Synthesis and Characterization of Biopolymeric Chitosan Derived from Land Snail Shells and Its Potential for Pb2+ Removal from Aqueous Solution
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Chitosan
2.3. Characterization of the Synthesized Chitosan
2.4. Adsorption and Kinetics
2.5. Desorption Studies
3. Results and Discussion
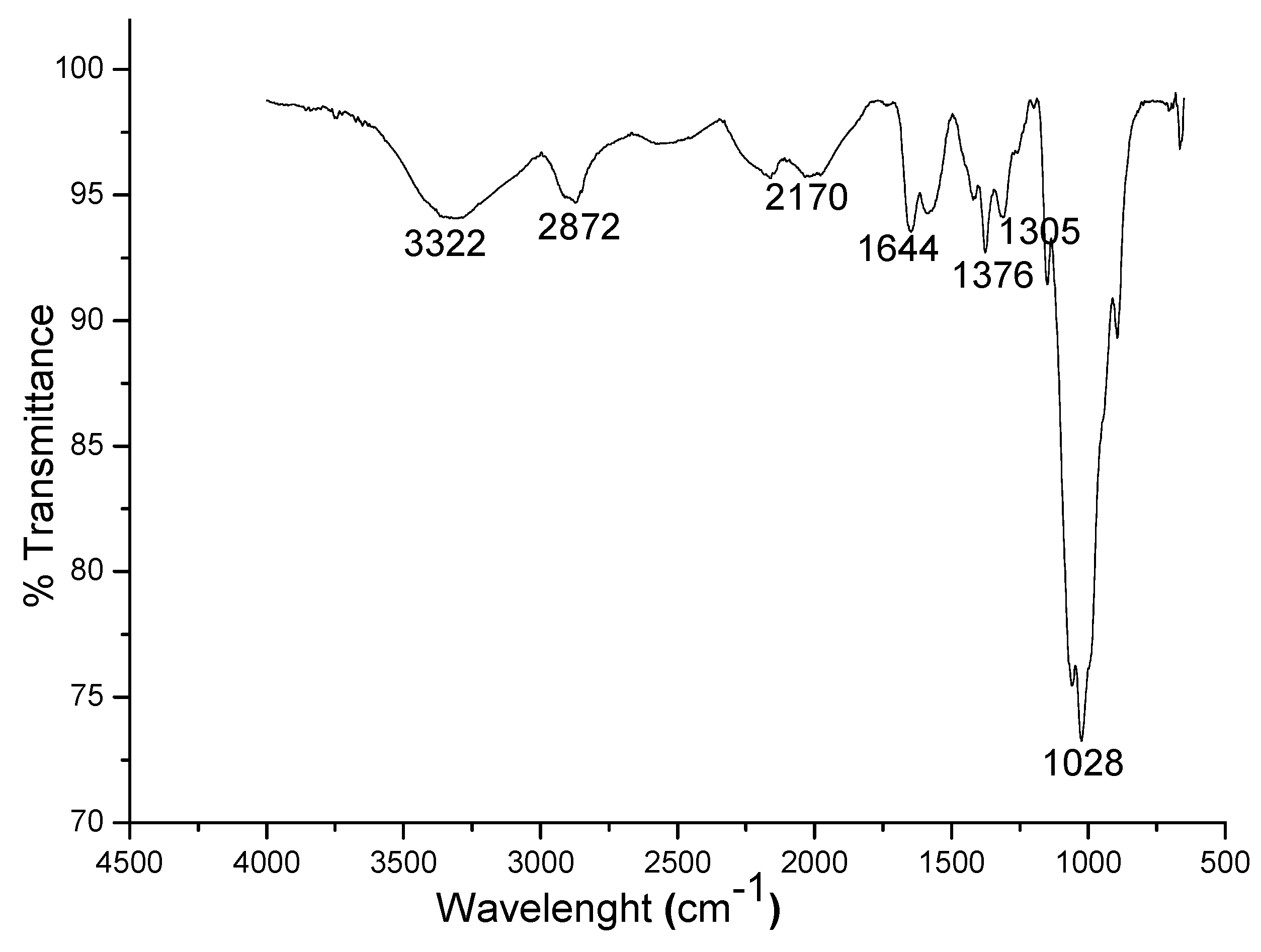
3.1. Characterization of the Synthesized Chitosan
| Component | wt % Composition |
|---|---|
| Na2O | 0.58 |
| MgO | 0.03 |
| Al2O3 | 0.15 |
| SiO2 | 0.34 |
| P2O5 | 0.07 |
| SO3 | 0.10 |
| Cl | 0.02 |
| K2O | 0.04 |
| CaO | 98.20 |
| Fe2O3 | 0.10 |
| SrO | 0.40 |


3.2. Effects of Experimental Conditions on Adsorption Efficiency


