Formation of Micro- and Nanostructures on the Nanotitanium Surface by Chemical Etching and Deposition of Titania Films by Atomic Layer Deposition (ALD)
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of Micro- and Nanoscale Structures on the Nanotitanium Surface by Controlled Liquid Phase Chemical Etching
2.2. Synthesis of Titanium Oxide Nanostructures by ALD on the Surface of Chemically Etched Nanotitanium
2.3. The Study of Morphology, Relief, Structure and Composition
2.4. Adhesive and Spreading Properties of the Human Osteoblasts MG-63 on the Titania Nanostructured Surface
3. Results and Discussion
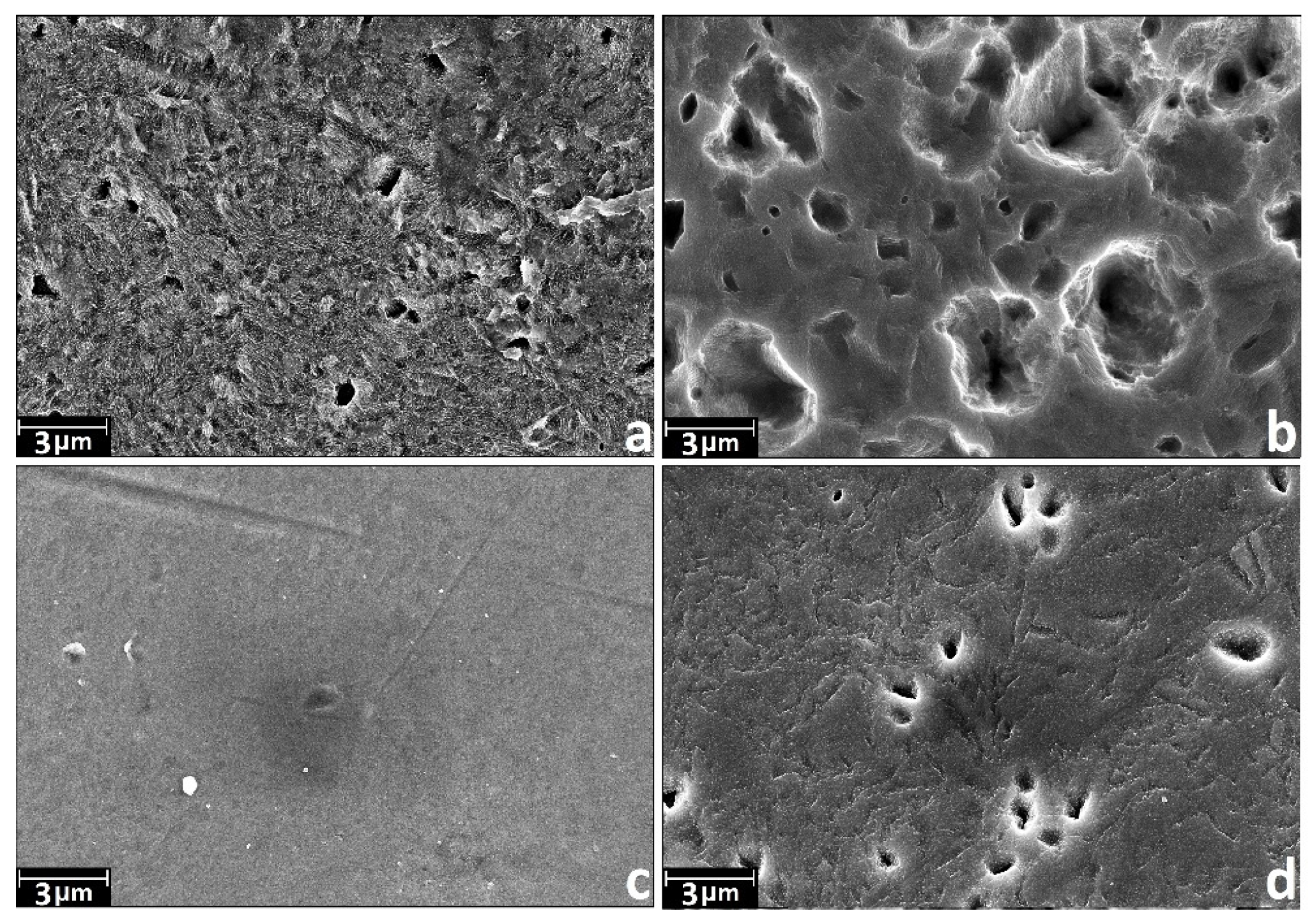
3.1. Chemical Etching, Composition, Morphology and Surface Relief

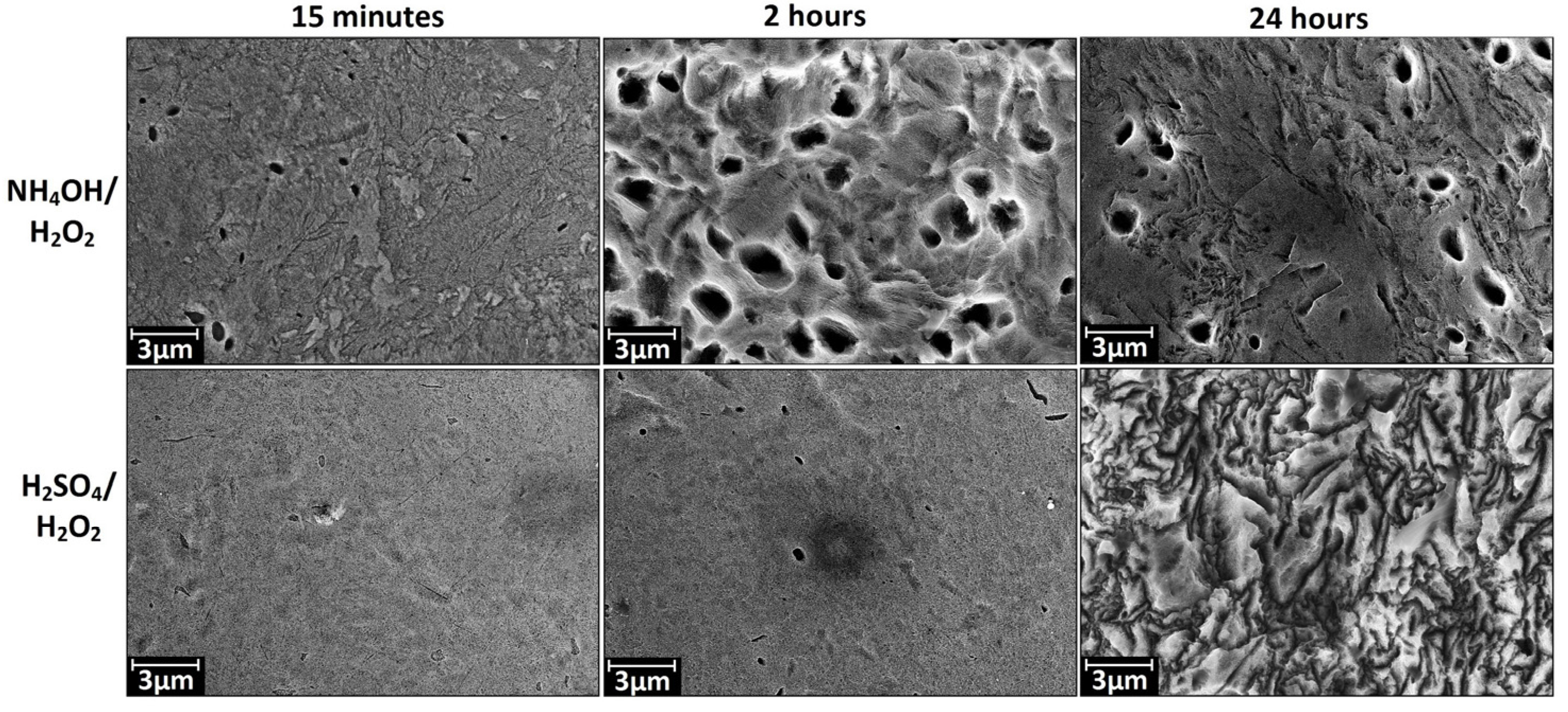
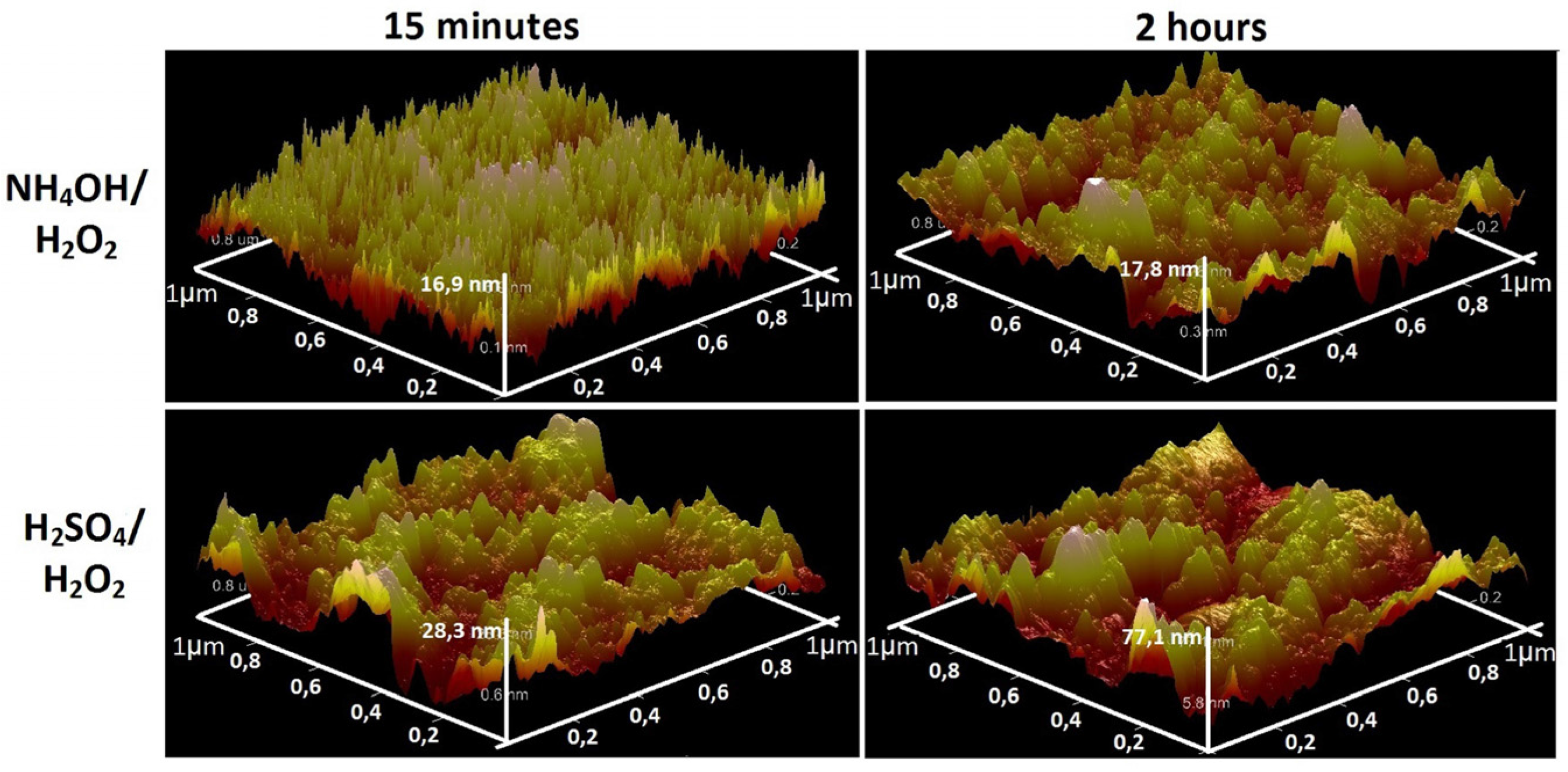
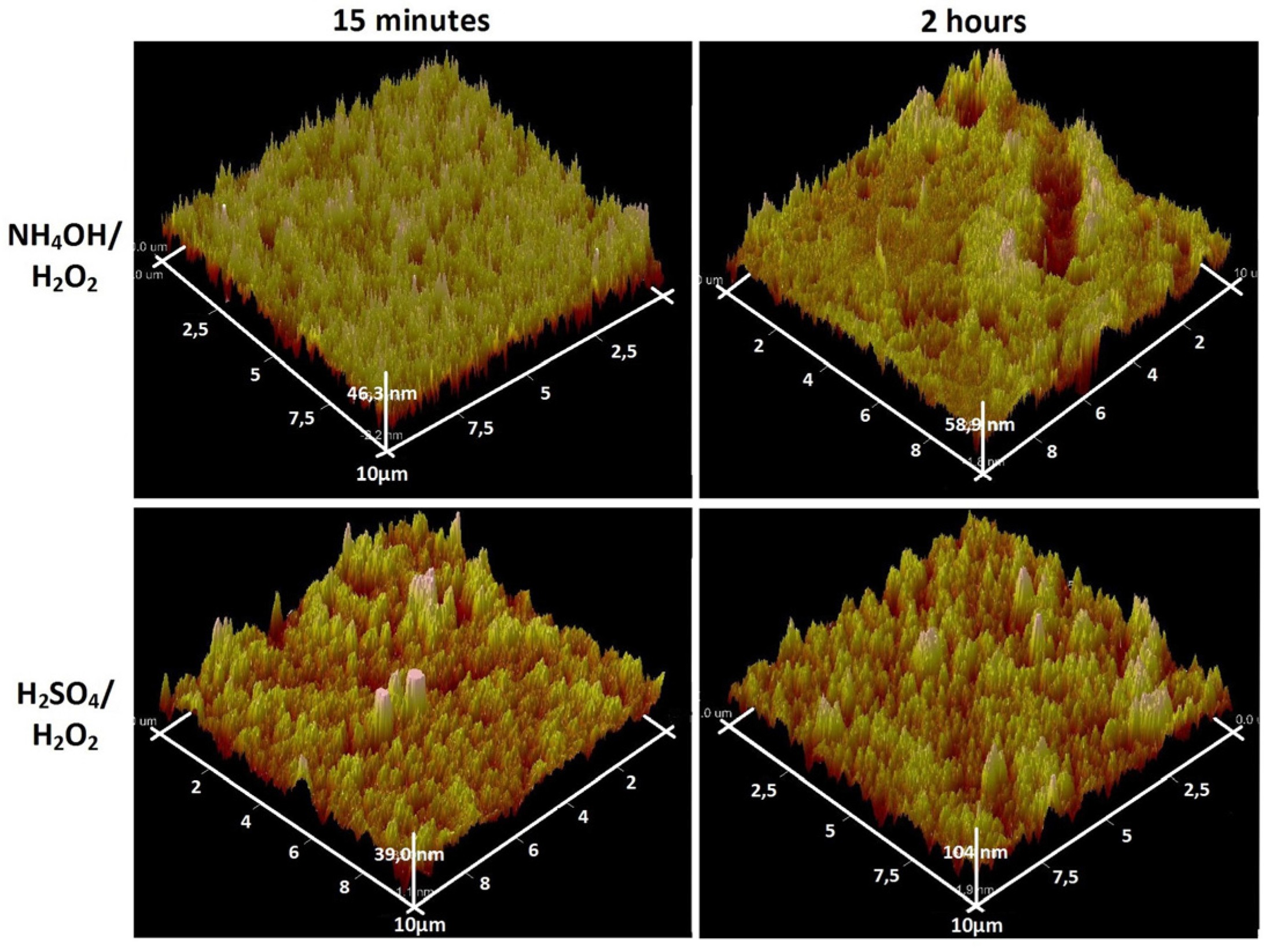

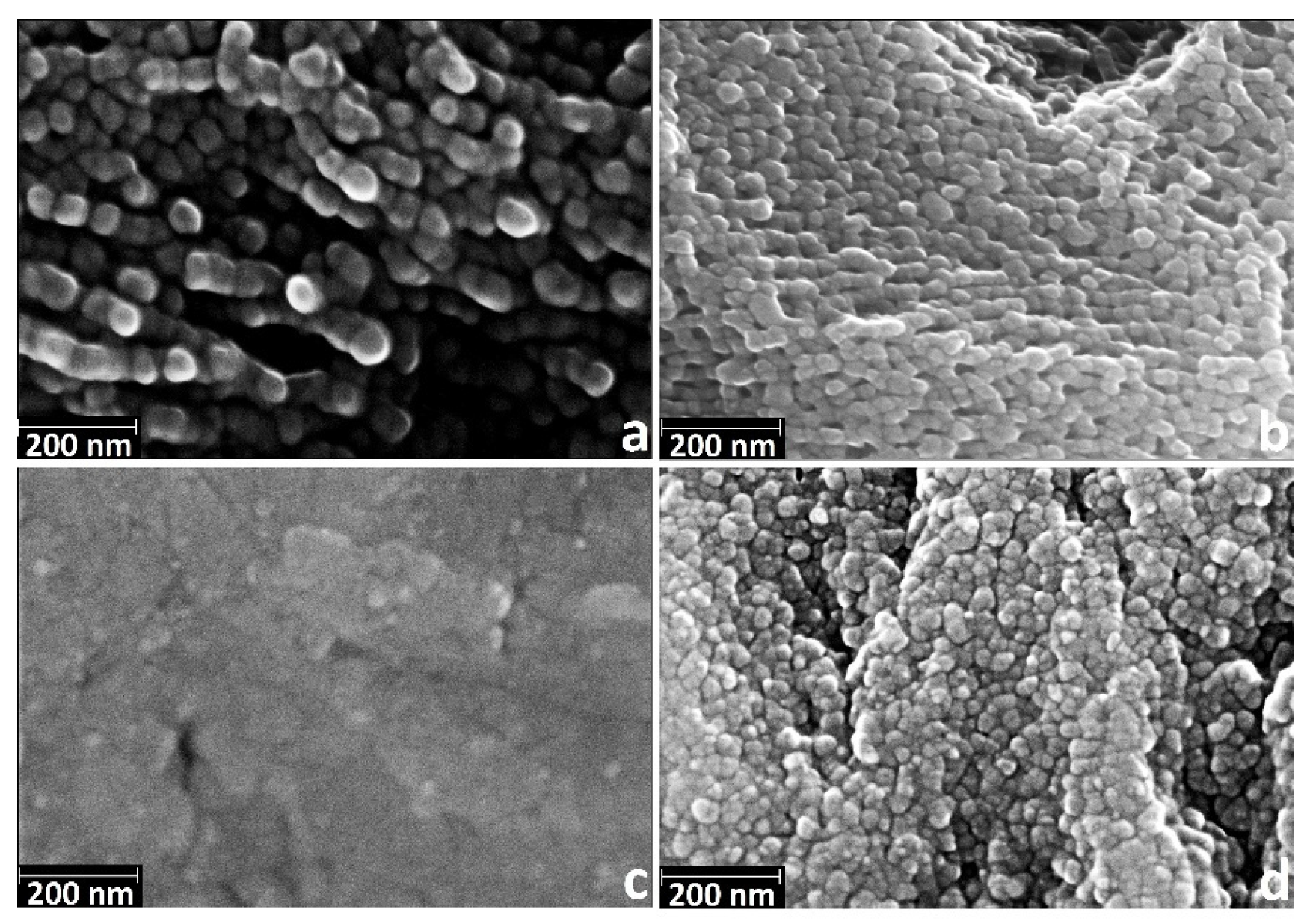
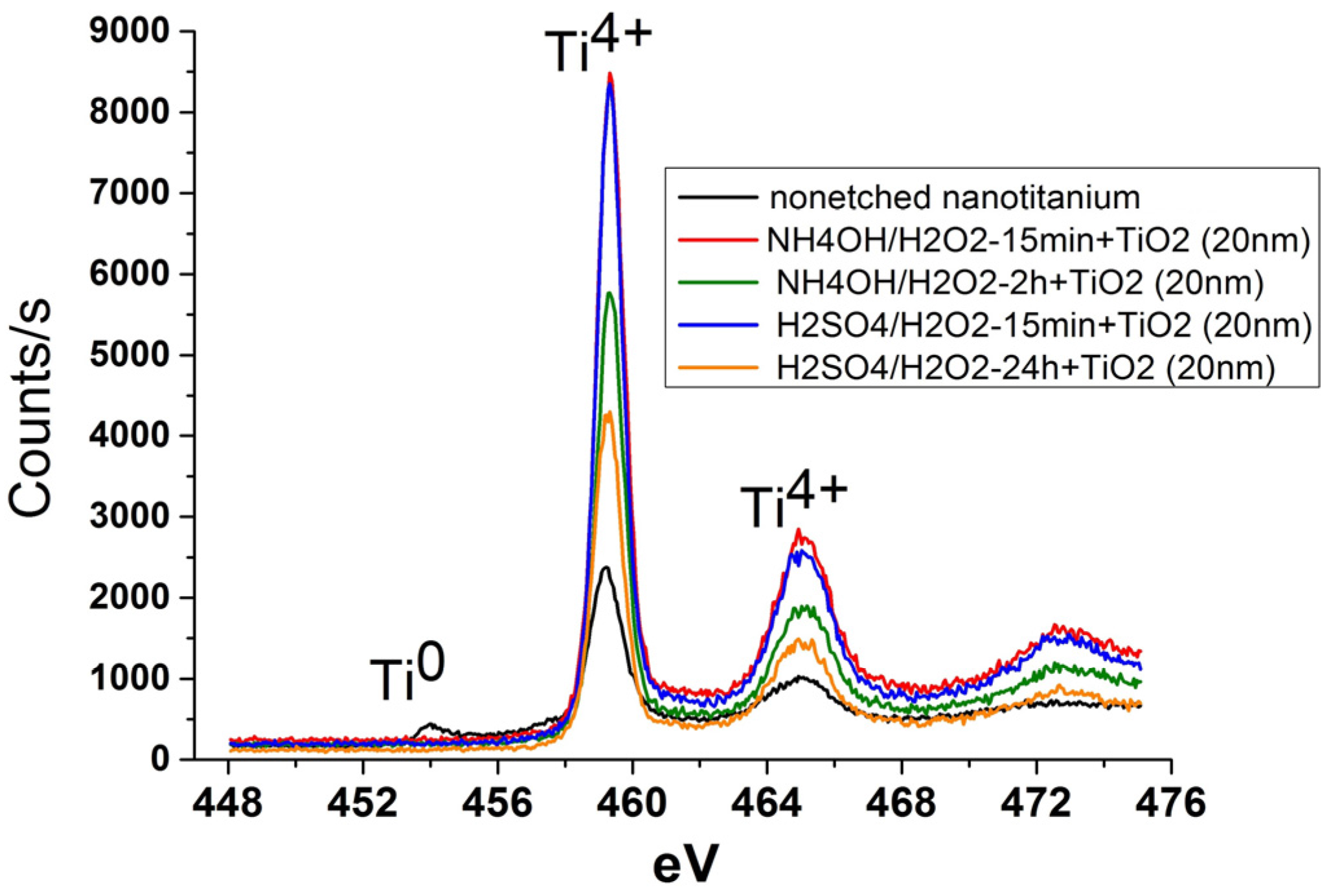
3.2. Deposition of TiO2 Nanocoatings, Structure, Composition and Morphology

- (1)
- NH4OH/H2O2-15 min—“coral” structure.
