Prediction Surface Morphology of Nanostructure Fabricated by Nano-Oxidation Technology
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Nano-Oxidation Experiments
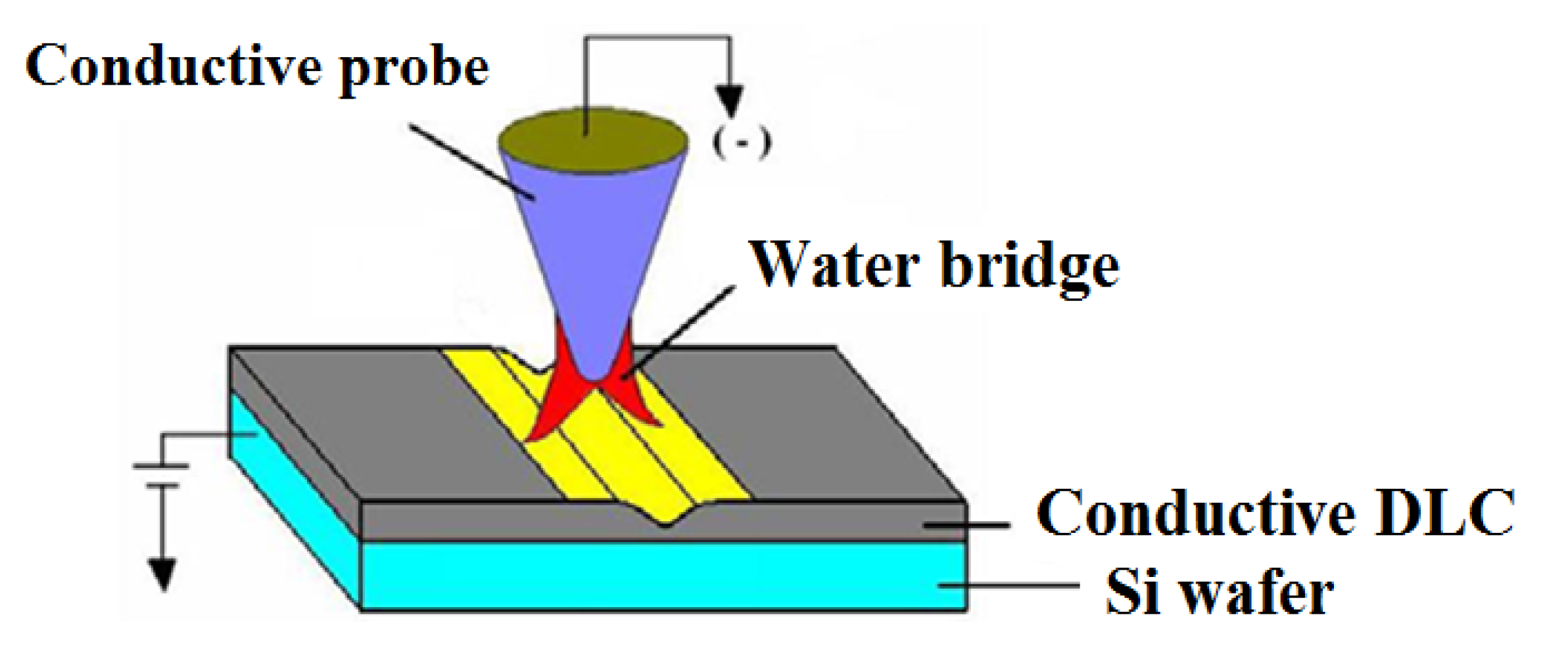
3. Establishment of Prediction Model
| No. | Independent Variable |
|---|---|
| Database 1 | v, t |
| Database 2 | v, t, v × t |
| Database 3 | v, t, v × v |
| Database 4 | v, t, t × t |
| Database 5 | v, t , v × t, v × v, t × t |
| No. | Independent Variable |
|---|---|
| Database 1 | v, s |
| Database 2 | v, s, v × s |
| Database 3 | v, s, v × v |
| Database 4 | v, t, s × s |
| Database 5 | v, s, v × s, v × v, s × s |
4. Results and Discussion
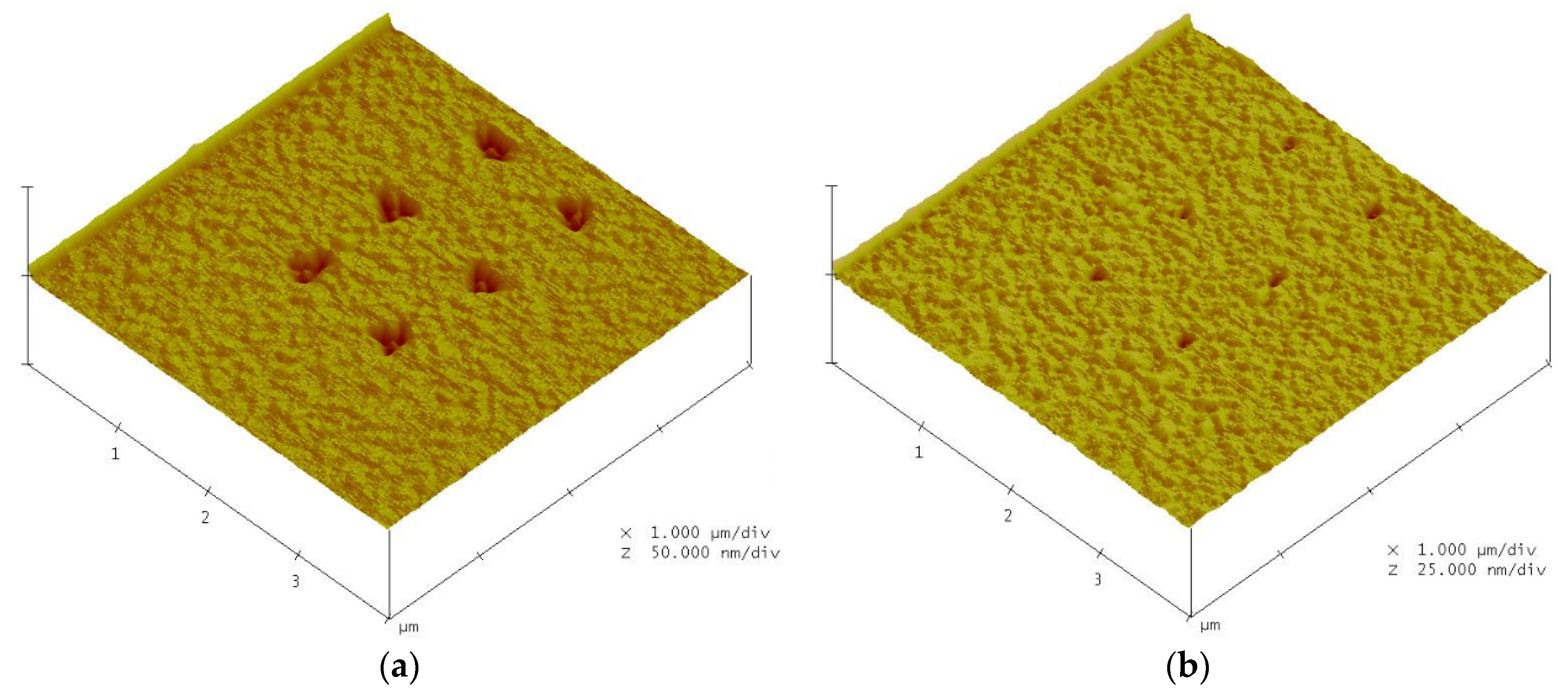
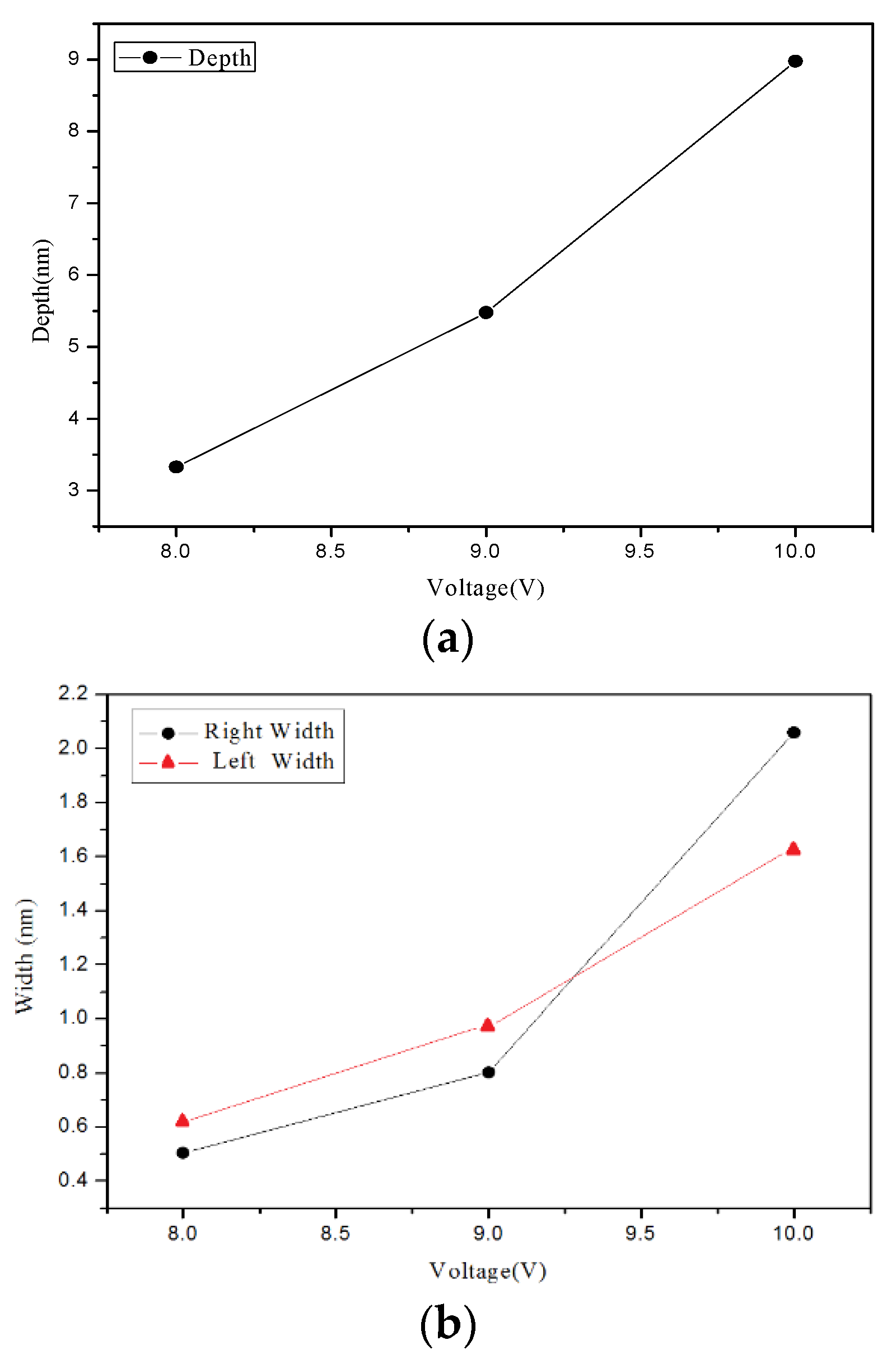
4.1. Nano-Oxidation Points

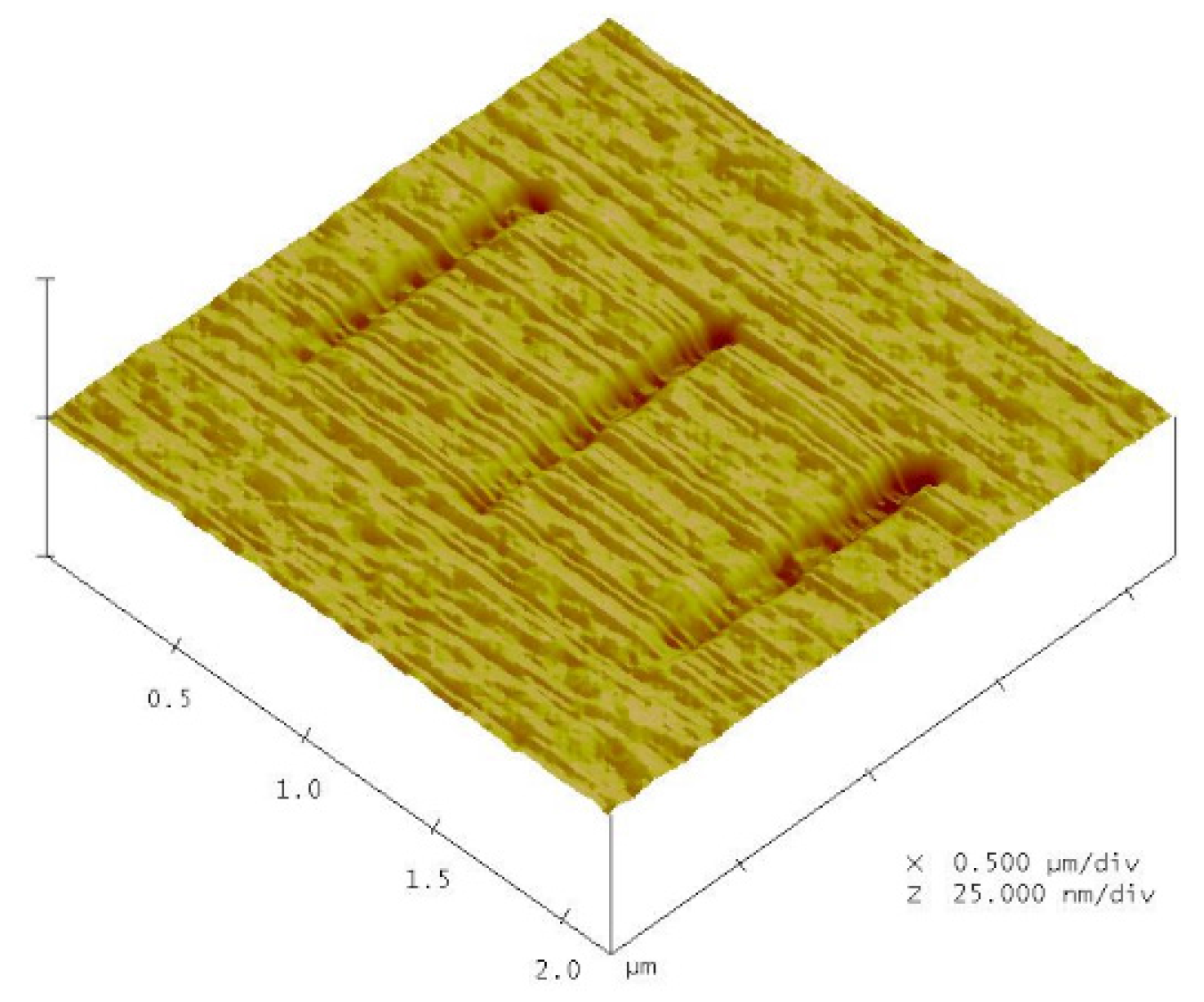
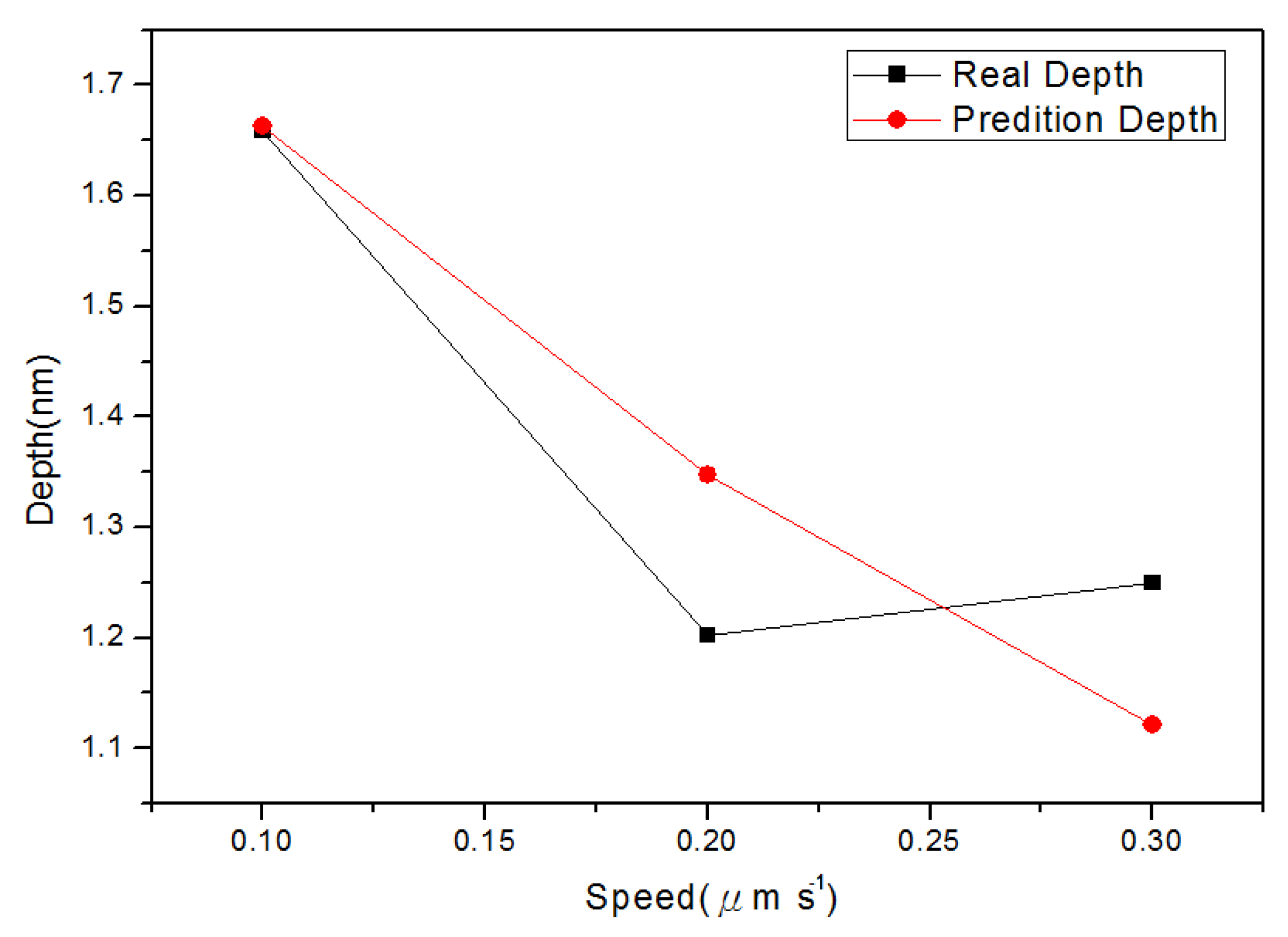
