Revealing New Structural Insights from Surfactant Micelles through DLS, Microrheology and Raman Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
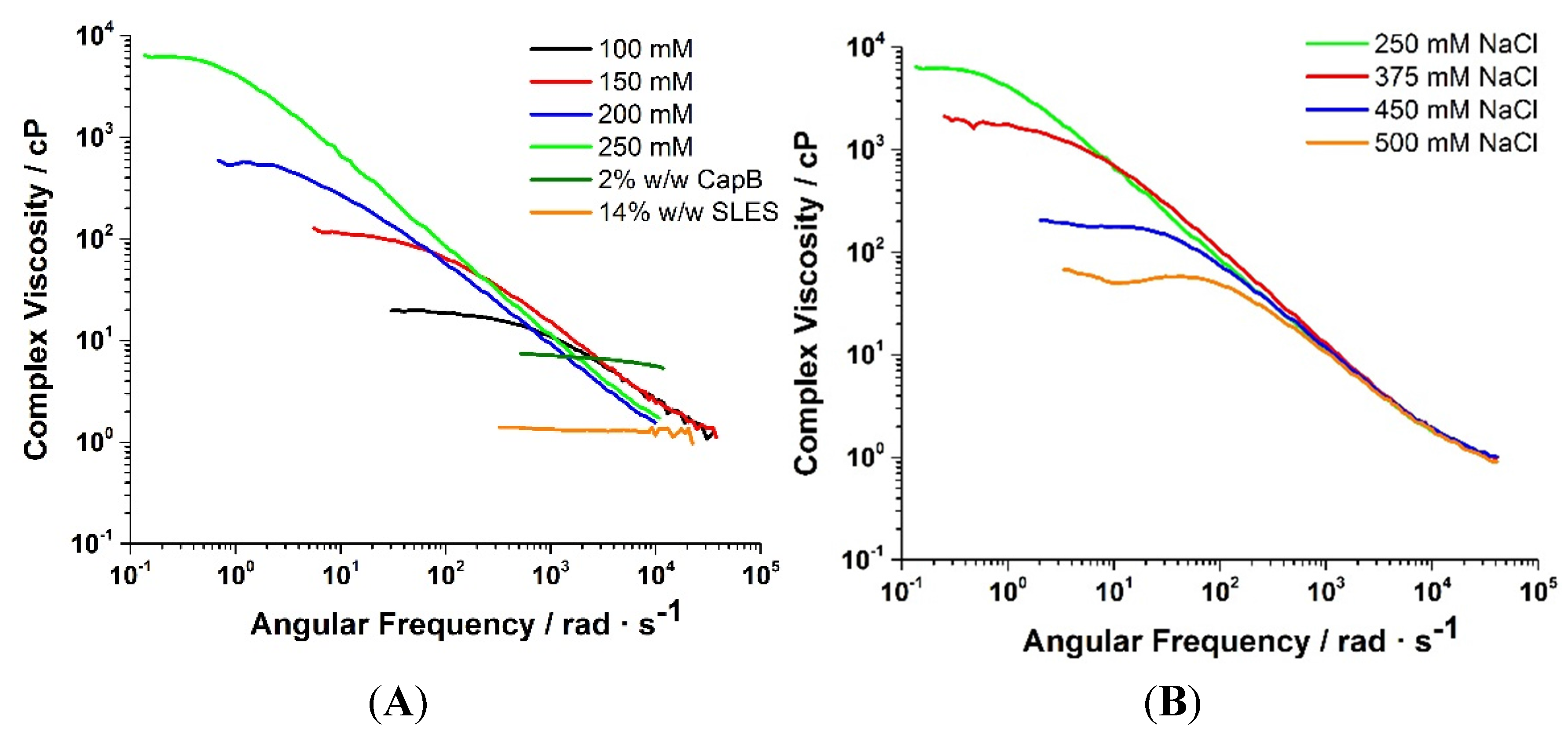
2.1. Dynamic Light Scattering
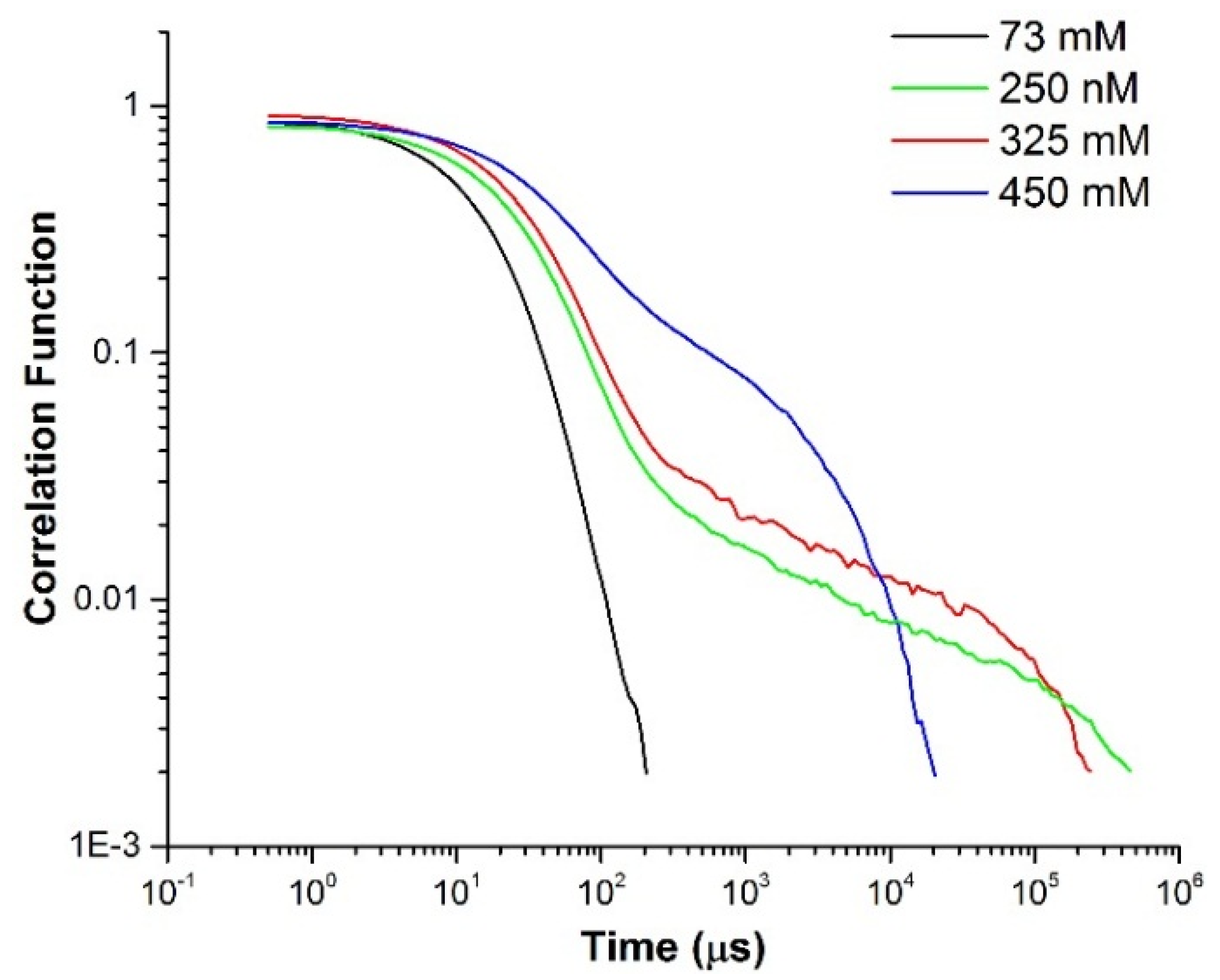
2.2. DLS-Optical Microrheology
| Samples | Diffusion Coefficient (µm2/s) |
|---|---|
| SLES (14% w/w with no NaCl) | 165 |
| CapB (2% w/w with 73 mM NaCl) | 94.1 |
| SLES/CapB (14% w/w :2% w/w with 73 mM NaCl | 46.6 |

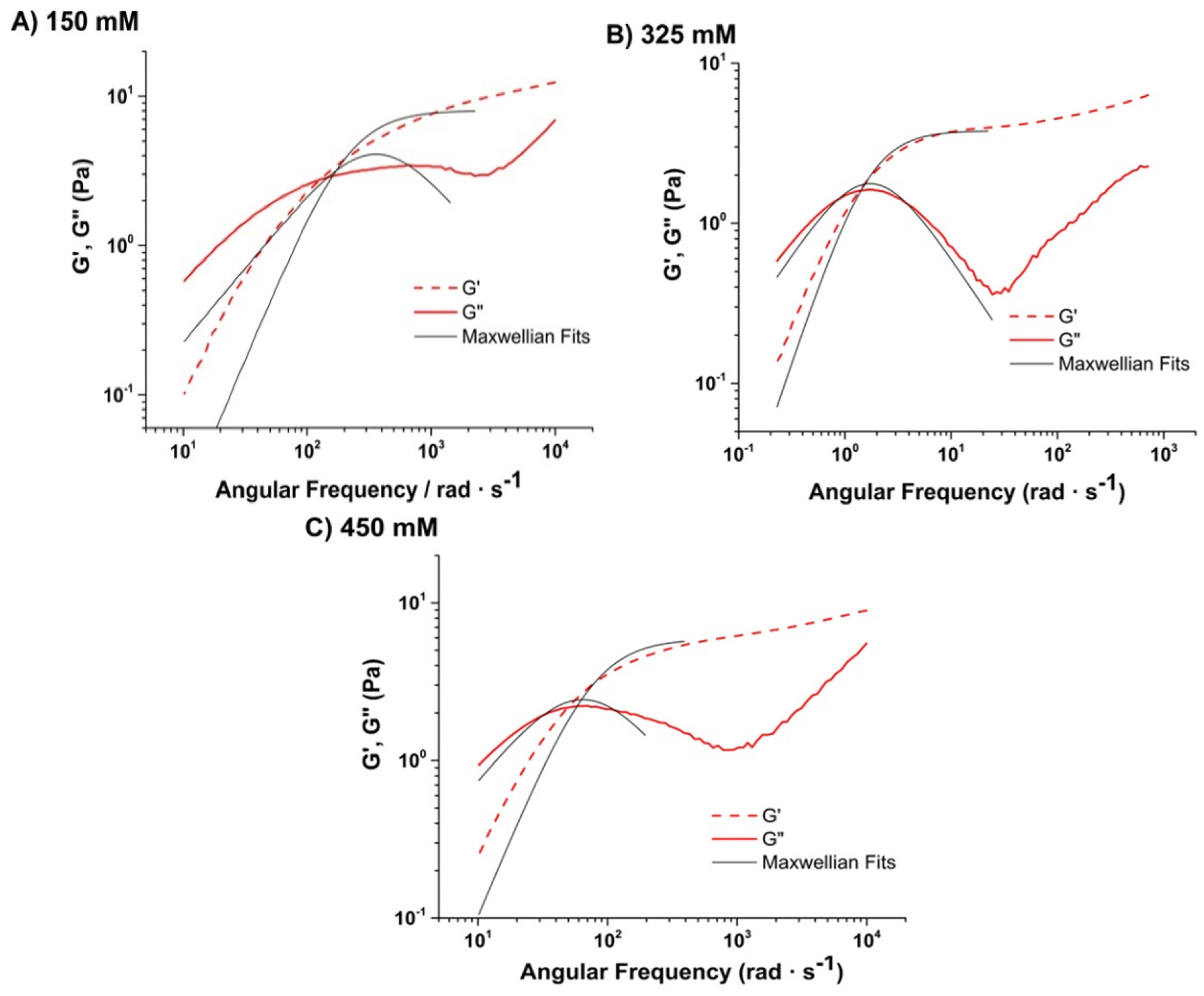
Viscoelastic Response and Evolution of Maxwellian Response
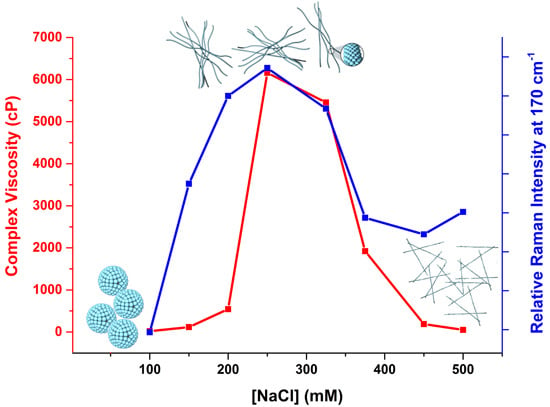
2.3. Raman Spectroscopy: Molecular Structural Changes

3. Experimental Section
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ezrahi, S.; Tuval, E.; Aserin, A. Properties, main applications and perspectives of worm micelles. Adv. Colloid Interface 2006, 128, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Zana, R.; Kaler, E.W. Giant Micelles: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Acharya, D.P.; Sharma, S.C.; Rodriguez-Abreu, C.; Aramaki, K. Viscoelastic micellar solutions in nonionic fluorinated surfactant systems. J. Phys. Chem. B 2006, 110, 20224–20234. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, C.A.; Söderman, O.; Ulvenlund, S. Aggregate morphology and flow behaviour of micellar alkylglycoside solutions. Colloid Polym. Sci. 2005, 283, 1313–1320. [Google Scholar] [CrossRef]
- Sato, T.; Acharya, D.P.; Kaneko, M.; Aramaki, K.; Singh, Y.; Ishitobi, M.; Kunieda, H. Oil-induced structural change of wormlike micelles in sugar surfactant systems. J. Dispers. Sci. Technol. 2006, 27, 611–616. [Google Scholar] [CrossRef]