- (2)
- NH4OH/H2O2-2 h—“mesh” structure.
- (3)
- H2SO4/H2O2-15 min—“sponge” structure.
- (4)
- H2SO4/H2O2-24 h—disordered, developed relief.
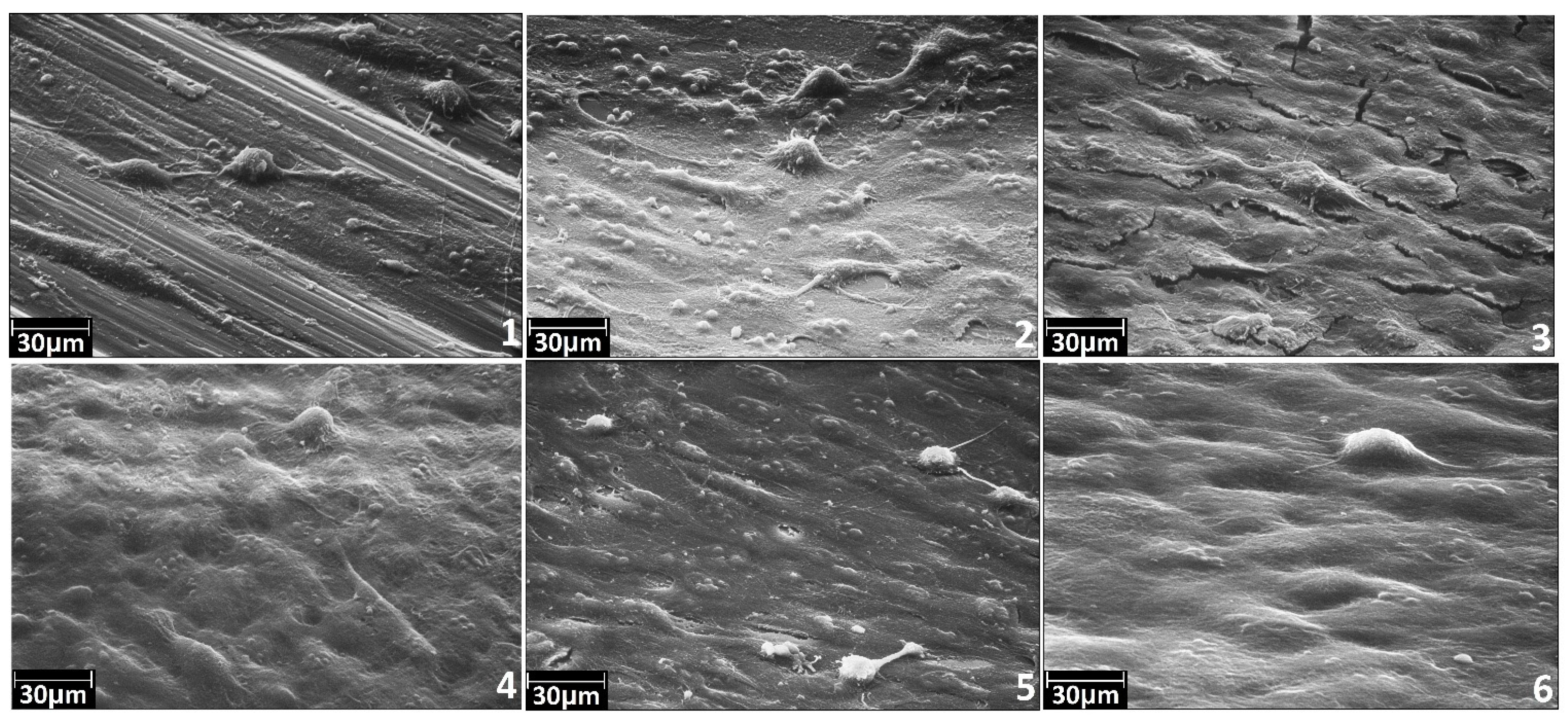
3.3. Adhesive and Spreading Properties of the Human Osteoblasts MG-63
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. (Eds.) Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses, and Medical Applications; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 2001; pp. 1–1019.
- Geetha, M. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.Z.; March, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Xie, K.Y.; Wang, Y.; Zhao, Y.; Chang, L.; Wang, G.; Chen, Z.; Cao, Y.; Liao, X.; Lavernia, E.J.; Valiev, R.Z.; et al. Nanocrystalline β-Ti alloy with high hardness low Young’s modulus and excellent in vitro biocompatibility for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.C.; Valiev, R.Z. Frontiers for bulk nanostructured metals in biomedical applications. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–52. [Google Scholar]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials: Fundamentals and Applications; John Wiley &. Sons, Inc: Hoboken. NJ, USA, 2014; pp. 1–440. [Google Scholar]
- Hench, L.L.; Jones, J.R. Biomaterials, Artificial Organs and Tissue Engineering; Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 15–25. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; KraljIglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Grigal, I.P.; Markeev, A.M.; Gudkova, S.A.; Chernikova, A.G.; Mityaev, A.S.; Alekhin, A.P. Correlation between bioactivity and structural properties of titanium dioxide coatings grown by atomic layer deposition. Appl. Surf. Sci. 2012, 258, 3415–3419. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kukubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. Histomorphometrical and mechanical evaluation of titanium plasma-spray-coated implants placed in the cortical bone of goats. J. Biomed. Mater. Res. 1998, 41, 41–48. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, J.K.; Kim, Y.D.; Choi, K.; Lee, K.H. In vivo behavior and mechanical stability of surface modified titanium implants by plasma spray coating and chemical treatments. J. Biomed. Mater. Res. 2004, 69, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, M.; Filatov, L.; Mishin, M.; Kondrateva, A.; Alexandrov, S. Formation of hydroxylapatite on CVD deposited titania layers. Phys. Status Solidi C 2015, 12, 918–922. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Arbenin, A.Y.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Characteristics of the synthesis of TiO2 films on a titanium surface by the sol-gel technique. Russ. J. Gen. Chem. 2014, 84, 2453–2454. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Smirnov, V.M.; Osmolovsky, M.G. Atomic layer deposition of tin oxide nanofilms: A review. Rev. Adv. Mater. Sci. 2015, 40, 262–275. [Google Scholar]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Osmolowskaya, O.M.; Smirnov, V.M. Growth mechanism of nanodimensional vanadium dioxide on silicon surface obtained by ML-ALD method. Rev. Adv. Mater. Sci. 2011, 27, 184–188. [Google Scholar]