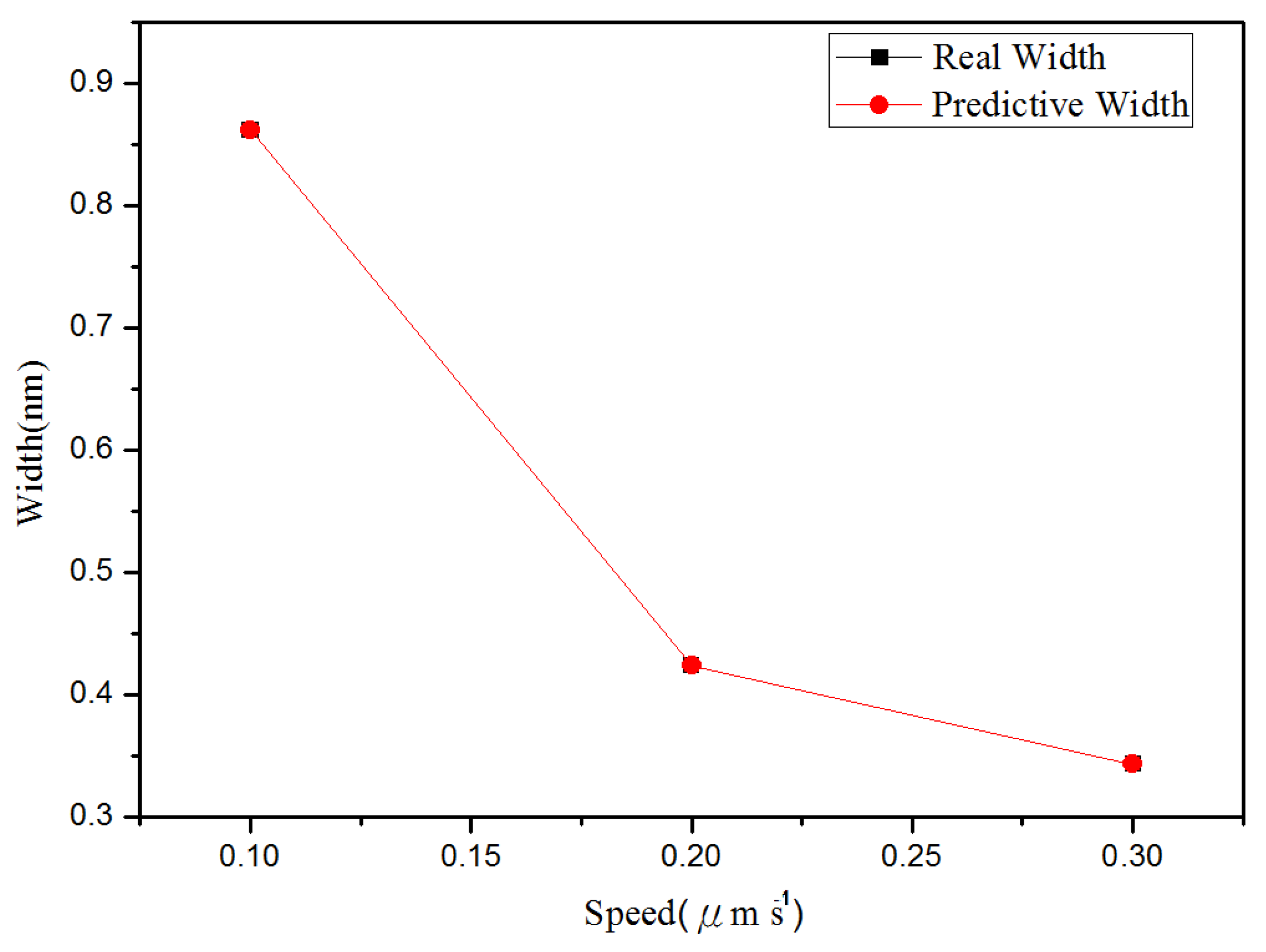
4.2. Nano-Oxidation Lines
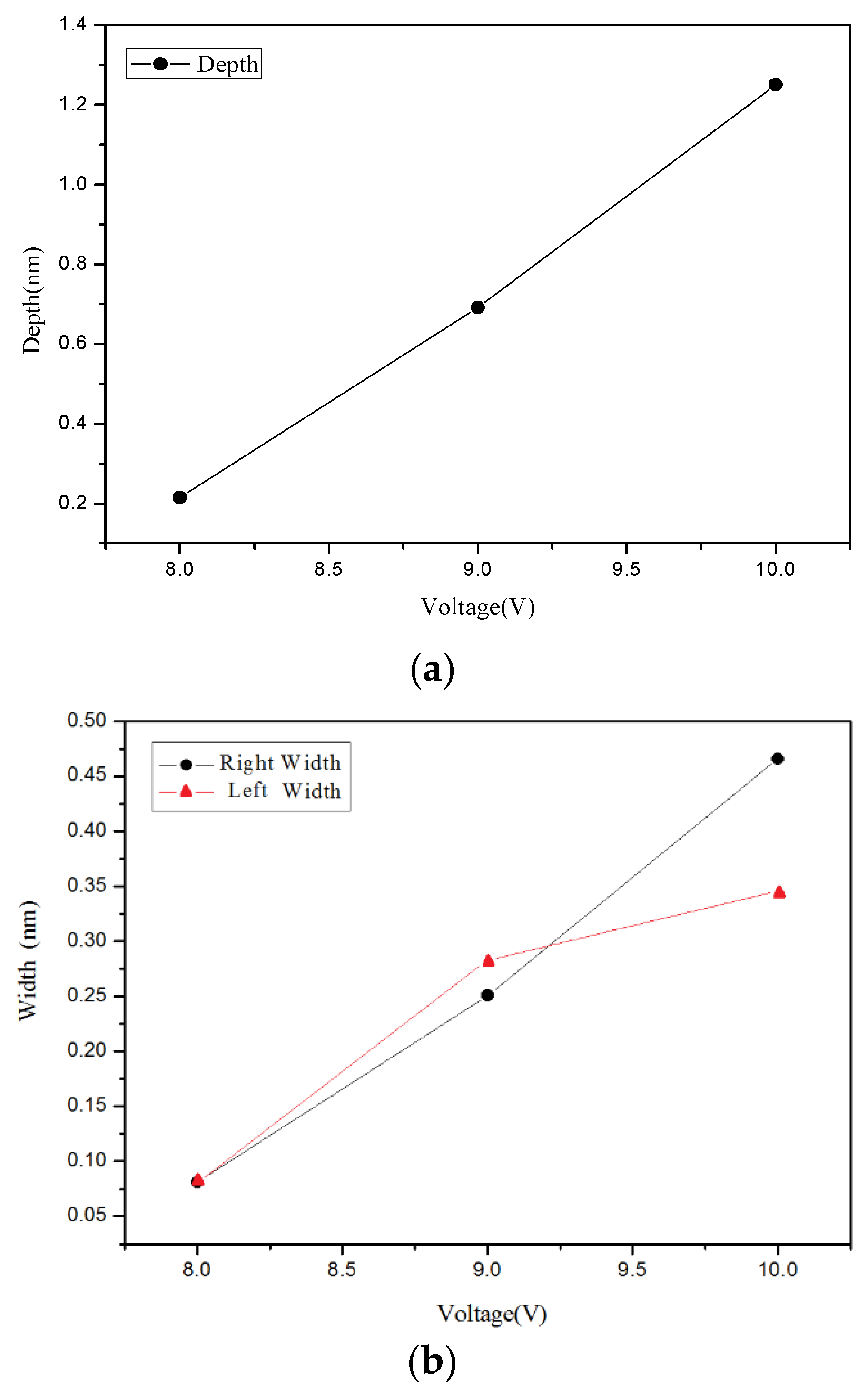

4.3. The Performance of Different Prediction Model






| Machined Morphology | POINT (%) | LINE (%) | |||||
|---|---|---|---|---|---|---|---|
| Prediction Model | Depth | Left | Right | Depth | Left | Right | |
| MRA | 16.18 | 20.94 | 18.96 | 14.22 | 18.96 | 19.6 | |
| BPN | 8.02 | 9.68 | 7.34 | 4.96 | 8.09 | 6.77 | |
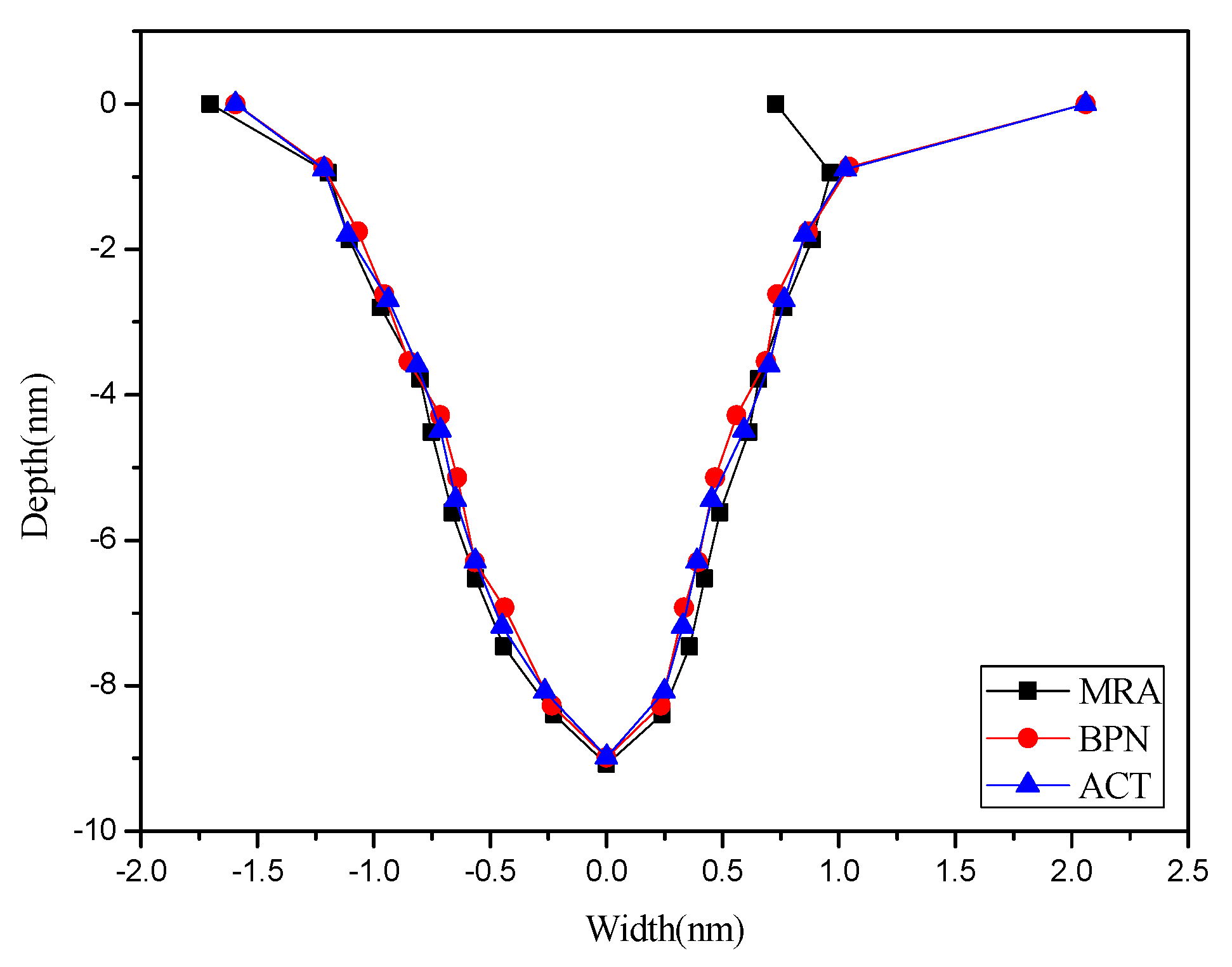
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, X.N.; Chung, H.J.; Sow, C.H.; Wee, A.T.S. Nanoscale materials patterning and engineering by atomic force microscopy nanolithography. Mater. Sci. Eng. R Rep. 2006, 54, 1–48. [Google Scholar] [CrossRef]
- Binning, G.; Quate, C.F. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Lee, J.W.; Li, C.L. Nano-scratching and nano-machining in different environments on Cr2N/Cu multilayer thin films. Thin Solid Films 2011, 519, 4992–4996. [Google Scholar] [CrossRef]