- Acharya, D.P.; Sato, T.; Kaneko, M.; Singh, Y.; Kunieda, H. Effect of added poly (oxyethylene) dodecyl ether on the phase and rheological behavior of wormlike micelles in aqueous SDS solutions. J. Phys. Chem. B 2006, 110, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Bucci, S.; Fagotti, C.; Degiorgio, V.; Piazza, R. Small-angle neutron-scattering study of ionic-nonionic mixed micelles. Langmuir 1991, 7, 824–826. [Google Scholar] [CrossRef]
- Penfold, J.; Staples, E.; Tucker, I. Neutron small angle scattering studies of micellar growth in mixed anionic-nonionic surfactants, sodium dodecyl sulfate, SDS, and hexaethylene glycol monododecyl ether, C12E6, in the presence and absence of solubilized alkane, hexadecane. J. Phys. Chem. B 2002, 106, 8891–8897. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Fritz, G.; Kaler, E.W. Wormlike micelles formed by synergistic self-assembly in mixtures of anionic and cationic surfactants. Langmuir 2002, 18, 3797–3803. [Google Scholar] [CrossRef]
- Schubert, B.A.; Kaler, E.W.; Wagner, N.J. The microstructure and rheology of mixed cationic/anionic wormlike micelles. Langmuir 2003, 19, 4079–4089. [Google Scholar] [CrossRef]
- Sakai, K.; Nomura, K.; Shrestha, R.G.; Endo, T.; Sakamoto, K.; Sakai, H.; Abe, M. Effects of spacer chain length of amino acid-based gemini surfactants on wormlike micelle formation. J. Oleo Sci. 2014, 63, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Nomura, K.; Yamamoto, M.; Yamawaki, Y.; Tamura, Y.; Sakai, K.; Sakamoto, K.; Sakai, H.; Abe, M. Peptide-based gemini amphiphiles: Phase behavior and rheology of wormlike micelles. Langmuir 2012, 28, 15472–15481. