL-K/MgO Solution Preparation
Lyophilized keratin powder was dissolved in DI water at a concentration of 10% (w/v). PCL was dissolved in TFE at a concentration of 10% (w/v). PCL-K solution was then created by first mixing PCL with keratin at a ratio of 90:10. This was followed by mixing PCL-K with MgO at a ratio of 1:1. The final solution mixture (i.e., PCL-K/MgO) was vortexed for 20 min.
2.5. Electrospinning of PCL/MgO and PCL-K/MgO Nanofibers
A previously prepared solution of PCL/MgO and PCL-K/MgO was individually fed into the syringe of 10 mL and then placed into a syringe pump (Model 78-01001, Fisher Scientific, Pittsburgh, PA, USA). The syringe pump was set to a flow rate of 1–2 mL/h for the PCL/MgO and PCL-K/MgO solutions. The positive lead from the high voltage power supply (Model CZE100PN30, Spellman High Voltage Electronics Corporation, Hauppauge, NY, USA) was fixed to a 21-gauge needle, and an 11-kV voltage was applied, which charged the polymer solution. Aluminum foil was wrapped around the collector for collecting the fiber. The tip to collector distance was maintained at 10 cm. The fibers formed were deposited onto a rotating grounded collector. Nanofiber samples were collected for 2–6 h.
2.6. Scanning Electron Microscopy
The electrospun nanofibers were sputter coated with gold by using a Polaron SEM Coating System for 1 min and 30 s at 15 mA. Then, these nanofibers were imaged using the SEM (Hitachi SU8000, Tokyo, Japan). The samples were observed at an accelerating voltage of 10 kV and a 5-µA current. The diameter of these electrospun fibers was determined by using Image-Pro Plus 6.0 software.
2.7. Characterization of Electrospun Nanofibers
To characterize the chemical bonding between PCL, MgO and keratin, FTIR spectra were obtained at 64 scans using a Varian 680i FTIR (Agilent Technologies, Santa Clara, CA, USA). The nanofiber membrane was placed in a sample compartment, and the system was purged with dry air before testing. Spectrum analysis was performed using standard Microcal Origin Software (Northampton, MA, USA).
2.8. Mechanical Tensile Test
Mechanical properties of the nanofibers were evaluated using a Shimadzu machine (North America Analytical and Measuring Instruments AGS-X series, Columbia, MD, USA). Trapezium Lite X software was used to collect data. Load and displacement values were collected every 500 milliseconds with a 50 N load cell at a displacement rate of 10 mm/min.
Mechanical characterization of electrospun nanofiber is extremely sensitive, as it involves measurement of very small changes in load required for deformation. These nanofibers are so delicate that any direct touch of the mat surface during sample preparation and testing can damage the fibers; hence, sufficient care must be taken. In order to avoid any such damage during handling and to maintain uniformity in loading conditions, a paper template of (38 mm × 25 mm) with an opening of (6 mm × 12 mm) was prepared as described in the literature [
28]. The template consisted of two halves, top and bottom, and held the sample with the aid of double-sided adhesive tape. Fiber samples were center aligned and placed within a gauge length (Lg) of a 6-mm window cut from a paper template. The axis of the specimen was maintained parallel with the load axis precisely to prevent specimen bending and premature failure. Five samples for each ratio of nanofiber were cut to a width of 5 mm and a length of 16 mm. The thickness of each sample was measured using a digital micrometer. Fiber samples were strained to breakage, and the ultimate tensile strength and Young’s modulus were derived from stress-strain curves.
2.9. Degradation and Release Study of Mg
Nanofibers of PCL/MgO and PCL-K/MgO were dried under reduced pressure at room temperature. These samples were incubated in 40 mL of 1X Phosphate Buffer Saline (PBS) solution for 1 week at 37 °C in a conical tube. At various time intervals, 10 mL of solution were extracted, and the medium in the tube was replenished with 10 mL of fresh 1X PBS. The concentration of Mg released in these solutions was determined using ICP-OES against standards made from High Purity Standards solutions (Catalog Number CCV-1, High Purity Standards, Charleston, SC, USA) and 2.0% nitric acid (Catalog Number CLBK-HNO3-250, SPEX CertiPrep, Metuchen, NJ, USA). These High Purity Standards were multi-element standards that contained Mg. Samples for ICP analysis were prepared with 1 mL of concentrated nitric acid (15.8 M) added to each solution. The data in ppm obtained from ICP analysis were used in determining the percent of Mg released in each solution.
Dried nanofibrous membranes were cut into squares (~30 mm × 30 mm), sterilized with 70% alcohol (10 min incubation) and washed thoroughly with DI water. Membrane stability was then tested by incubating samples in 15 mL PBS (pH 7.5 at 37 °C). The buffer was replaced every 3 days. Nanofibers after incubation for a requisite time were removed from the PBS solution, rinsed with DI water and lyophilized. For in vitro degradation testing, these samples were observed for morphological changes under an SEM. SEM experimental parameters were the same as discussed earlier in the SEM section.
2.10. Statistical Analysis
Statistical analysis was performed using a one-way analysis of variance (ANOVA). p-values less than 0.05 were considered statistically significant, and the Tukey test method was conducted for pairwise comparisons. SPSS Statistics 17.0 software was used to conduct the statistical analysis.