- Huang, J.C.; Weng, Y.J.; Lee, J.W. Evaluation of the nanofabrication and corrosion on copper by in situ ECAFM. J. Chin. Soc. Mech. Eng. 2011, 32, 61–66. [Google Scholar]
- Huang, J.C.; Li, C.L.; Lee, J.W. The study of nanoscratch and nanomachining on hard multilayer thin films using atomic force microscope. Scanning 2012, 34, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Weng, Y.J. The study on the nanomachining property and cutting model of single-crystal sapphire by atomic force microscopy. Scanning 2014, 36, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Weng, Y.J.; Liu, H.S. A study of the nanomachining and nanopatterning on different materials using atomic force microscopy. Int. J. Mater. Prod. Technol. 2014, 49, 57–71. [Google Scholar] [CrossRef]
- Huang, J.C.; Chen, C.M. High voltage nano-oxidation in deionized water and atmospheric environments by Atomic Force Microscopy. Scanning 2012, 34, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Weng, Y.J. A study on the construction of nano-oxidation process parameter prediction model by combining a processing database and multiple regression equation. Microsys. Technol. 2014, 20, 367–378. [Google Scholar] [CrossRef]
- Huang, J.C.; Liu, H.S.; Xue, C.S.; Zhang, G.J.; Zhan, X.M. The study on the fabrication of nanostructure and nanomold by nano-oxidation and hydrofluoric acid etching on TiAlN thin film. J. Chin. Soc. Mech. Eng. 2013, 34, 353–359. [Google Scholar]
- Huang, J.C.; Chen, C.M. The study on the atomic force microscopy base nanoscale electrical discharge machining. Scanning 2012, 34, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.B.; Chiang, H.L.; Huang, J.C. Study