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.A.; Calabrese, M.A.; Wagner, N.J. Rheology of Branched Wormlike Micelles. Curr. Opin. Colloid Interface Sci. 2014, 19, 530–535. [Google Scholar] [CrossRef]
- Mason, T.G.; Weitz, D. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys. Rev. Lett. 1995, 74. [Google Scholar] [CrossRef]
- Hassan, P.; Bhattacharya, K.; Kulshreshtha, S.; Raghavan, S. Microrheology of wormlike micellar fluids from the diffusion of colloidal probes. J. Phys. Chem. B 2005, 109, 8744–8748. [Google Scholar] [CrossRef] [PubMed]
- Martiel, I.; Sagalowicz, L.; Mezzenga, R. Viscoelasticity and interface bending properties of lecithin reverse wormlike micelles studied by diffusive wave spectroscopy in hydrophobic environment. Langmuir 2014, 30, 10751–10759. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, C.; Schopferer, M.; Scheffold, F.; Willenbacher, N. Linear-to-branched micelles transition: A rheometry and diffusing wave spectroscopy (DWS) study. Langmuir 2008, 25, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, P.; Pashkovski, E.E. Rheology of viscoelastic mixed surfactant solutions: Effect of scission on nonlinear flow and rheochaos. Langmuir 2006, 22, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Gomez, E.; Lopez-Diaz, D.; Castillo, R. Microrheology and characteristic lengths in wormlike micelles made of a zwitterionic surfactant and SDS in brine. J. Phys. Chem. B 2010, 114, 12193–12202. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, N.; Oelschlaeger, C.; Schopferer, M.; Fischer, P.; Cardinaux, F.; Scheffold, F. Broad bandwidth optical and mechanical rheometry of wormlike micelle solutions. Phys. Rev. Lett. 2007, 99. [Google Scholar] [CrossRef]