4. Discussion
Dissolving PCL in TFE and keratin powder in DI water both at a concentration of 10% (w/v) was acquired from a previous study [
6]. The preparation of a homogeneous polymer solution is one of the most important criteria to obtain uniform polyblend nanofibers of PCL and keratin. In several previous studies, miscible polymer solutions have been achieved with PCL, blended with natural polymers, such as chitosan, collagen, gelatin, silk,
etc. [
6,
30,
31]. TFE was used in this research, because it has been found to be a good organic solvent in the fabrication of electrospun nanofibers [
2]. Through hydrogen bonding, TFE helps in forming stable complexes between PCL and keratin, which then results in the viscosity suitable for electrospinning [
6].
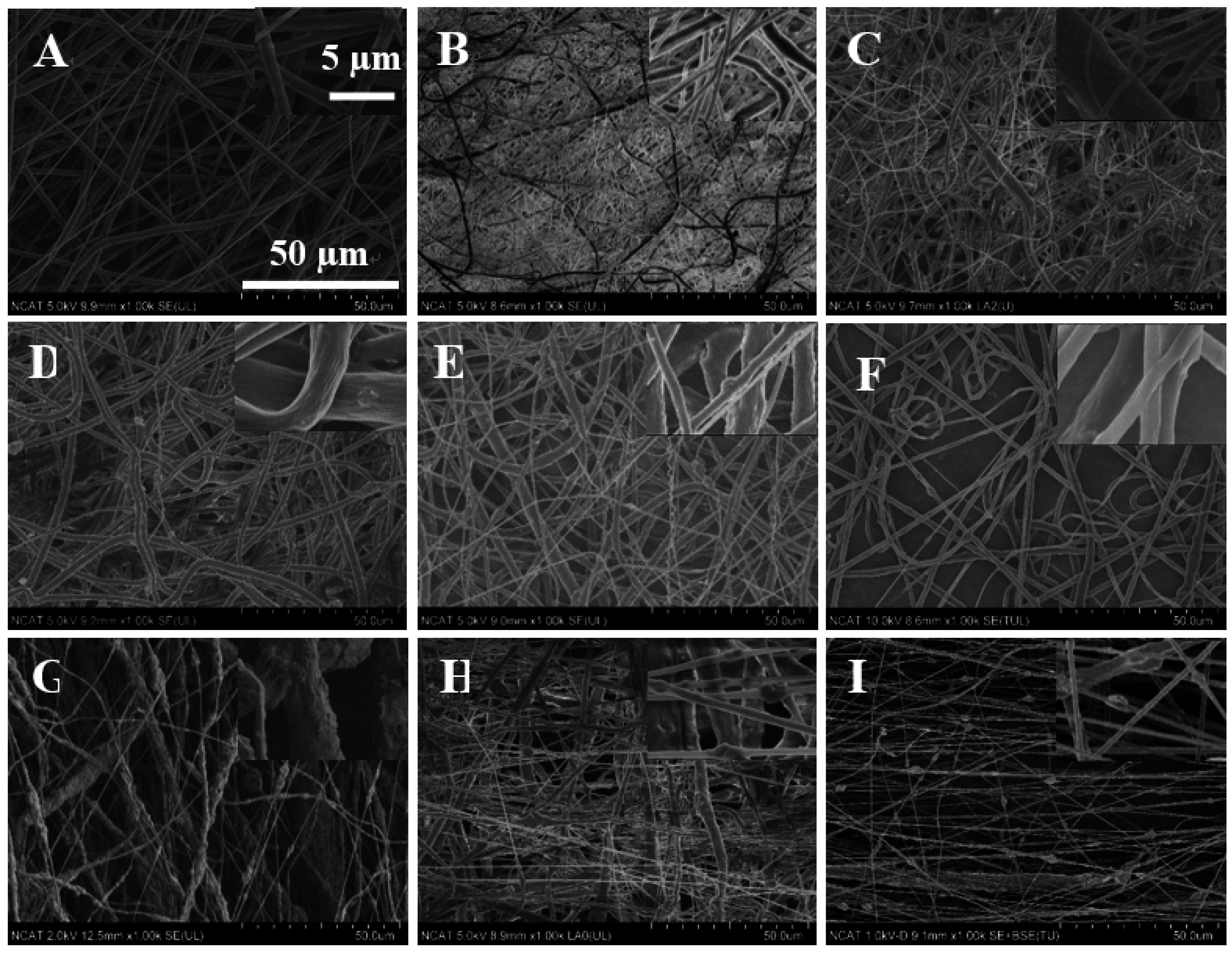
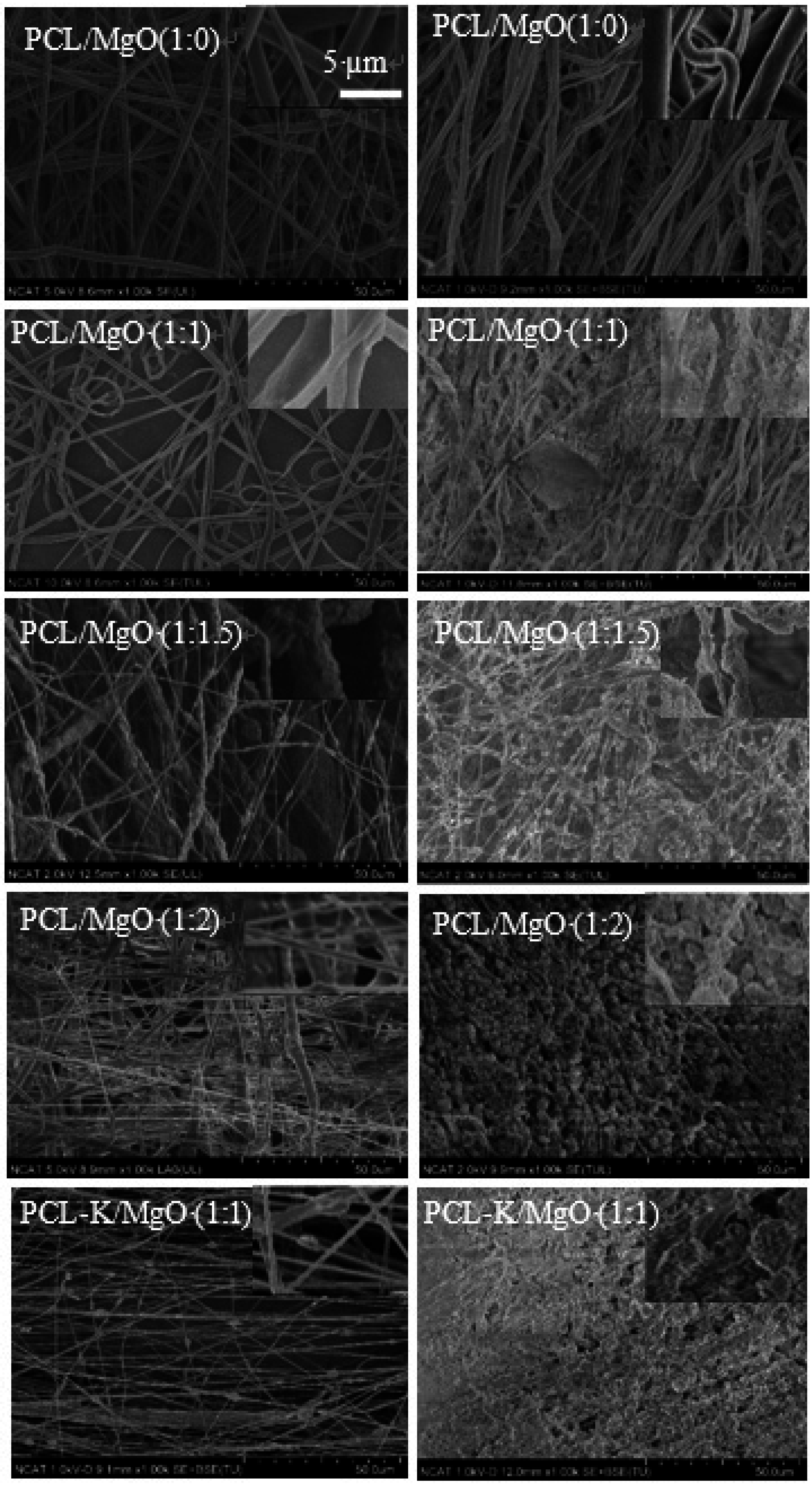
Figure 8.