- Dendooven, J.; Devloo-Casier, K.; Ide, M.; Grandfield, K.; Kurttepeli, M.; Ludwig, K.F.; Bals, S.; van der Voort, P.; Detaverniera, C. Atomic layer deposition-based tuning of the pore size in mesoporous thin films studied by in situ grazing incidence small angle X-ray scattering. Nanoscale 2014, 6, 14991–14998. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, E.G.; Arbenin, A.Y.; Plotnikov, A.F.; Smirnov, V.M. Pore radius fine tuning of a silica matrix (MCM-41) based on the synthesis of alumina nanolayers with different thicknesses by atomic layer deposition. J. Vac. Sci. Technol. A 2015, 33, 021519. [Google Scholar] [CrossRef]
- Hwang, C.S.; Yoo, C.Y. Atomic Layer Deposition for Semiconductors; Springer: New York, NY, USA, 2013; pp. 1–263. [Google Scholar]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst design with atomic layer deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Niu, W.; Li, X.; Karuturi, S.K.; Fam, D.W.; Fan, H.; Shrestha, S.; Wong, L.H.; Tok, A.I.Y. Applications of atomic layer deposition in solar cells. Nanotechnology 2015, 26, 064001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Finch, D.S.; Oreskovic, T.; Ramadurai, K.; Herrmann, C.F.; George, S.M.; Mahajan, R.L. Biocompatibility of atomic layer-deposited alumina thin films. J. Biomed. Mater. Res. A 2008, 87, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lynn, A.D.; King, D.M.; Bryant, S.J.; Weimer, A.W. Biocompatible interface films deposited within porous polymers by atomic layer deposition (ALD). ACS Appl. Mater. Interfaces 2009, 1, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Putkonen, M.; Sajavaara, T.; Rahkila, P.; Xu, L.; Cheng, S.; Niinistö, L.; Whitlow, H.J. Atomic layer deposition and characterization of biocompatible hydroxyapatite thin films. Thin Solid Films 2009, 517, 5819–5824. [Google Scholar] [CrossRef]
- Patel, S.; Butt, A.; Tao, Q.; Royhman, D.; Sukotjo, C.; Takoudis, C.G. Novel functionalization of Ti-V alloy and Ti-II using atomic layerdeposition for improved surface wettability. Colloids Surf. B