- Christov, N.; Denkov, N.; Kralchevsky, P.; Ananthapadmanabhan, K.; Lips, A. Synergistic sphere-to-rod micelle transition in mixed solutions of sodium dodecyl sulfate and cocoamidopropyl betaine. Langmuir 2004, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Kaneda, I.; Hiwatari, Y.; Masunaga, H.; Sakurai, K. Salt-concentration dependence of the structure and form factors for the wormlike micelle made from a dual surfactant in aqueous solutions. J. Appl. Crystallogr. 2007, 40, S264–S268. [Google Scholar] [CrossRef]
- Amin, S.; Kermis, T.W.; van Zanten, R.M.; Dees, S.J.; van Zanten, J.H. Concentration fluctuations in CTAB/NaSal solutions. Langmuir 2001, 17, 8055–8061. [Google Scholar] [CrossRef]
- Amin, S.; Rega, C.A.; Jankevics, H. Detection of viscoelasticity in aggregating dilute protein solutions through dynamic light scattering-based optical microrheology. Rheol. Acta 2012, 51, 329–342. [Google Scholar] [CrossRef]
- Kumar, R.; Kalur, G.C.; Ziserman, L.; Danino, D.; Raghavan, S.R. Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: from viscoelastic solutions to elastic gels. Langmuir 2007, 23, 12849–12856. [Google Scholar] [CrossRef] [PubMed]
- Cates, M.; Candau, S. Statics and dynamics of worm-like surfactant micelles. J. Phys. Condens. Matter 1990, 2, 6869–6873. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Sood, A. Effect of screening of intermicellar interactions on the linear and nonlinear rheology of a viscoelastic gel. Langmuir 2003, 19, 3121–3127. [Google Scholar] [CrossRef]