SEM images of in vitro degradation of nanofibers. Left images are from Day 0; right images are from Day 7. The scale bar is 50 µm.
Figure 8.
SEM images of in vitro degradation of nanofibers. Left images are from Day 0; right images are from Day 7. The scale bar is 50 µm.
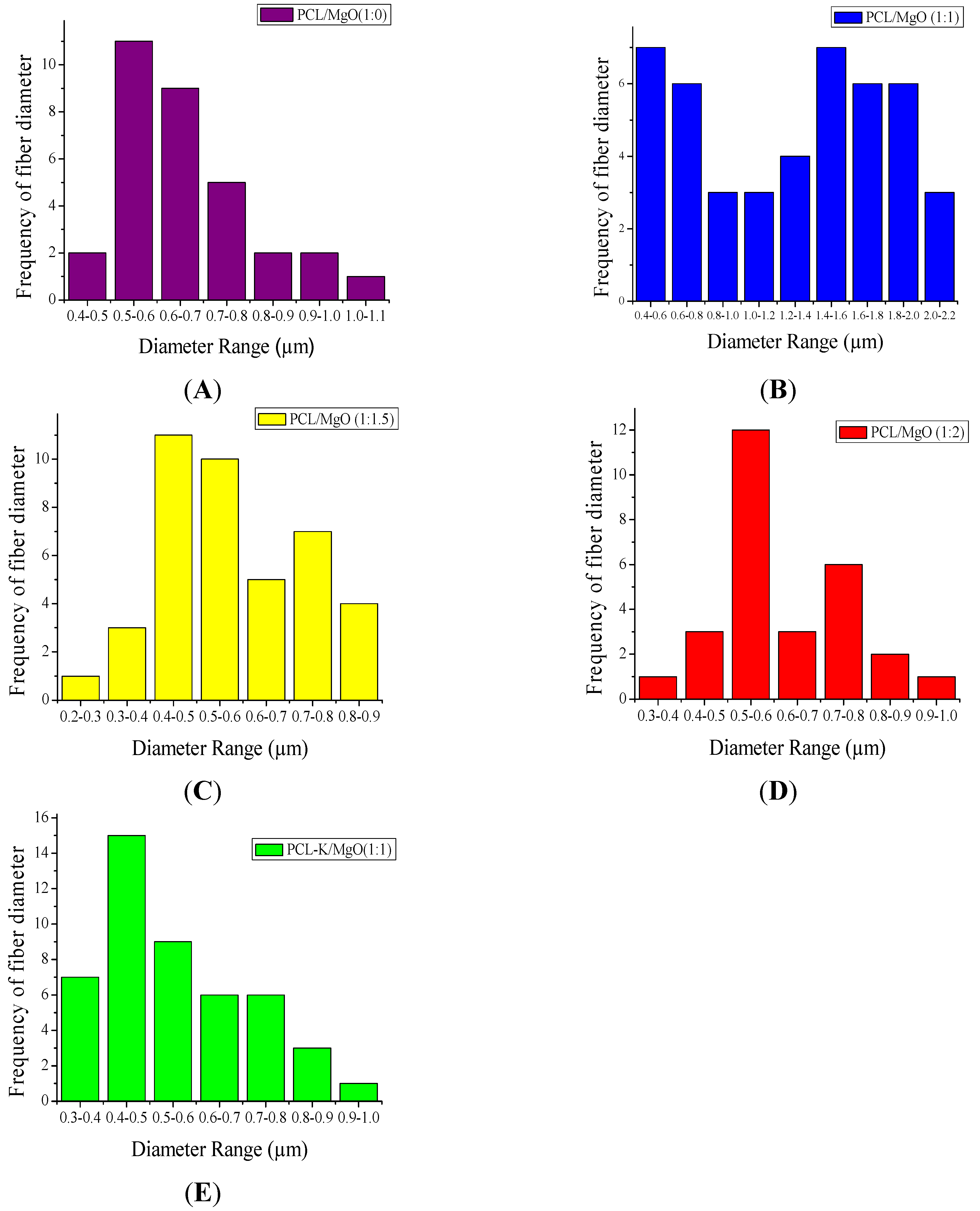
In this research, PCL-based composite nanofibers were fabricated by the electrospinning technique. Solutions of PCL/MgO in ratios of 1:0–1:3 were prepared and yielded nanofibers. Overall, the thickness of the pure PCL nanofiber was thicker than the other nanofiber ratios produced, as shown in
Figure 1. The distinct result of nanofibers produced from the solution of PCL alone was contributed by solution conductivity and solution viscosity [
7]. A decreasing trend in the average diameter of the nanofiber was observed as the ratio of MgO was increased, as shown in