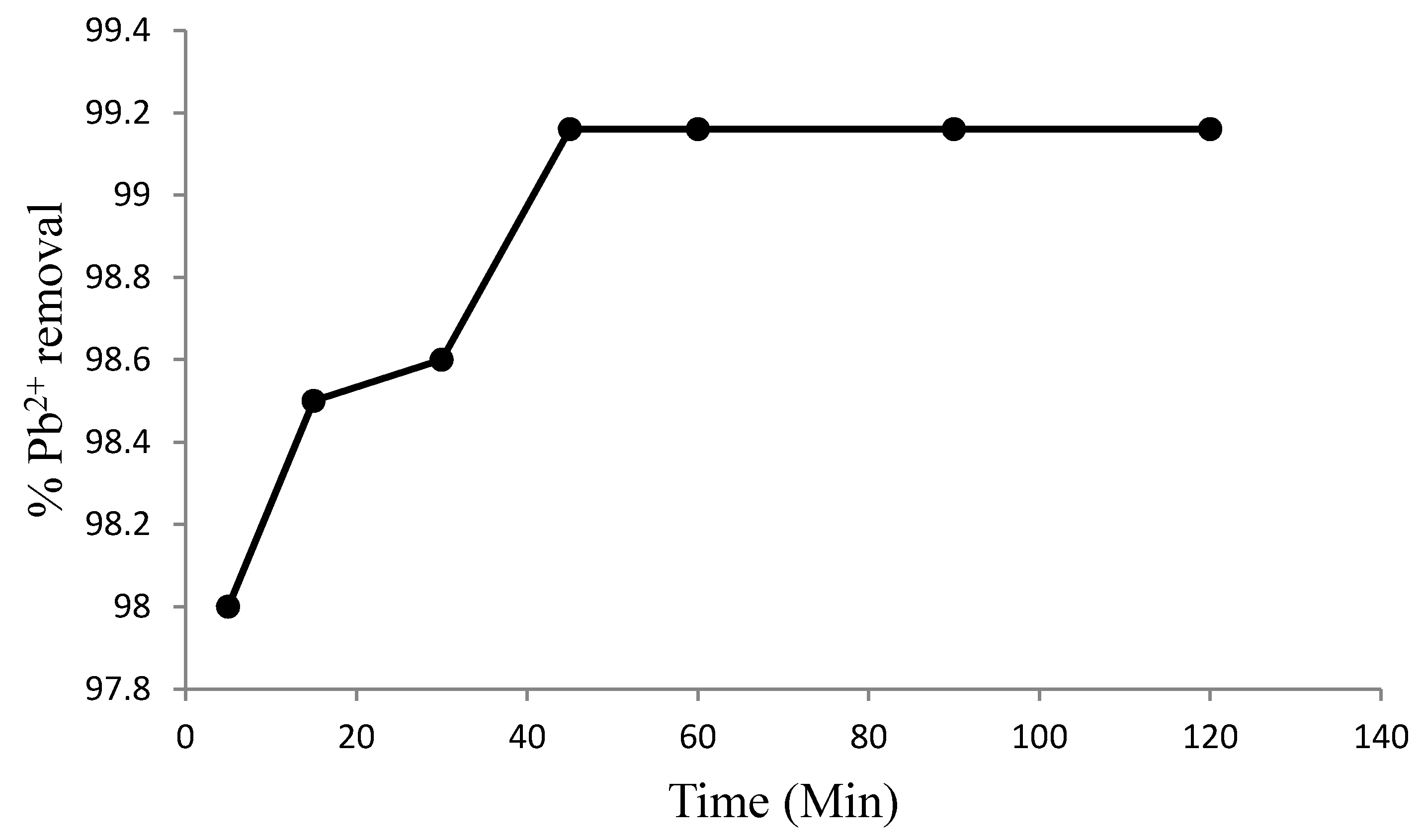
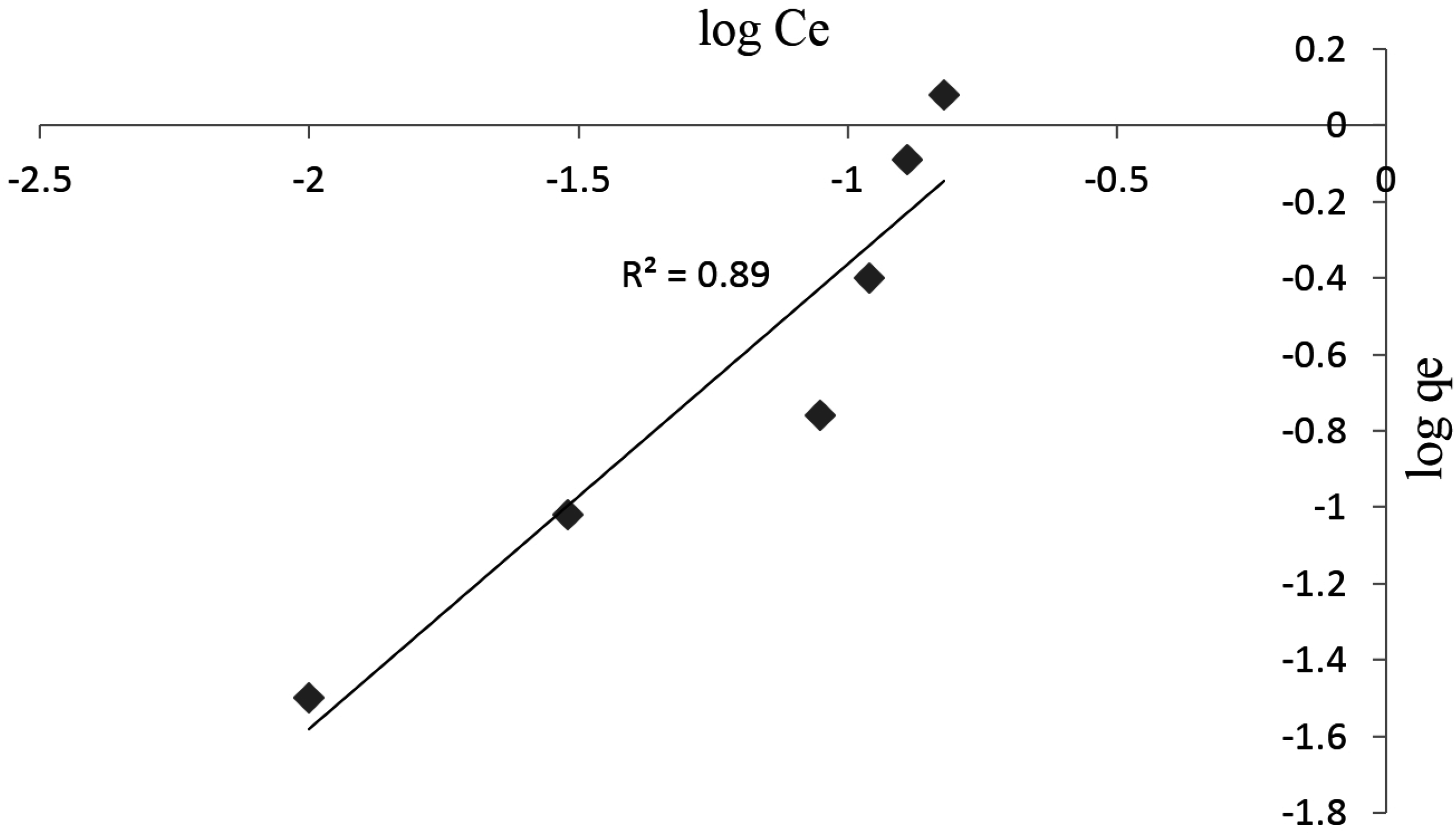
3.3. Adsorption Studies

3.4. Kinetic Studies


3.5. Desorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez-Montoya, V.; Perez-Cruz, M.A.; Mendoza-Casillo, D.I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of dyes and heavy metals on zeolitic structure. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Karthiica, P.; Vimala, R.; Vinidhini, V. Use of natural products as biosorbent of heavy metals, An overview. Nat. Prod. Radiance 2008, 7, 133–138. [Google Scholar]
- Cataldo, S.; Muratore, N.; Orecchio, S.; Pettignano, A. Enhancement of adsorption ability of calcium alginate gel beads towards Pd(II) ion. A kinetic and equilibrium study on hybrid Laponite and Montmorillonite-alginate gel beads. Appl. Clay Sci. 2015, 118, 162–170. [Google Scholar] [CrossRef]
- Koh, L.L.; Wong, M.K.; Gan, L.M. Factors affecting the leaching of lead from UPVC pipes. Environ. Monit. Asses. 1991, 19, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sublet, R.; Simonnot, M.; Boireau, A.; Sardin, M. Selection of an adsorbent for lead removal from drinking water by a point-of-use treatment device. Water Res. 2003, 37, 4904–4912. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Health. Point-of-Use Water Treatment Units for Lead Reduction. 141–0717. August 2010. Available online: http://www.health.state.mn.us/divs/eh/water/factsheet/com/poulead.html (accessed on 15 December 2014).