- Koehler, R.D.; Raghavan, S.R.; Kaler, E.W. Microstructure and dynamics of wormlike micellar solutions formed by mixing cationic and anionic surfactants. J. Phys. Chem. B 2000, 104, 11035–11044. [Google Scholar] [CrossRef]
- Angelico, R.; Amin, S.; Monduzzi, M.; Murgia, S.; Olsson, U.; Palazzo, G. Impact of branching on the viscoelasticity of wormlike reverse micelles. Soft Matter 2012, 8, 10941–10949. [Google Scholar] [CrossRef]
- Khatory, A.; Kern, F.; Lequeux, F.; Appell, J.; Porte, G.; Morie, N.; Ott, A.; Urbach, W. Entangled versus multiconnected network of wormlike micelles. Langmuir 1993, 9, 933–939. [Google Scholar] [CrossRef]
- Lequeux, F. Reptation of connected wormlike micelles. Europhys. Lett. 1992, 19. [Google Scholar] [CrossRef]
- Walrafen, G. Raman spectral studies of the effects of temperature on water structure. J. Chem. Phys. 1967, 47, 114–126. [Google Scholar] [CrossRef]
- Amin, S.; Blake, S.; Kenyon, S.M.; Kennel, R.C.; Lewis, E.N. A novel combination of DLS-optical microrheology and low frequency Raman Spectroscopy to reveal underlying biopolymer self-assembly and gelation mechanisms. J. Chem. Phys. 2014, 141, 234201–234205. [Google Scholar] [CrossRef] [PubMed]
- Cataliotti, R.S. On the low-frequency vibrational spectrum of water. Phil. Mag. Lett. 2009, 89, 641–648. [Google Scholar] [CrossRef]