on advanced nano-scale near field photolithography. Scanning 2010, 32, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Shirakashi, J.I.; Jou, S.; Huang, J.C.; Chen, T.P. Scratch properties of nickel thin films using atomic force microscopy. J. Vac. Sci. Technol. B 2010, 28, 202–210. [Google Scholar] [CrossRef]
- Hsu, J.H.; Lin, C.Y.; Lin, H.N. Fabrication of metallic nanostructures by atomic force microscopy nanomachining and lift-off process. J. Vac. Sci. Technol. B 2004, 22, 2768–2771. [Google Scholar] [CrossRef]
- Chen, J.M.; Liao, S.W.; Tsai, Y.C. Electrochemical synthesis of polypyrrole within PMMA nanochannels produced by AFM mechanical lithography. Synth. Metals 2005, 155, 11–17. [Google Scholar] [CrossRef]
- Garcia, R.; Martinez, R.V.; Martinez, J. Nano-chemistry and scanning probe nanolithographies. Chem. Soc. Rev. 2006, 35, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Snow, E.S.; Campbell, P.M. Fabrication of Si nanostructures with an atomic force microscope. Appl. Phys. Lett. 1994, 64, 1932–1934. [Google Scholar] [CrossRef]
- Huang, J.C.; Weng, Y.J.; Yang, S.Y.; Weng, Y.C.; Wang, J.Y. Fabricating nanostructure by atomic force microscopy. Jpn. J. Appl. Phys. 2009, 48. [Google Scholar] [CrossRef]
- Huang, J.C.; Tsai, C.L.; Tseng, A.A. The influence of the bias type, doping condition and pattern geometry on AFM tip-induced local oxidation. J. Chin. Inst. Eng. 2010, 33, 55–61. [Google Scholar] [CrossRef]
- Huang, J.C.; Wang, J.Y. The fabrication and preservation of nanostructures on silicon wafers with a native oxide layer. Scanning 2012, 34, 347–356. [Google Scholar] [PubMed]