Biointerfaces 2014, 115, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, 2nd ed.; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1995; pp. 1–261. [Google Scholar]
- Vetrone, F.; Variola, F.; de Oliveira, P.T.; Zalzal, S.F.; Yi, J.H.; Sam, J.; Bombonato-Prado, K.F.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Nanoscale oxidative patterning of metallic surfaces to modulate cell activity and fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Brunski, J.B.; Orsini, G.; Tambasco de Oliveira, P.; Wazen, R.; Nanci, A. Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives. Nanoscale 2011, 3, 335–353. [Google Scholar] [CrossRef] [PubMed]
- D’jkonov, G.S.; Stenina, E.V.; Sviridova, E.V.; Salimgareeva, G.C.; Semenova, I.P.; Zemtsova, E.G.; Valiev, R.Z. Regulation of the surface microrelief of coarse-grained and ultrafine-grained titanium by etching method. Mater. Phys. Mech. 2014, 21, 259–265. [Google Scholar]
- Solovyev, A.A.; Ovchinnikov, D.V.; Korostelev, E.V.; Markeev, A.M. Correlation between structural and bioactive properties of titanium dioxide formed by atomic layer deposition. Nanotechnol. Russ. 2013, 8, 388–391. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, D.V.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Formation of Micro- and Nanostructures on the Nanotitanium Surface by Chemical Etching and Deposition of Titania Films by Atomic Layer Deposition (ALD). Materials 2015, 8, 8366-8377. https://doi.org/10.3390/ma8125460
Nazarov DV, Zemtsova EG, Valiev RZ, Smirnov VM. Formation of Micro- and Nanostructures on the Nanotitanium Surface by Chemical Etching and Deposition of Titania Films by Atomic Layer Deposition (ALD). Materials. 2015; 8(12):8366-8377. https://doi.org/10.3390/ma8125460
Chicago/Turabian StyleNazarov, Denis V., Elena G. Zemtsova, Ruslan Z. Valiev, and Vladimir M. Smirnov. 2015. "Formation of Micro- and Nanostructures on the Nanotitanium Surface by Chemical Etching and Deposition of Titania Films by Atomic Layer Deposition (ALD)" Materials 8, no. 12: 8366-8377. https://doi.org/10.3390/ma8125460