Figure 2. Magnesium ions’ presence in the precursor solution enhanced the overall conductivity, which produced smaller diameter nanofiber. Water-soluble keratin extracted from human hair was miscible with PCL and MgO in a PCL-K/MgO ratio of 1:1 and yielded nanofibers. Nanofibers of PCL-K/MgO in a ratio of 1:1 and PCL/MgO in ratios of 1:0–1:2 were further investigated. PCL-K/MgO with a ratio of 1:1 was the composition selected with keratin due to a limited supply of keratin and for the simplicity of this study. The average diameter of the keratin-based nanofiber was higher compared to other nanofibers consisting of MgO. The solutions of PCL-K/MgO with a ratio of 1:1 appeared more viscous compared to the other solutions of PCL/MgO without keratin. This is due to the nature of keratin having recurring integrin-binding domains [
19].
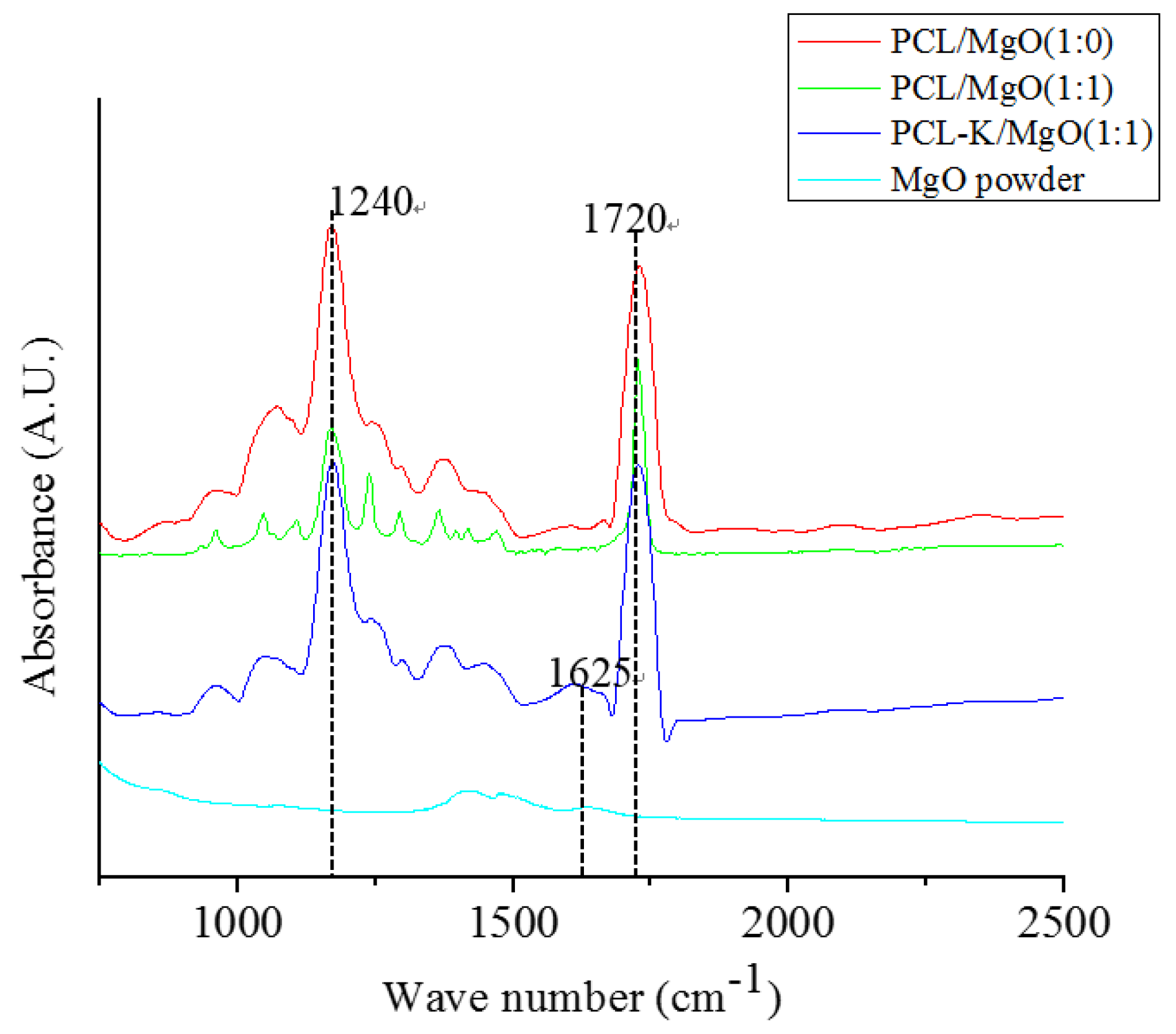
In this study, the characteristic FTIR absorption bands at 1720 cm
−1 and 1625 cm