- Huang, J.C. Fabricating nanostructures through a combination of nano-oxidation and wet etching on the different surface conditions of silicon wafer. Scanning 2012, 34, 264–270. [Google Scholar] [PubMed]
- Watson, G.S.; Myhra, S.; Watson, J.A. Parallel and serial generated patterned DLC surfaces for creating three-dimensional polymeric stamp structures. Surf. Sci. 2011, 605, 989–993. [Google Scholar]
- Crossley, A.; Johnston, C.; Watson, G.S.; Myhra, S. Tribology of diamond-like carbon films from generic fabrication routes investigated by lateral force microscopy. J. Phys. D Appl. Phys. 1998, 31, 1955–1962. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, S. Use of a neural network to link AFM etch patterns to plasma parameters. Vacuum 2007, 82, 78–83. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Garmarudi, A.B.; Khoddami, N.; Shabani, K.; Khanlari, M. A novel technique based on diffuse reflectance near-infrared spectrometry and back-propagation artificial neural network for estimation of particle size in TiO2 nano particle samples. Microchem. J. 2010, 95, 337–340. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-C.; Chang, H.; Kuo, C.-G.; Li, J.-F.; You, Y.-C. Prediction Surface Morphology of Nanostructure Fabricated by Nano-Oxidation Technology. Materials 2015, 8, 8437-8451. https://doi.org/10.3390/ma8125468
Huang J-C, Chang H, Kuo C-G, Li J-F, You Y-C. Prediction Surface Morphology of Nanostructure Fabricated by Nano-Oxidation Technology. Materials. 2015; 8(12):8437-8451. https://doi.org/10.3390/ma8125468
Chicago/Turabian StyleHuang, Jen-Ching, Ho Chang, Chin-Guo Kuo, Jeen-Fong Li, and Yong-Chin You. 2015. "Prediction Surface Morphology of Nanostructure Fabricated by Nano-Oxidation Technology" Materials 8, no. 12: 8437-8451. https://doi.org/10.3390/ma8125468