- Edokpayi, J.N.; Odiyo, J.O.; Olasoji, S.O. Assessment of heavy metal contamination of Dzindi River, in Limpopo Province, South Africa. Int. J. Nat. Sci. Res. 2014, 2, 185–194. [Google Scholar]
- Zvinowanda, C.M.; Okonkwo, J.O.; Agyei, N.M.; Shabalala, P.N. Physicochemical characterization of maize tassel as an adsorbent. I. surface texture, microstructure and thermal stability. J. Appl. Polym. Sci. 2009, 111, 1923–1930. [Google Scholar] [CrossRef]
- Mihajlovic, M.T.; Lazarevic, S.S.; Jankovic-Castvan, I.M.; Kovac, J.; Jokic, B.M.; Janackovic, D.T.; Petrovic, R.D. Kinetics, thermodynamics, and structural investigations on the removal of Pb2+, Cd2+, and Zn2+ from multicomponent solutions onto natural and Fe(III)-modified zeolites. Clean Technol. Environ. Policy 2015, 14, 407–419. [Google Scholar] [CrossRef]
- Rorrer, G. Heavy metal ions. In Removal from Wastewater: Encyclopaedia of Environmental Analysis and Remediation; Meyers, R.A., Ed.; Wiley: New York, NY, USA, 1998; Volume 4, pp. 2101–2125. [Google Scholar]
- Johnson, P.D.; Padmanabhan, G.P.; Ohlinger, K.N.; Ritchie, S.; Teuber, L.; Kirby, J. Enhanced removal of heavy metals in primary treatment using coagulation and flocculation. Water Environ. Res. 2008, 80, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.F. Standard Handbook of Hazardous Waste Treatment and Disposal; McGraw-Hill: New York, NY, USA, 1989. [Google Scholar]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Technologies; Butterworth-Heinemann: Woburn, MA, USA, 2002. [Google Scholar]
- Geselbarcht, J. Micro filtration/reverse osmosis pilot trials for Livermore, California, advanced water reclamation. In Proceedings of the AWWA 1996 Water reuse conference, San Diego, CA, USA, 25–28 February 1996; p. 187.
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Sewvandi, G.A.; Adikary, S.U. Removal of Heavy Metals from Wastewater Using Chitosan. Society for Social Management Systems Internet Journal. 2011. Available online: http://hdl.handle.net/10173/836 (accessed on 18 December 2014).