−1 confirmed the presence of PCL and keratin, respectively, as shown in
Figure 3 [
6,
29]. PCL/MgO and PCL-K/MgO nanofibers showed the characteristic bands of PCL and keratin. Absorption peaks of MgO are not clearly visible in composite nanofibers, because of their weak intensity compared to PCL and keratin. Peak intensities of keratin are also weak compared to PCL because of its small composition. Keratin proteins give rise to several characteristic absorption bands known as amide I (1600–1700 cm
−1) and amide II (1480–1580 cm
−1). The amide I band is mainly associated with the C = O stretching vibration, whereas amide II results from the N–H bending vibration and from the C–N stretching vibration. The characteristic absorption band at amide I at 1625 cm
−1 was used as a reference peak to confirm the bonding interactions between PCL carbonyl groups and keratin amine groups.
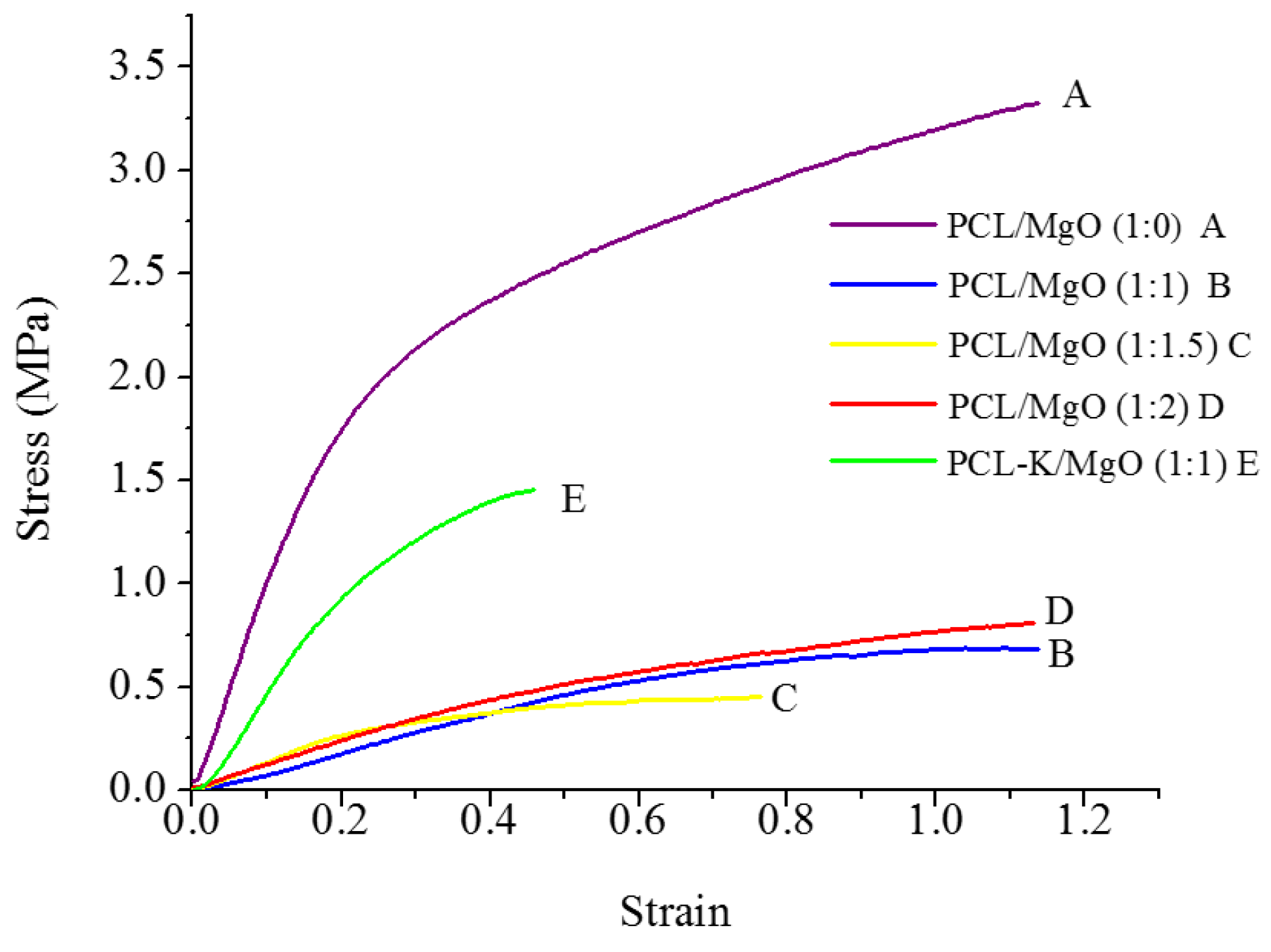