- Guégan, R. Confinement effects on water structure in membrane lyotropic phases. J. Colloid Interface Sci. 2011, 358, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolantoni, M.; Sassi, P.; Morresi, A.; Santini, S. Hydrogen bond dynamics and water structure in glucose-water solutions by depolarized Rayleigh scattering and low-frequency Raman Spectroscopy. J. Chem. Phys. 2007, 127. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, S.E.; Nielsen, O.F. Low-frequency Raman Spectroscopy. J. Mol. Struct. 1995, 347, 267–283. [Google Scholar] [CrossRef]
- Faurskov Nielsen, O. Low-frequency spectroscopic studies and intermolecular vibrational energy transfer in liquids. J. Chem. Phys. 1996, 93, 57–99. [Google Scholar] [CrossRef]
- Tominaga, Y.; Fujiwara, A.; Amo, Y. Dynamical structure of water by Raman Spectroscopy. Fluid Phase Equilib. 1998, 144, 323–330. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, S.; Blake, S.; Kennel, R.C.; Lewis, E.N. Revealing New Structural Insights from Surfactant Micelles through DLS, Microrheology and Raman Spectroscopy. Materials 2015, 8, 3754-3766. https://doi.org/10.3390/ma8063754
Amin S, Blake S, Kennel RC, Lewis EN. Revealing New Structural Insights from Surfactant Micelles through DLS, Microrheology and Raman Spectroscopy. Materials. 2015; 8(6):3754-3766. https://doi.org/10.3390/ma8063754
Chicago/Turabian StyleAmin, Samiul, Steven Blake, Rachel C. Kennel, and E. Neil Lewis. 2015. "Revealing New Structural Insights from Surfactant Micelles through DLS, Microrheology and Raman Spectroscopy" Materials 8, no. 6: 3754-3766. https://doi.org/10.3390/ma8063754
APA StyleAmin, S., Blake, S., Kennel, R. C., & Lewis, E. N. (2015). Revealing New Structural Insights from Surfactant Micelles through DLS, Microrheology and Raman Spectroscopy. Materials, 8(6), 3754-3766. https://doi.org/10.3390/ma8063754