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. 3 Biotech. 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Jatto, E.O.; Asia, I.O.; Egbon, E.E.; Otutu, J.O.; Chukwuedo, M.E.; Ewansiha, C.J. Treatment of waste water from food industry using snail shell. Acaedmia Arena 2010, 2, 32–36. [Google Scholar]
- Coughlin, R.W.; Deshaies, M.R.; Davis, E.M. Preparation of chitosan for heavy metal removal. Environ. Prog. 1990, 9, 35–39. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. APS PharmSciTech 2006, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Anis, K.P.A.; Banthia, A.K. Development and characterization of chitosan based polymeric hydrogel membranes. Des. Monomers Polym. 2010, 13, 93–206. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Özbay, İ.; Özdemir, U.; Özbay, B.; Veli, S. Kinetic, thermodynamic, and equilibrium studies for adsorption of azo reactive dye onto a novel waste adsorbent: Charcoal ash. Desalin. Water Treat. 2013, 51, 6091–6100. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Msagati, T.A.M.; Popoola, E.O. A Novel Approach for the removal of lead(II) ion from wastewater using mucilaginous leaves of diceriocaryum eriocarpum plant. Sustainability 2015, 7, 14026–14041. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Jyothi, R.K.; Chang, Y.; Yang, J. Factors affect on bioremediation of Co(II) and Pb(II) onto Lonicera japonica flowers powder. Desalin. Water Treat. 2015. [Google Scholar] [CrossRef]
- Koduru, J.R.; Chang, Y.; Yang, J.; Kim, I. Iron oxide impregnated morus alba l. fruit peel for biosorption of Co(II): Biosorption properties and mechanism. Sci. World J. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dambies, L.; Guimon, C.; Yiacoumi, S.; Guiba, E. Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Coll. Surf. A Physicochem. Eng. Asp. 2001, 177, 203–214. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Puri. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Hikmat, N.A.; Qassim, B.B.; Khethi, M.T. Thermodynamic and kinetic studies of lead adsorption from aqueous solution onto petiole and fibre of palm tree. Am. J. Chem. 2014, 4, 116–124. [Google Scholar]
- Raya, I.; Zakir, M. The adsorption of Pb (II) ions on activated carbon from rice husk, irradiated by ultrasonic waves: Kinetic and thermodynamics studies. J. Nat. Sci. Res. 2014, 4, 18–24. [Google Scholar]
- Tahiruddin, N.S.M.; Ab Rahman, S.Z. Adsorption of lead in aqueous solution by a mixture of activated charcoal and peanut shell. World J. Sci. Technol. Res. 2013, 1, 102–109. [Google Scholar]
- Kannamba, B.; Reddy, K.L.; AppaRao, B.V. Removal of Cu (II) from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.B.; Vieira, R.S.; Luna, F.M.T.; Guibal, E.; Beppu, M.M. Adsorption of copper(II) and mercury(II) ions onto chemically-modified chitosan membranes: Equilibrium and kinetic properties. Adsorpt. Sci. Technol. 2012, 30, 1–12. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R.; Laskar, M.A. Adsorptive removal of Pb2+ form aqueous solution by macrocyclic calyx[4]naphthalene: Kinetic, thermodynamic, and isotherm analysis. Environ. Sci. Pollut. Res. 2013, 20, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V K.; Ali, I. Removal of endosulfan and methoxychlor from water on carbon slurry. Environ. Sci. Technol. 2008, 42, 766–770. [Google Scholar]
- Gupta, V.K.; Gupta, M.; Shamar, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminium industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Asandei, D.; Bulgariu, L.; Bobu, E. Lead(II) removal from aqueous solutions by adsorption onto chitosan. Cellul. Chem. Technol. 2009, 43, 211–216. [Google Scholar]
- Ng, J.C.Y.; Cheung, W.H.; Mckay, G. Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J. Colloid Interface Sci. 2002, 255, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Chikazaza, L. Bioremediation of lead(II) from polluted wastewaters employing sulphuric acid treated maize tassel biomass. Am. J. Anal. Chem. 2013, 4, 689–695. [Google Scholar] [CrossRef]
- Chen, D.; Hu, B.; He, M.; Haung, C. Micro-column preconcentration/separation using thiacalix[4]arene tetracarboxylate derivative modified mesoporous TiO2 as packing materials on-line coupled to inductively coupled plasma optical emission spectrometry for the determination of trace heavy metals in environmental water samples. Microchem. J. 2010, 95, 90–95. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edokpayi, J.N.; Odiyo, J.O.; Popoola, E.O.; Alayande, O.S.; Msagati, T.A.M. Synthesis and Characterization of Biopolymeric Chitosan Derived from Land Snail Shells and Its Potential for Pb2+ Removal from Aqueous Solution. Materials 2015, 8, 8630-8640. https://doi.org/10.3390/ma8125482
Edokpayi JN, Odiyo JO, Popoola EO, Alayande OS, Msagati TAM. Synthesis and Characterization of Biopolymeric Chitosan Derived from Land Snail Shells and Its Potential for Pb2+ Removal from Aqueous Solution. Materials. 2015; 8(12):8630-8640. https://doi.org/10.3390/ma8125482
Chicago/Turabian StyleEdokpayi, Joshua N., John O. Odiyo, Elizabeth O. Popoola, Oluwagbemiga S. Alayande, and Titus A. M. Msagati. 2015. "Synthesis and Characterization of Biopolymeric Chitosan Derived from Land Snail Shells and Its Potential for Pb2+ Removal from Aqueous Solution" Materials 8, no. 12: 8630-8640. https://doi.org/10.3390/ma8125482