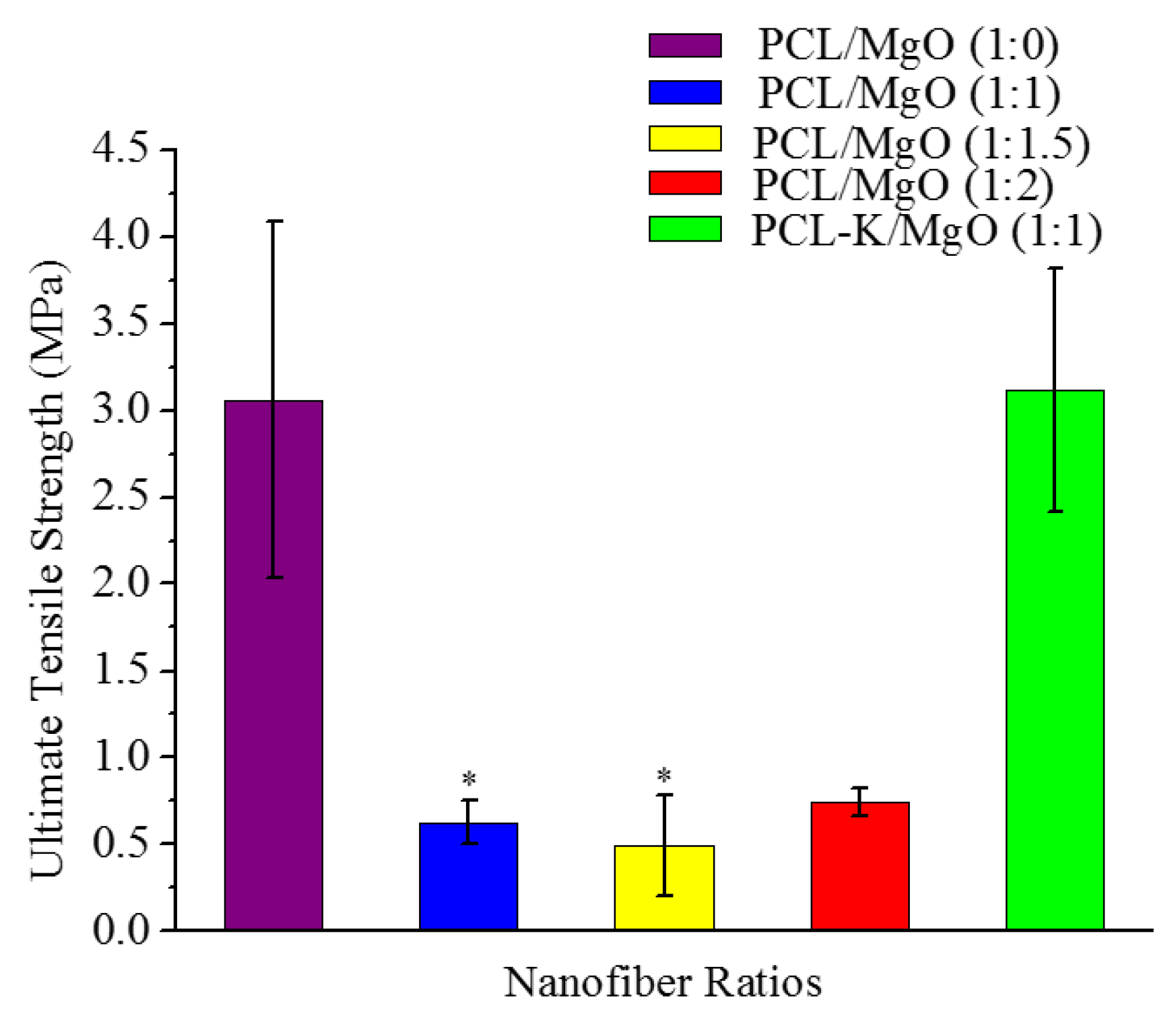
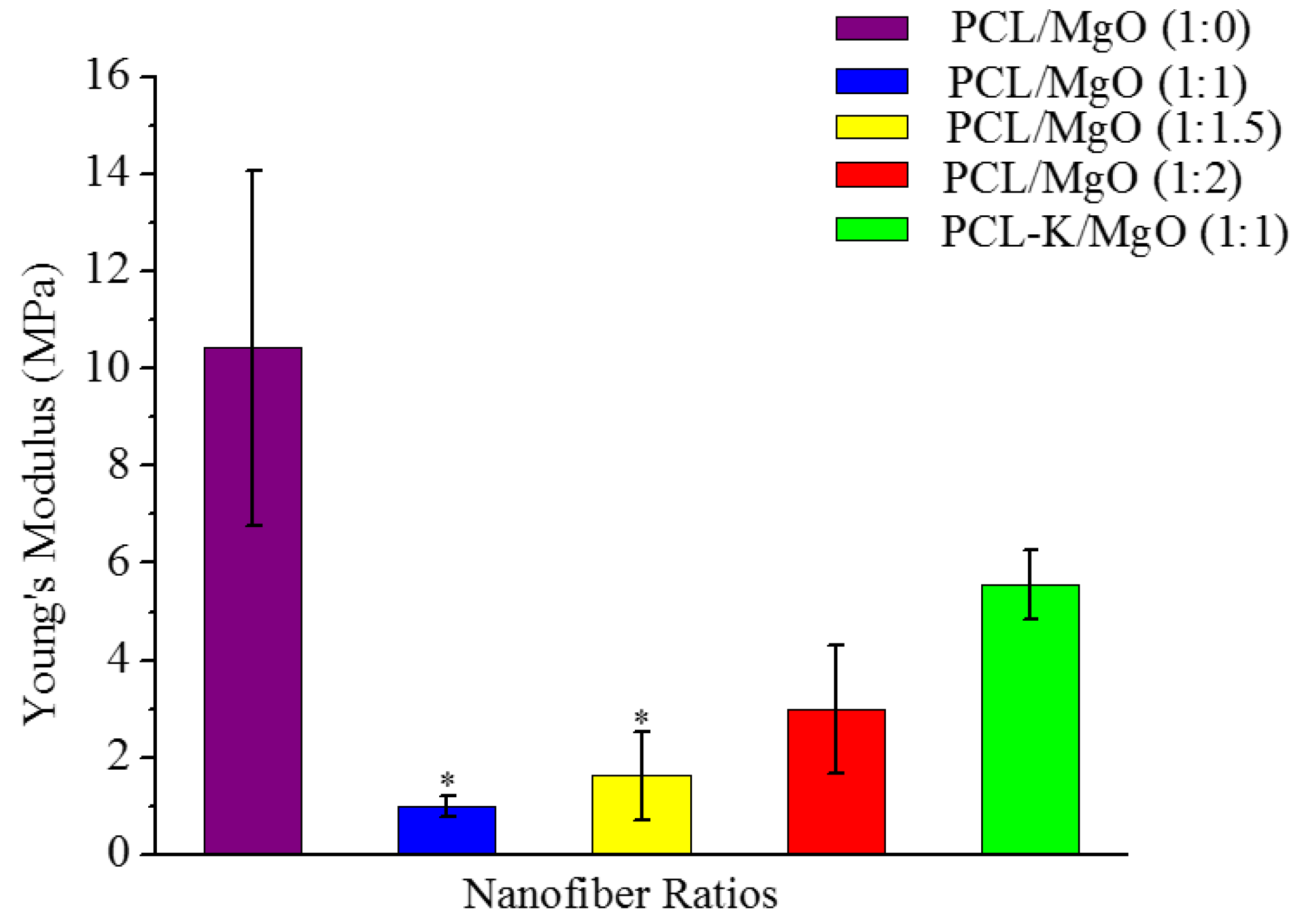
A suitable nanofiber needs to have good mechanical properties for tissue regeneration application. A material modulus close to the target tissue is crucial in order to avoid stress-shielding effect. This allows maintaining the mechanical integrity of the fiber in
in vivo and
in vitro applications. The data suggest that PCL nanofibers had the highest Young’s modulus (10.5 MPa) and that PCL-K/MgO nanofibers had the highest ultimate tensile strength (3.2 MPa). The PCL-K/MgO nanofibers had a slightly higher ultimate tensile strength than PCL nanofibers. Young’s modulus and ultimate tensile strength of the PCL-K/MgO nanofibers were significantly higher compared to that of the nanofibers of PCL/MgO with a ratio of 1:1, as shown in
Figure 5 and
Figure 6. It is expected that a PCL-based composite nanofiber made of natural materials can cause improved mechanical strength compared with nanofibers made of natural or synthetic materials alone [
32]. Therefore, a combination of a synthetic polymer (PCL) and a natural polymer (keratin) is the cause of PCL-K/MgO nanofibers having the highest ultimate tensile strength compared to all of the PCL/MgO nanofibers. This is also the reason that PCL-K/MgO nanofibers synthesized in this study had higher mechanical properties compared to PCL/MgO nanofibers with a ratio of 1:1. The increase of strength is due the strong intermolecular hydrogen bonding interactions between PCL carbonyl groups and keratin amine groups. As shown in
Figure 5 and
Figure 6, the mechanical properties are comparable to those of previously developed PCL-based composite nanofibers for various tissue engineering applications, such as PCL/chitosan [
30,
33] and PCL/collagen [
34].
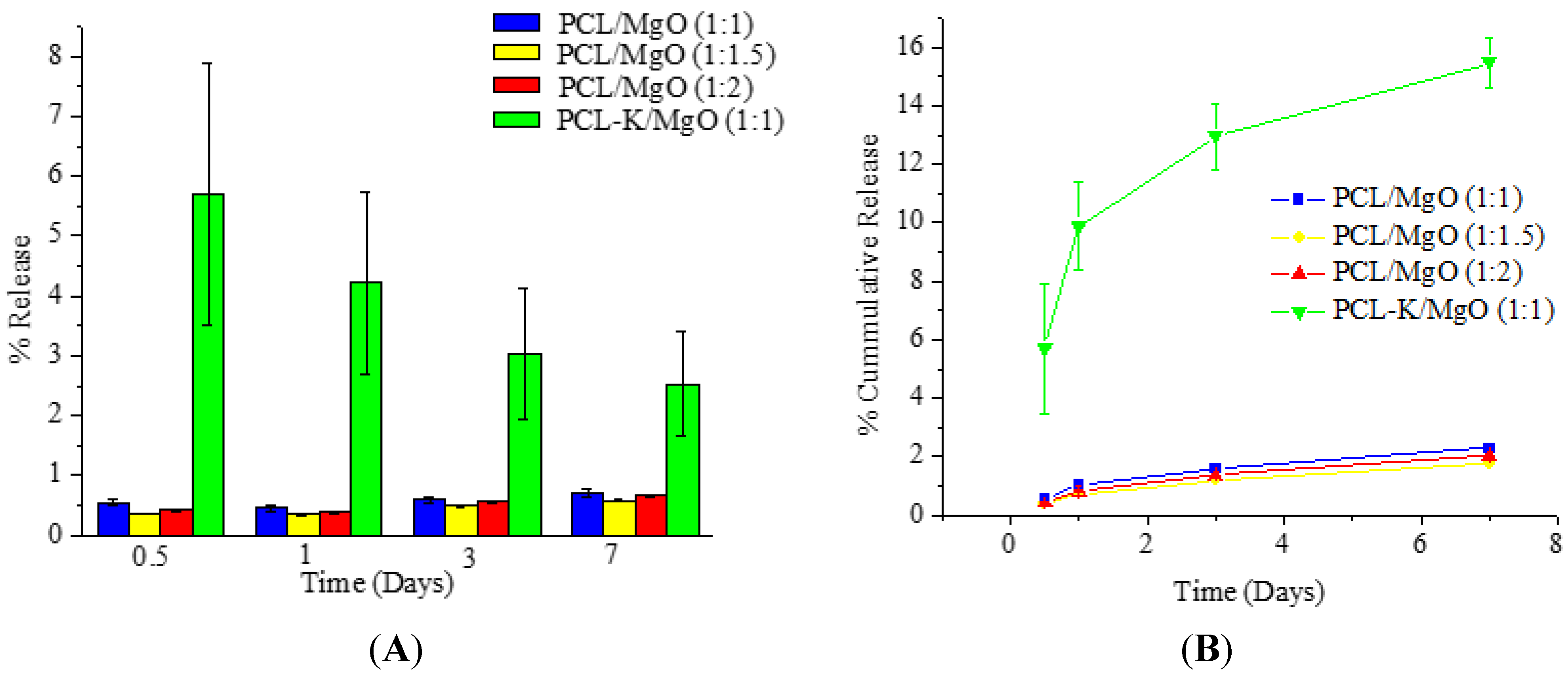
When PCL/MgO and PCL-K/MgO nanofibers degrade, Mg is released into the degradation medium. The release study of Mg from nanofibers was done with the use of an ICP-OES. PCL-K/MgO nanofiber samples released magnesium significantly more over time compared to all other PCL/MgO nanofiber samples, as shown in
Figure 7. However, for the PCL-K/MgO nanofibers, the individual daily release of magnesium decreased over time. PCL is characterized as being hydrophobic, whereas keratin is characterized as being hydrophilic. Due to the hydrophilic nature of the polymer and the presence of hydrolysable amide bonds, keratin shows bulk eroding characteristics, readily allowing permeation of water into the polymer matrix, and degrades throughout the nanofiber [
35,
36]. Bulk-eroding polymers are often characterized by a burst of drug release during the first few hours of incubation, followed by a slow, diffusion-controlled release [
37]. In contrast to keratin, PCL shows surface eroding behavior and degrades very slowly due to the presence of five hydrophobic CH
2 moieties in its repeating units and a high degree of crystallinity [
38]. This is the reason for very low magnesium release in PCL/MgO nanofibers when compared to PCL-K/MgO nanofibers, which shows greater release of magnesium contributed by the high degradation rate of keratin.
The structural integrity for pure PCL nanofiber was unchanged after seven days in PBS medium. However, for other samples, the structural integrity was deformed with the maximum change observed in the PCL-K/MgO sample. This change is supported by the maximum loss of Mg.
The degradation study of Mg demonstrated how PCL/MgO nanofibers and PCL-K/MgO nanofibers would be affected in physiological conditions for a week. At Day 7, the SEM image of the PCL nanofibers did not show any changes, suggesting that the fibers did not degrade. This is consistent with the fact that the polymer PCL can take up to two years to degrade [
17]. At Day 7, it can be seen from SEM images for the PCL/MgO nanofibers of ratios of 1:1, 1:1.5 and 1:2 and PCL-K/MgO nanofibers that the fibers retained their surface morphology, as shown in
Figure 8. The greater the amount of MgO in the fibers of the PCL/MgO nanofibers, the less intact the fibers were, meaning that the fibers degraded. MgO is a sparingly soluble oxide in water. The degradation of the nanofibers with MgO may not occur in a week, although the fiber morphology changed with a higher composition of MgO. For tissue engineering applications that need a significant amount of time to heal, the degradation time of nanofiber matrix needs to be longer than a week. This rapid degradation was observed when introduced into the PBS solution for over a week, which caused a higher release rate of Mg. In the research discussed by Johnson and Liu (2013), after three days of incubation in PBS solution, magnesium-based samples with oxides on their surface were completely degraded [
39]. The degradation of the PCL-K/MgO nanofibers is due to keratin’s hydrophilic nature releasing more Mg into the solution, and then, perhaps, this reaction influences the degradative behavior of the nanofiber.